Abstract
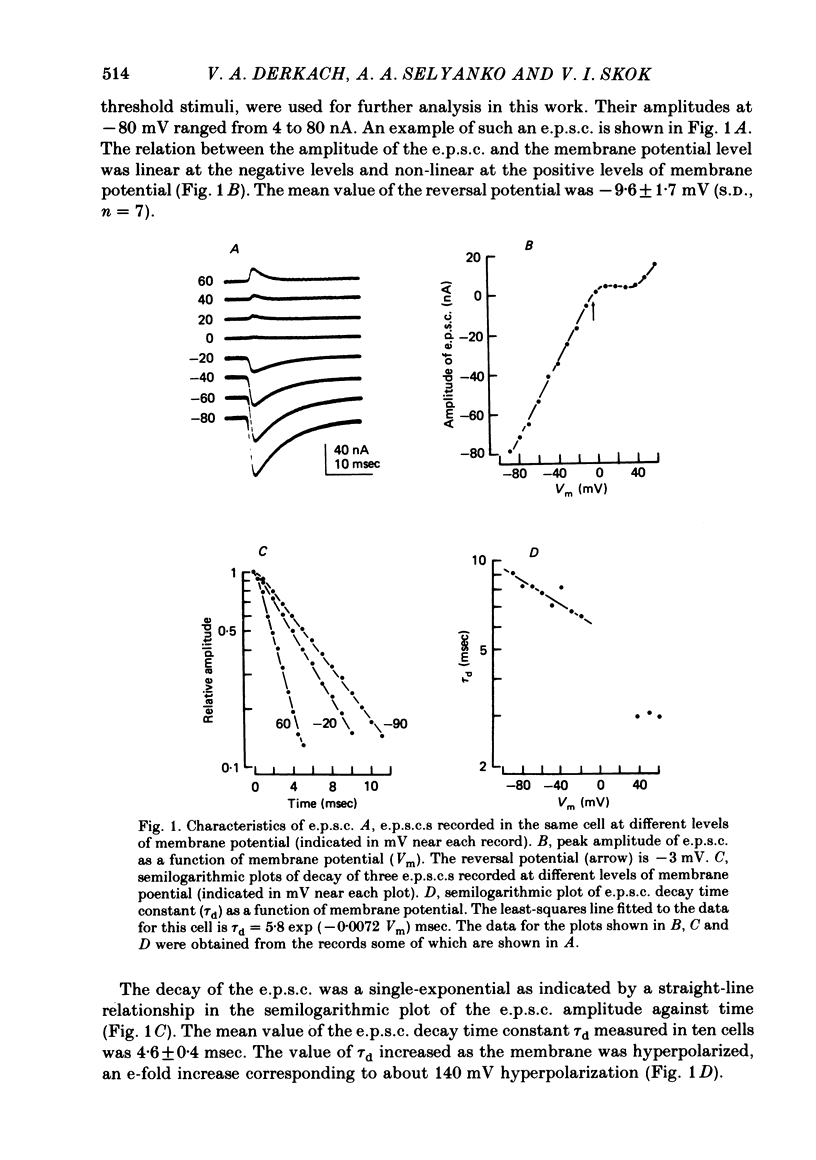
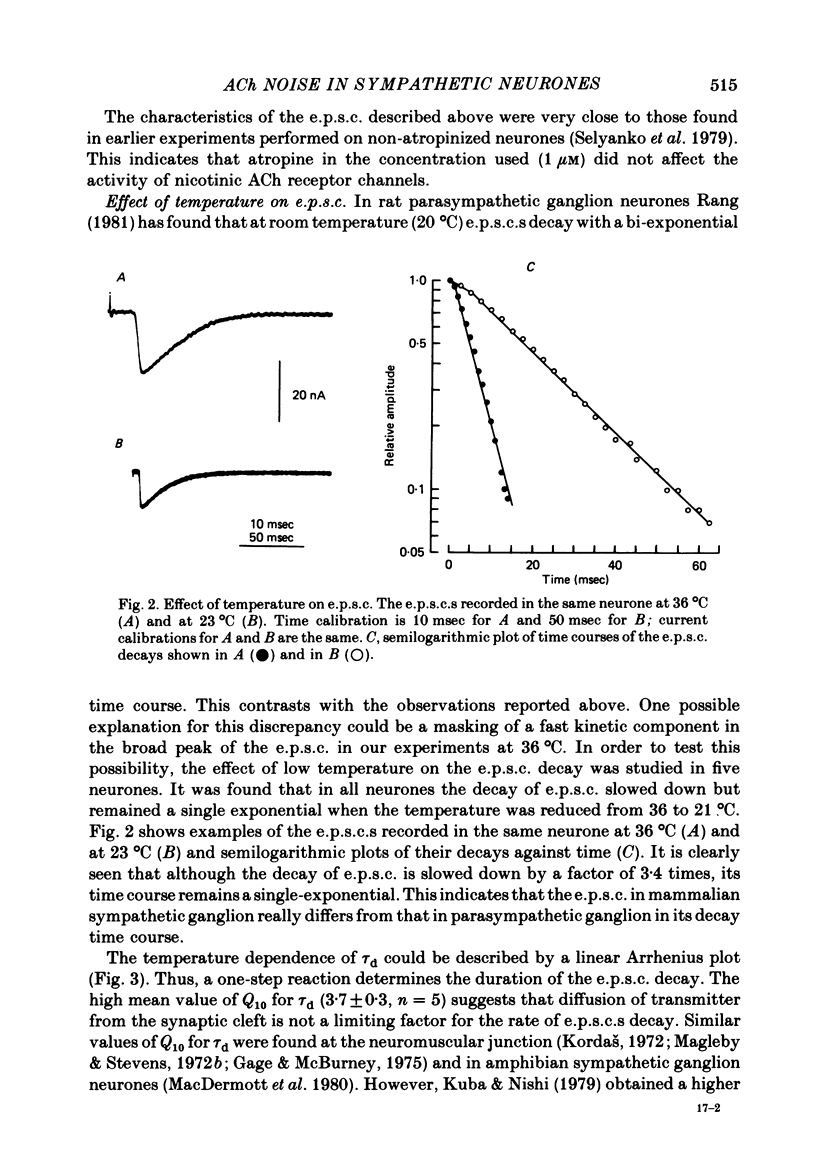
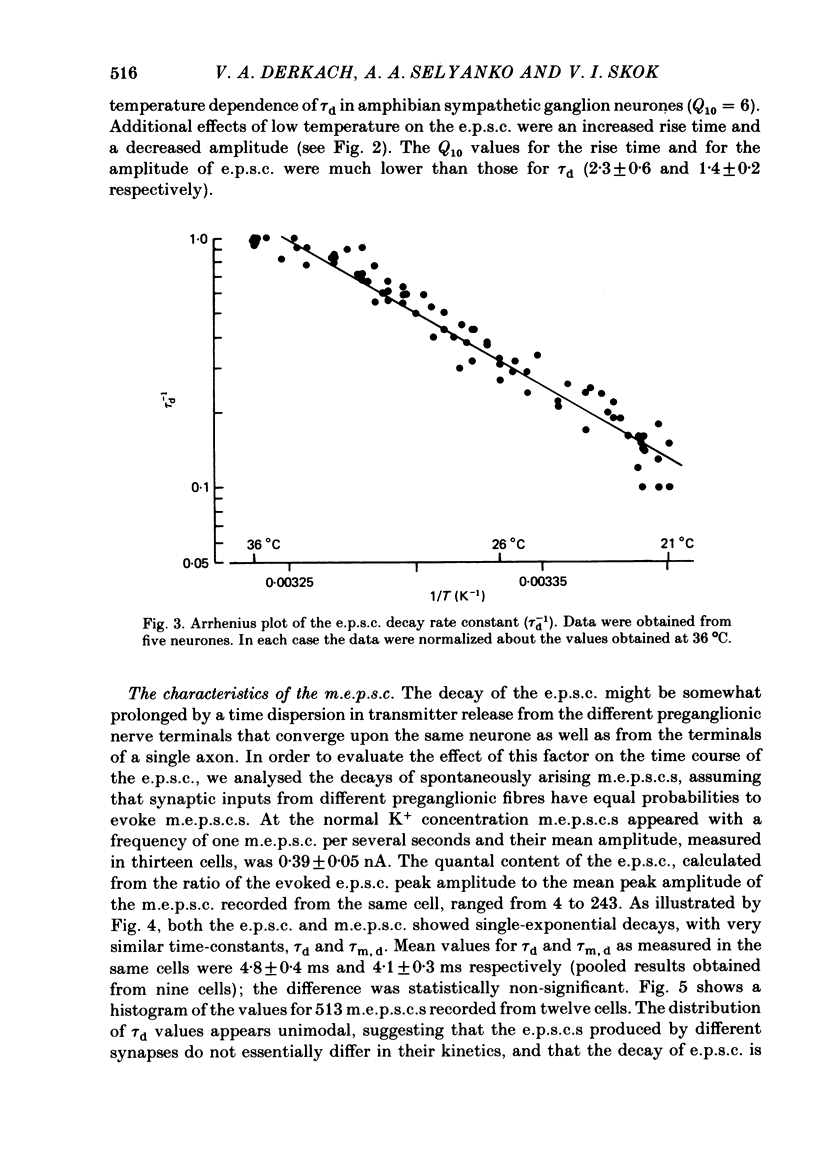
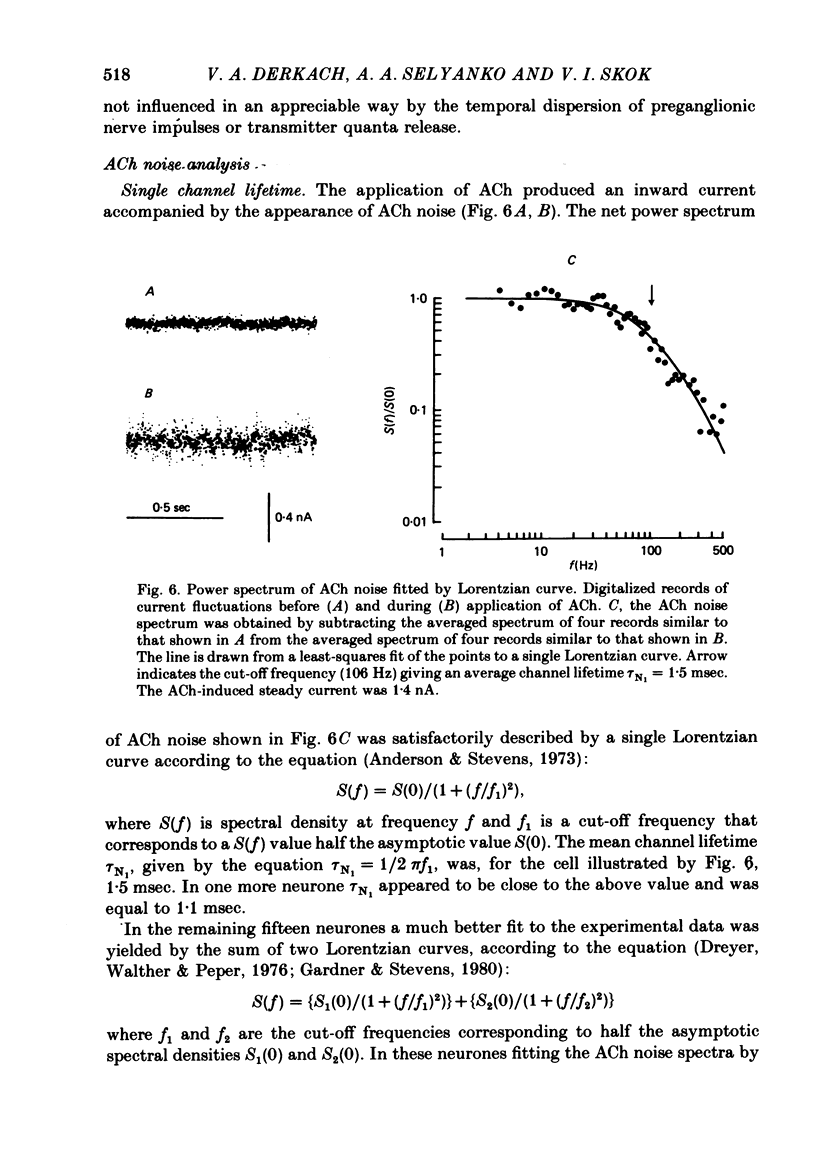
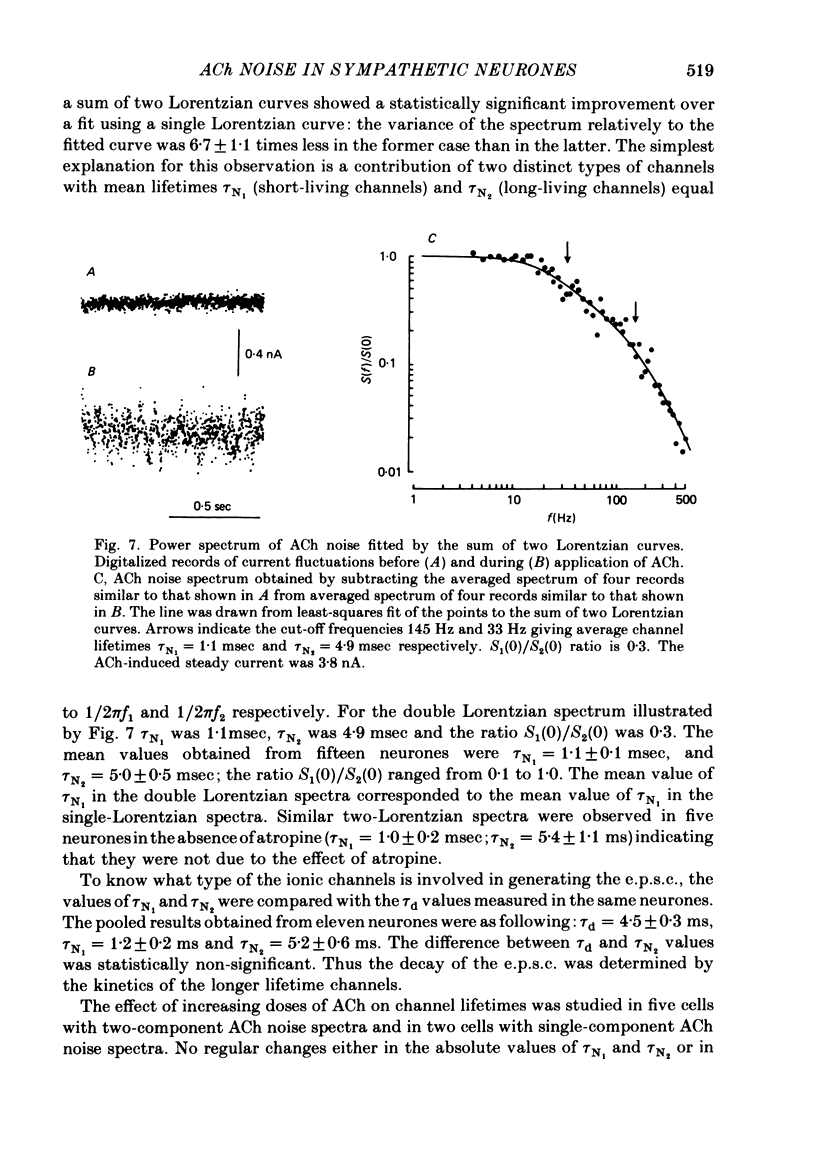
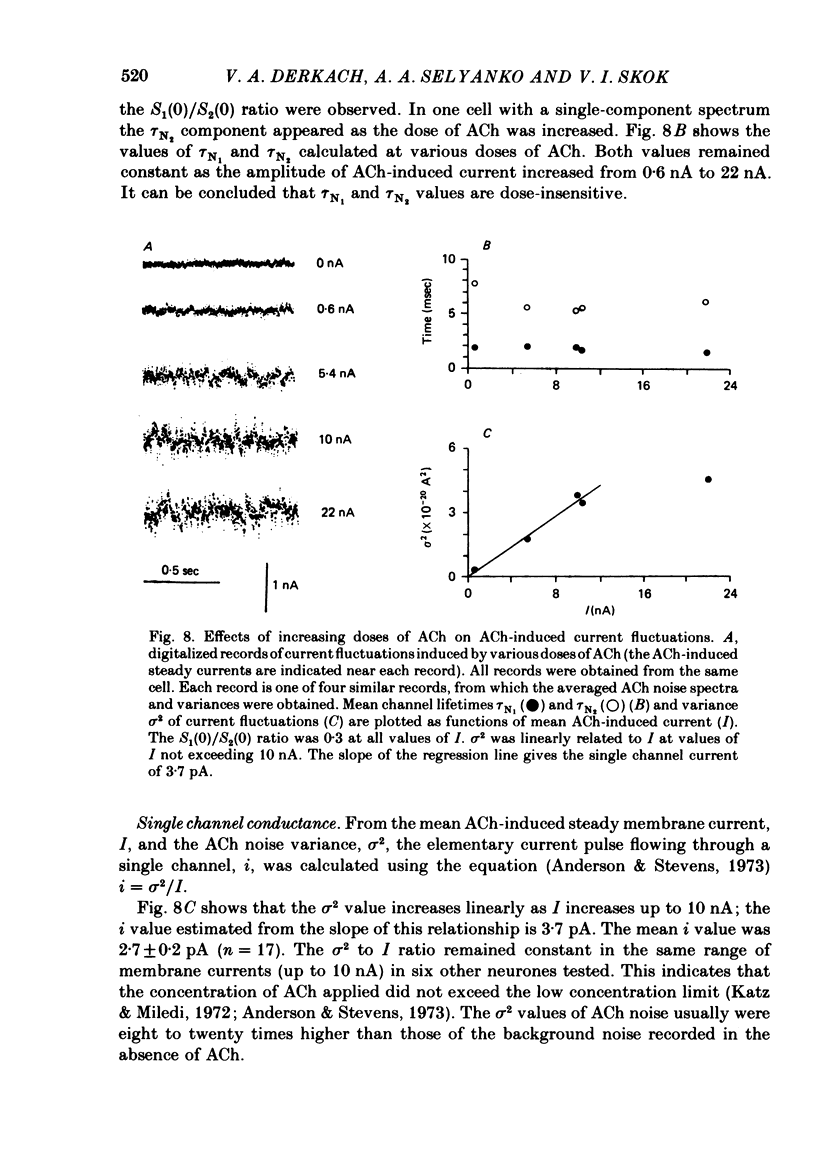
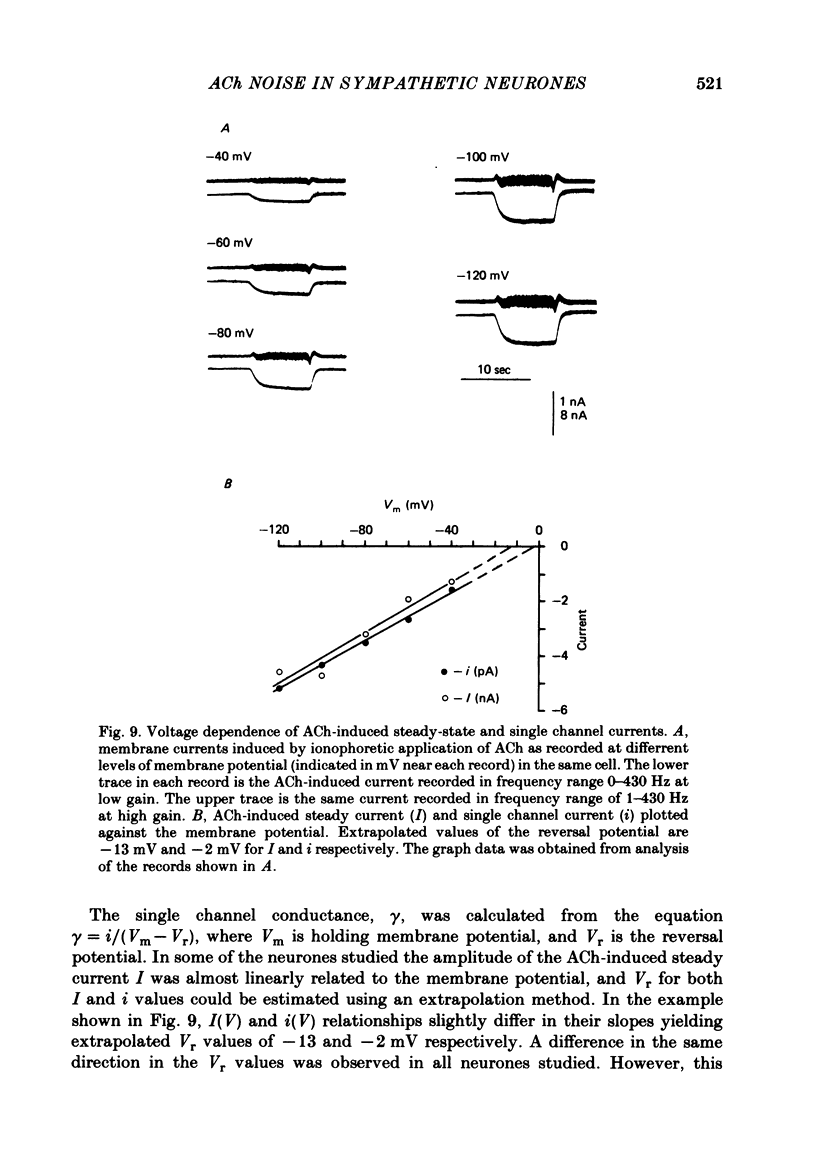
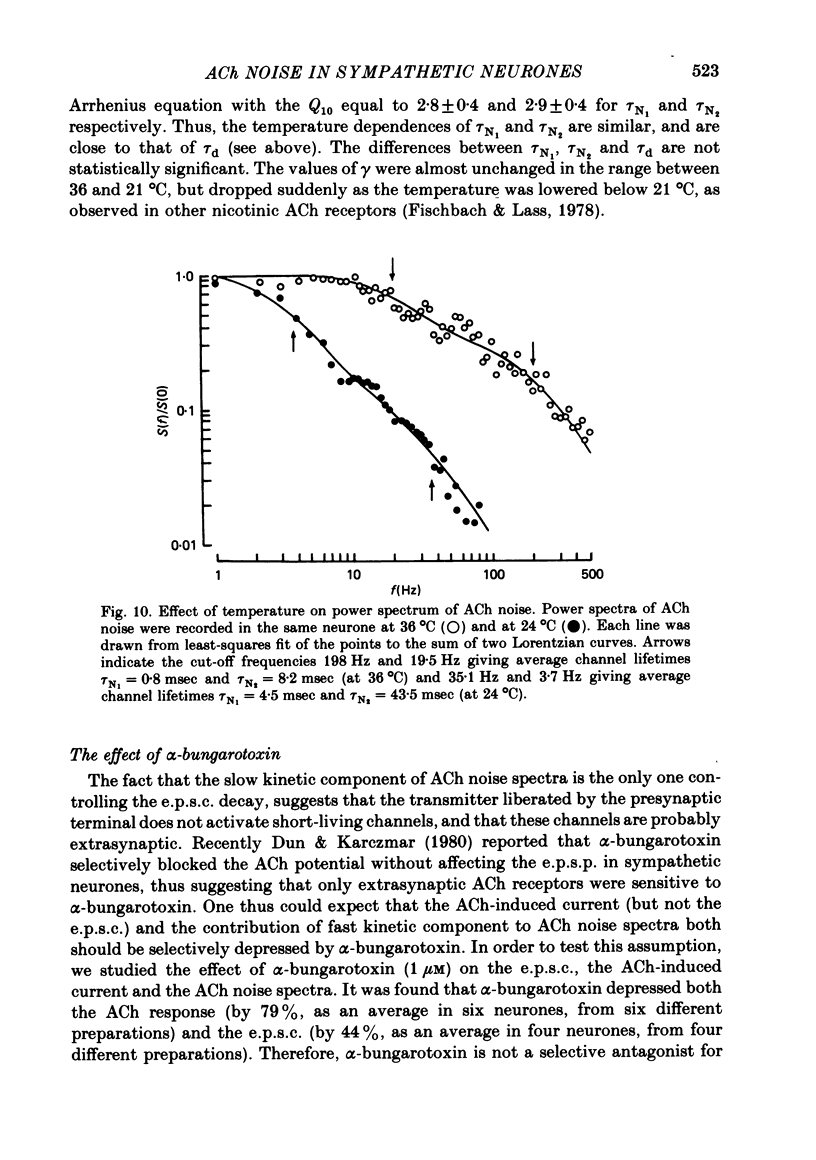
Post-synaptic currents and responses to ionophoretically applied acetylcholine (ACh) were recorded at 34-37 degrees C from rabbit superior cervical ganglion neurones clamped at -80 mV membrane potential. Atropine (1 microM) was used to block muscarinic receptors. The fast excitatory post-synaptic current (e.p.s.c.) reversed at -9.6 +/- 1.7 mV and decayed with a single exponential time course. The e.p.s.c. decay time constant, tau d, was 4.5 +/- 0.3 msec and increased as the membrane was hyperpolarized (e-fold increase in tau d corresponded to 140 