Abstract
1. The effects of cocaine on the neuromuscular transmission and smooth muscle cells of the guinea-pig mesenteric artery were observed using various experimental procedures.
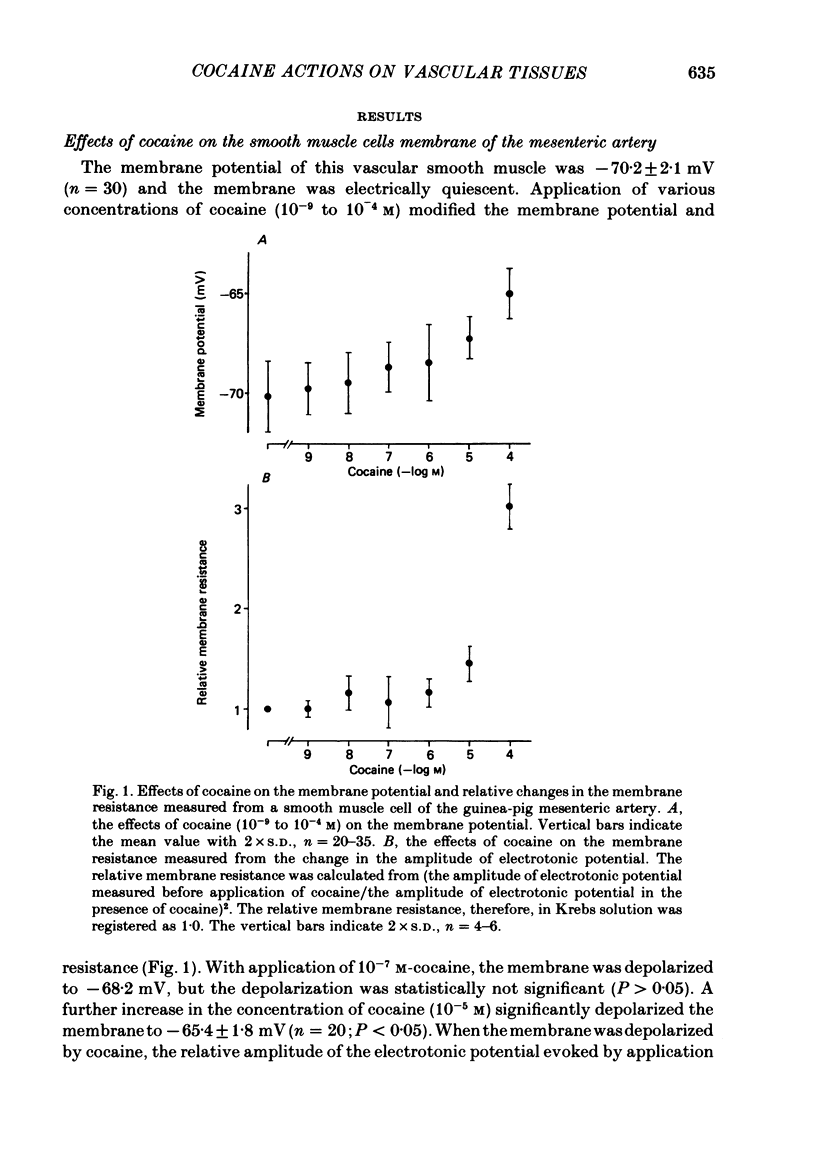
2. Cocaine (10-7 M) depolarized the membrane and increased the membrane resistance of single smooth muscle cells. Outward current pulses produced neither spikes nor graded responses in Krebs solution, but in the presence of 10-5 M-cocaine, outward current did produce spikes.
