Abstract
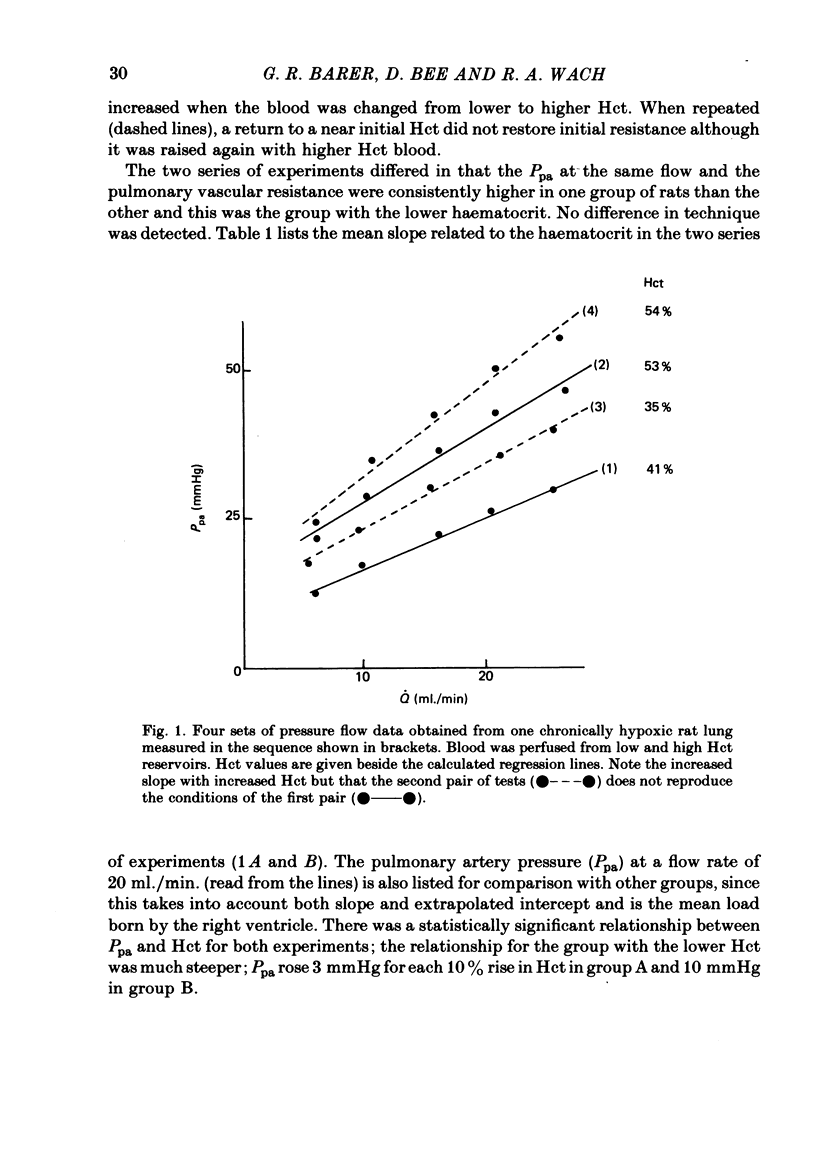
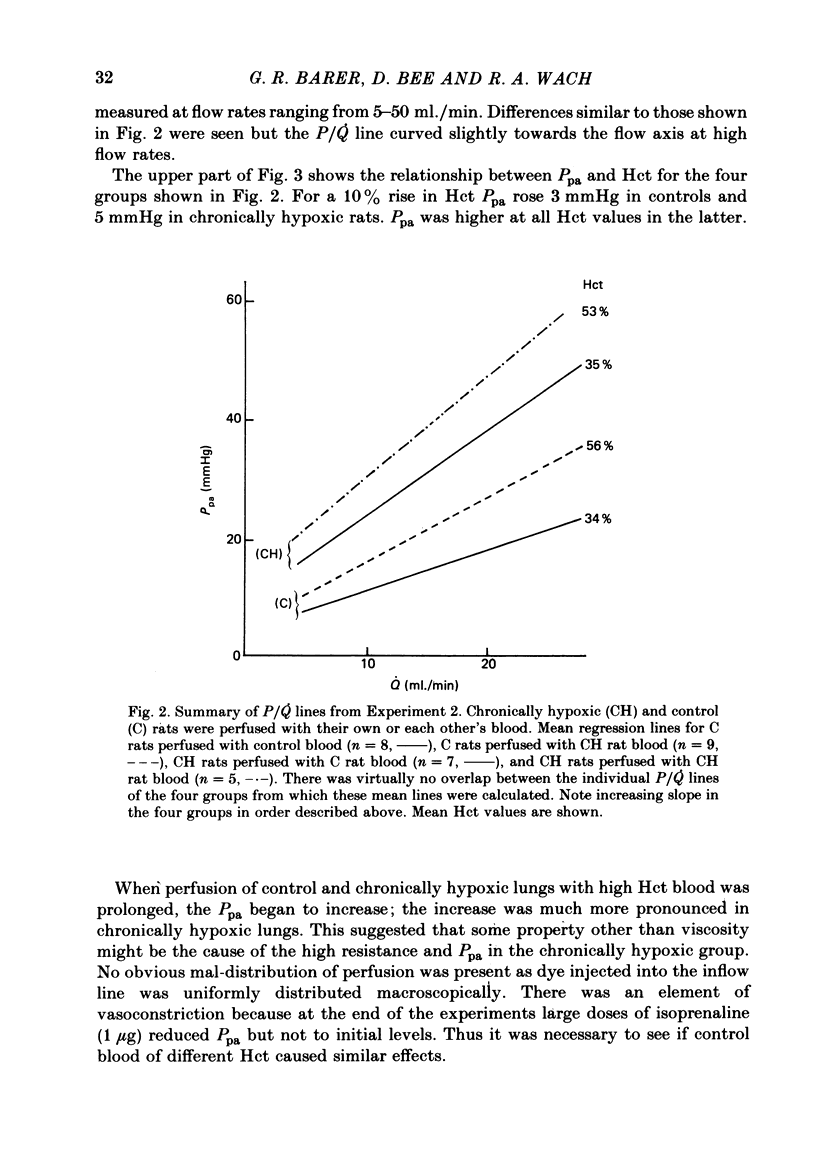
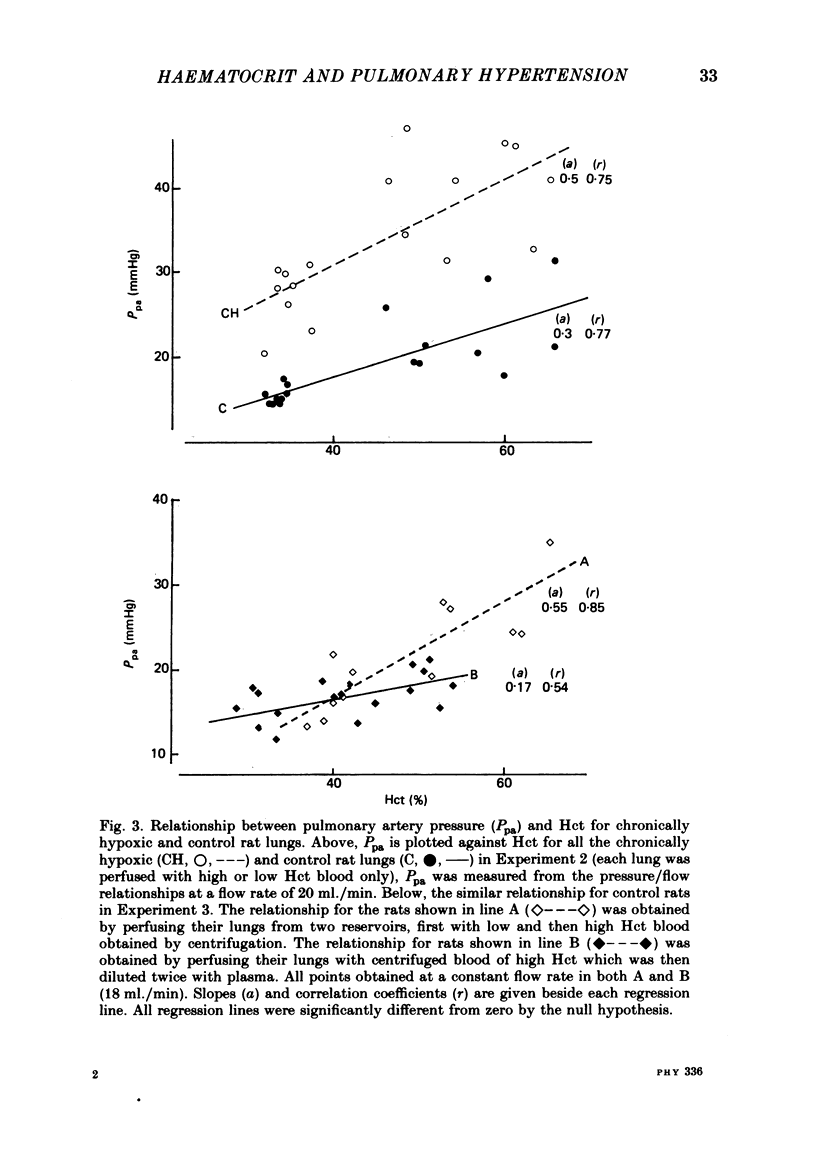
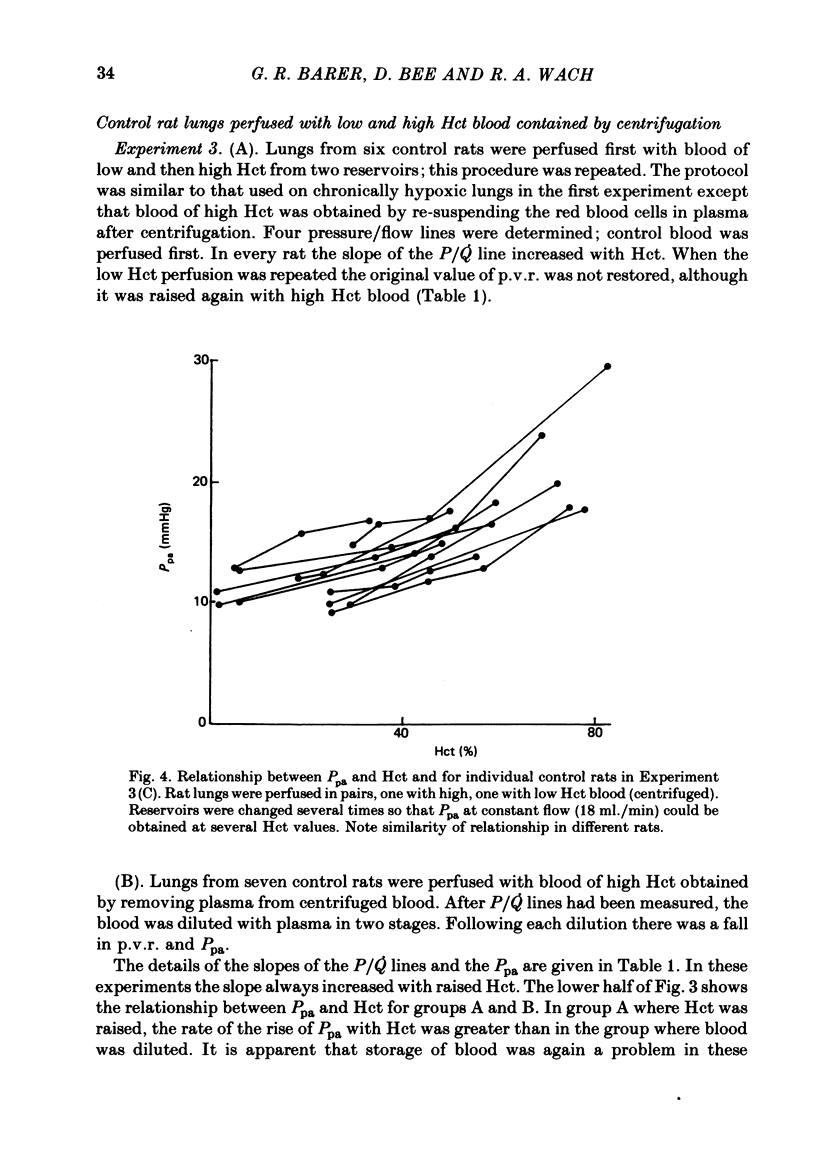
A rat model was used to assess the viscosity factor in pulmonary hypertension of high altitude. Rats exposed to 10% O2 for three weeks developed increased pulmonary vascular resistance (p.v.r.) and polycythaemia; the haematocrit (Hct) was 50-60%, values similar to those in normal men at high altitudes. The contribution of high Hct to the increased p.v.r. was assessed in isolated perfused lungs of chronically hypoxic rats perfused with their own high Hct blood, or normal Hct blood from control rats. Pressure/flow relationships were measured over a wide range and the slope (P/Q) of this relationship and its extrapolated intercept on the pressure axis were increased by high Hct blood. A return to low Hct blood did not restore initial conditions although a second perfusion with high Hct blood again increased p.v.r. and intercept. Lack of reversibility was attributed to changes with time in blood or lung. In a second experiment designed to eliminate time changes, chronically hypoxic or litter-mate control rats were each perfused with only one blood, their own or each other's