mV hyperpolarization). Miniature e.p.s.c.s. (m.e.p.s.c.s) decayed with time constants similar to those of the e.p.s.c. The decay of the e.p.s.c. was slowed by lowering temperature but remained a single exponential; the changes of tau d with temperature followed the Arrhenius equation (Q10 = 3.7). In most of the neurones studied the analysis of ACh noise spectra revealed two kinetic components with mean time constants tau N1 = 1.1 +/- 0.1 msec and tau N2 = 5.0 +/- 0.5 msec. In a few neurones only the tau N1 component was found. Similar two-component ACh noise spectra were observed in the neurones not treated with atropine. tau N1 and tau N2 components revealed temperature dependences similar to each other and close to that of tau d. The values of tau N1 and tau N2 and the ratio between the contributions of the tau N1 and tau N2 components to the ACh noise spectrum did not depend on the dose of ACh. The single channel conductance is 36 +/- 3 pS. A single ACh quantum opens about 150 ionic channels and the e.p.s.c. consists of 4-243 quanta. It is suggested that in mammalian sympathetic ganglion neurones there are two types of nicotinic ACh receptor channels, with short and long lifetimes, and that the kinetics of e.p.s.c. and m.e.p.s.c. are determined by the activity of the longer lifetime channel type.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Large W. A., Rang H. P. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol. 1979 Oct;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. Life time and elementary conductance of the channels mediating the excitatory effects of acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:177–206. doi: 10.1113/jphysiol.1978.sp012299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. The mode of action