and P/Q relations were rapidly measured. The P/Q slope and pressure intercept increased progressively in the following groups: control rats perfused with their own blood (Hct 34%), control rats perfused with chronically hypoxic blood (Hct 56%), chronically hypoxic rats perfused with control blood (Hct 35%) and chronically hypoxic rats perfused with chronically hypoxic blood (Hct 53%). To exclude factors in chronically hypoxic blood other than high Hct which might increase p.v.r., control rats were perfused with blood of different Hct obtained by centrifugation. High Hct again increased p.v.r. There was a significant relationship in all rats between pulmonary artery pressure (Ppa), which takes into account both P/Q slope, intercept and Hct. There was substantial batch variation which may reflect sensitivity to hypoxia. In chronically hypoxic rats with high Hct blood, Ppa varied from 29-47 mmHg; with low Hct blood the range was 26-38 mmHg. Comparable values for control rats were 21-29 and 17-20 mmHg. We conclude that the polycythaemic blood of chronic hypoxia contributes substantially to pulmonary hypertension. Where it is excessive, it may prejudice tissue blood flow.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham A. S., Cole R. B., Bishop J. M. Reversal of pulmonary hypertension by prolonged oxygen administration to patients with chronic bronchitis. Circ Res. 1968 Jul;23(1):147–157. doi: 10.1161/01.res.23.1.147. [DOI] [PubMed] [Google Scholar]
- Abraham A. S., Cumming A. G., Horsfield R., Prowse K. Regional hypoxia and distribution of pulmonary blood flow. Scand J Respir Dis. 1970;51(1):33–36. [PubMed] [Google Scholar]
- Barer G. R., Barer R., Robinson K. A solid-state 50 Hz electromagnetic flowmeter. J Physiol. 1972 Apr;222(1):8P–9P. [PubMed] [Google Scholar]
- Emery C. J., Bee D., Barer G. R. Mechanical properties and reactivity of vessels in isolated perfused lungs of chronically hypoxic rats. Clin Sci (Lond) 1981 Nov;61(5):569–580. doi: 10.1042/cs0610569. [DOI] [PubMed] [Google Scholar]
- Hauge A. Role of histamine in hypoxic pulmonary hypertension in the rat. I. Blockade or potentiation of endogenous amines, kinins, and ATP. Circ Res. 1968 Mar;22(3):371–383. doi: 10.1161/01.res.22.3.371. [DOI] [PubMed] [Google Scholar]
- Herget J., Suggett A. J., Leach E., Barer G. R. Resolution of pulmonary hypertension and other features induced by chronic hypoxia in rats during complete and intermittent normoxia. Thorax. 1978 Aug;33(4):468–473. doi: 10.1136/thx.33.