of antagonists of the excitatory response to acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:207–235. doi: 10.1113/jphysiol.1978.sp012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Large W. A., Rang H. P. An analysis of the action of a false transmitter at the neuromuscular junction. J Physiol. 1977 Apr;266(2):361–395. doi: 10.1113/jphysiol.1977.sp011772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V. A., Selianko A. A., Skok V. I. Analiz atsetilkholinovogo shuma v neironakh simpaticheskogo gangliia mlekopitaiushchego. Dokl Akad Nauk SSSR. 1981;259(4):981–984. [PubMed] [Google Scholar]
- Dionne V. E., Parsons R. L. Characteristics of the acetylcholine-operated channel at twitch and slow fibre neuromuscular junctions of the garter snake. J Physiol. 1981 Jan;310:145–158. doi: 10.1113/jphysiol.1981.sp013541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Karczmar A. G. Blockade of ACh potentials by alpha-bungarotoxin in rat superior cervical ganglion cells. Brain Res. 1980 Sep 8;196(2):536–540. doi: 10.1016/0006-8993(80)90421-7. [DOI] [PubMed] [Google Scholar]
- Fischbach G. D., Lass Y. A transition temperature for acetylcholine channel conductance in chick myoballs. J Physiol. 1978 Jul;280:527–536. doi: 10.1113/jphysiol.1978.sp012399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D., Stevens C. F. Rate-limiting step of inhibitory post-synaptic current decay in Aplysia buccal ganglia. J Physiol. 1980 Jul;304:145–164. doi: 10.1113/jphysiol.1980.sp013316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D. Time integral of synaptic conductance. J Physiol. 1980 Jul;304:181–191. doi: 10.1113/jphysiol.1980.sp013318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., NELSON P. G. STRUCTURAL AND FUNCTIONAL CHANGES IN THE FROG SYMPATHETIC GANGLION FOLLOWING CUTTING OF THE PRESYNAPTIC NERVE FIBRES. J Physiol. 1965 Mar;177:1–20. doi: 10.1113/jphysiol.1965.sp007571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koketsu K., Nishi S., Soeda H. Calcium and acetylcholine-potential of bullfrog sympathetic ganglion cell membrane. Life Sci. 1968 Sep 1;7(17):955–963. doi: 10.1016/0024-3205(68)90102-1. [DOI] [PubMed] [Google Scholar]
- Kuba K., Nishi S. Characteristics of fast excitatory postsynaptic current in bullfrog sympathetic ganglion cells. Effects of membrane potential, temperature and Ca ions. Pflugers Arch. 1979 Jan 31;378(3):205–212. doi: 10.1007/BF00592737. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Connor E. A., Dionne V. E., Parsons R. L. Voltage clamp study of fast excitatory synaptic currents in bullfrog sympathetic ganglion cells. J Gen Physiol. 1980 Jan;75(1):39–60. doi: 10.1085/jgp.75.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. A quantitative description of end-plate currents. J Physiol. 1972 May;223(1):173–197. doi: 10.1113/jphysiol.1972.sp009840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Dreyer F., Peper K. Current-voltage relation and reversal potential at junctional and extrajunctional ACh-receptors of the frog neuromuscular junction. Pflugers Arch. 1976 Mar 11;362(1):43–47. doi: 10.1007/BF00588679. [DOI] [PubMed] [Google Scholar]
- McAfee D. A., Yarowsky P. J. Calcium-dependent potentials in the mammalian sympathetic neurone. J Physiol. 1979 May;290(2):507–523. doi: 10.1113/jphysiol.1979.sp012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Noise analysis of drug induced voltage clamp currents in denervated frog muscle fibres. J Physiol. 1976 Jul;258(3):705–729. doi: 10.1113/jphysiol.1976.sp011442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D. J., Sachs F. Single ionic channels observed in tissue-cultured muscle. Nature. 1979 Dec 20;282(5741):861–863. doi: 10.1038/282861a0. [DOI] [PubMed] [Google Scholar]
- Pappano A. J., Volle R. L. Observations on the role of calcium ions in ganglionic responses to acetylcholine. J Pharmacol Exp Ther. 1966 May;152(2):171–180. [PubMed] [Google Scholar]
- Rang H. P. The characteristics of synaptic currents and responses to acetylcholine of rat submandibular ganglion cells. J Physiol. 1981 Feb;311:23–55. doi: 10.1113/jphysiol.1981.sp013571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Selyanko A. A., Derkach V. A., Skok V. I. Fast excitatory postsynaptic currents in voltage-clamped mammalian sympathetic ganglion neurones. J Auton Nerv Syst. 1979 Dec;1(2):127–137. doi: 10.1016/0165-1838(79)90011-0. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]