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter C., Barer G. R., Shaw J. W., Clegg E. J. Growth of the heart and lungs in hypoxic rodents: a model of human hypoxic disease. Clin Sci Mol Med. 1974 Mar;46(3):375–391. doi: 10.1042/cs0460375. [DOI] [PubMed] [Google Scholar]
- Kentera D., Susić D., Cvetković A., Djordjević G. Effects of SQ 14.225, an orally active inhibitor of angiotensin-converting enzyme, on hypoxic pulmonary hypertension and right ventricular hypertrophy in rats. Basic Res Cardiol. 1981 May-Jun;76(3):344–351. doi: 10.1007/BF01907777. [DOI] [PubMed] [Google Scholar]
- Leach E., Howard P., Barer G. R. Resolution of hypoxic changes in the heart and pulmonary arterioles of rats during intermittent correction of hypoxia. Clin Sci Mol Med. 1977 Feb;52(2):153–162. doi: 10.1042/cs0520153. [DOI] [PubMed] [Google Scholar]
- Lockhart A., Zelter M., Mensch-Dechene J., Antezana G., Paz-Zamora M., Vargas E., Coudert J. Pressure-flow-volume relationships in pulmonary circulation of normal highlanders. J Appl Physiol. 1976 Oct;41(4):449–456. doi: 10.1152/jappl.1976.41.4.449. [DOI] [PubMed] [Google Scholar]
- McGrath R. L., Weil J. V. Adverse effects of normovolemic polycythemia and hypoxia on hemodynamics in the dog. Circ Res. 1978 Nov;43(5):793–798. doi: 10.1161/01.res.43.5.793. [DOI] [PubMed] [Google Scholar]
- Monge C. C., Whittembury J. Chronic mountain sickness. Johns Hopkins Med J. 1976 Dec;139(Suppl):87–89. [PubMed] [Google Scholar]
- Murray J. F., Karp R. B., Nadel J. A. Viscosity effects on pressure-flow relations and vascular resistance in dogs' lungs. J Appl Physiol. 1969 Sep;27(3):336–341. doi: 10.1152/jappl.1969.27.3.336. [DOI] [PubMed] [Google Scholar]
- Wade J. P., Pearson T. C., Russell R. W., Wetherley-Mein G. Cerebral blood flow and blood viscosity in patients with polycythaemia secondary to hypoxic lung disease. Br Med J (Clin Res Ed) 1981 Sep 12;283(6293):689–692. doi: 10.1136/bmj.283.6293.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will D. H., Hicks J. L., Card C. S., Alexander A. F. Inherited susceptibility of cattle to high-altitude pulmonary hypertension. J Appl Physiol. 1975 Mar;38(3):491–494. doi: 10.1152/jappl.1975.38.3.491. [DOI] [PubMed] [Google Scholar]
- Willison J. R., Thomas D. J., du Boulay G. H., Marshall J., Paul E. A., Pearson T. C., Russell R. W., Symon L., Wetherley-Mein G. Effect of high haematocrit on alertness. Lancet. 1980 Apr 19;1(8173):846–848. doi: 10.1016/s0140-6736(80)91353-7. [DOI] [PubMed] [Google Scholar]