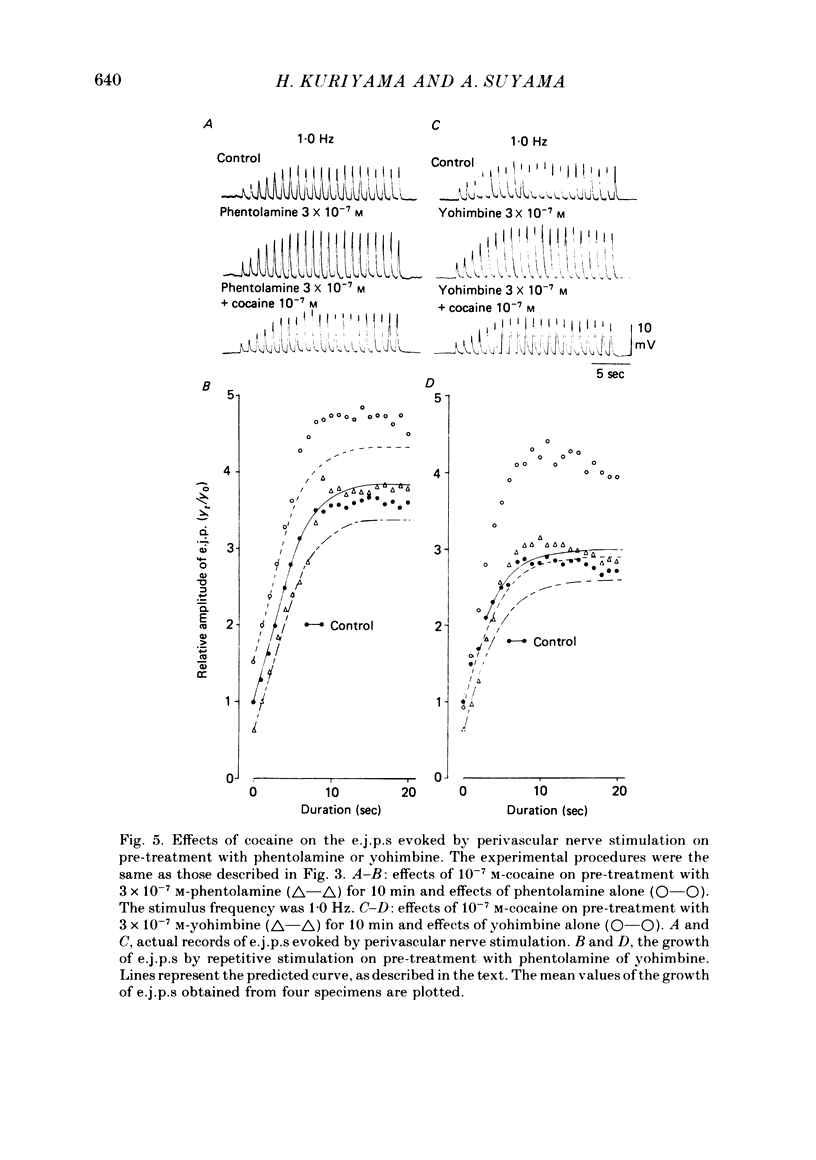
3. Perivascular nerve stimulation evoked excitatory junction potentials (e.j.p.s). Repetitive stimulation (0·25-1·0 Hz) produced a frequency-dependent facilitation. Application of cocaine (10-7 M) reduced the amplitude of the first e.j.p. (e.j.p.(f)) and also after completion of facilitation (e.j.p.(s)). However, the facilitation process was not affected by cocaine (10-5 M).
4. On pre-treatment with phentolamine (3 × 10-7 M), both e.j.p.(f) and e.j.p.(s) were enhanced, but on pre-treatment with yohimbine (3 × 10-7 M), e.j.p.(f) was inhibited and e.j.p.(s) was enhanced. Both phentolamine and yohimbine accelerated the facilitation of e.j.p.s in the absence and presence of cocaine (10-7 M).
5. The conduction velocity of nerve excitation measured from the latency of generation of e.j.p.s was slightly lowered by cocaine. The number of nerve fibres or varicosities contributing to the generation of an e.j.p. was not reduced in the presence of cocaine (10-5 M).
6. Mechanical responses could be recorded on perivascular nerve stimulation, and direct muscle stimulation on treatment with tetrodotoxin. Cocaine (10-7 to 10-4 M) enhanced the contraction evoked by direct muscle stimulation and inhibited the contraction evoked by perivascular nerve stimulation.
7. Cocaine (10-5 M) enhanced the contraction evoked by 5 × 10-6 M-noradrenaline (NA) and direct muscle stimulation (5 sec pulse) but no effect was observed on the K-induced contraction (39·2 mM-K). On pre-treatment with guanethidine (10-6 M) these effects of cocaine were not affected.
8. In the presence of cocaine (10-5 M), the depolarization of the membrane induced by NA was additively increased, and the dose response curve for NA was shifted to the left with no change in the maximum amplitude of contraction.
9. When 10-5 M-cocaine was applied during contractions evoked by alternate perivascular nerve stimulation and exogenously applied NA, the contraction evoked by perivascular nerve stimulation was reduced, while that evoked by NA was enhanced.
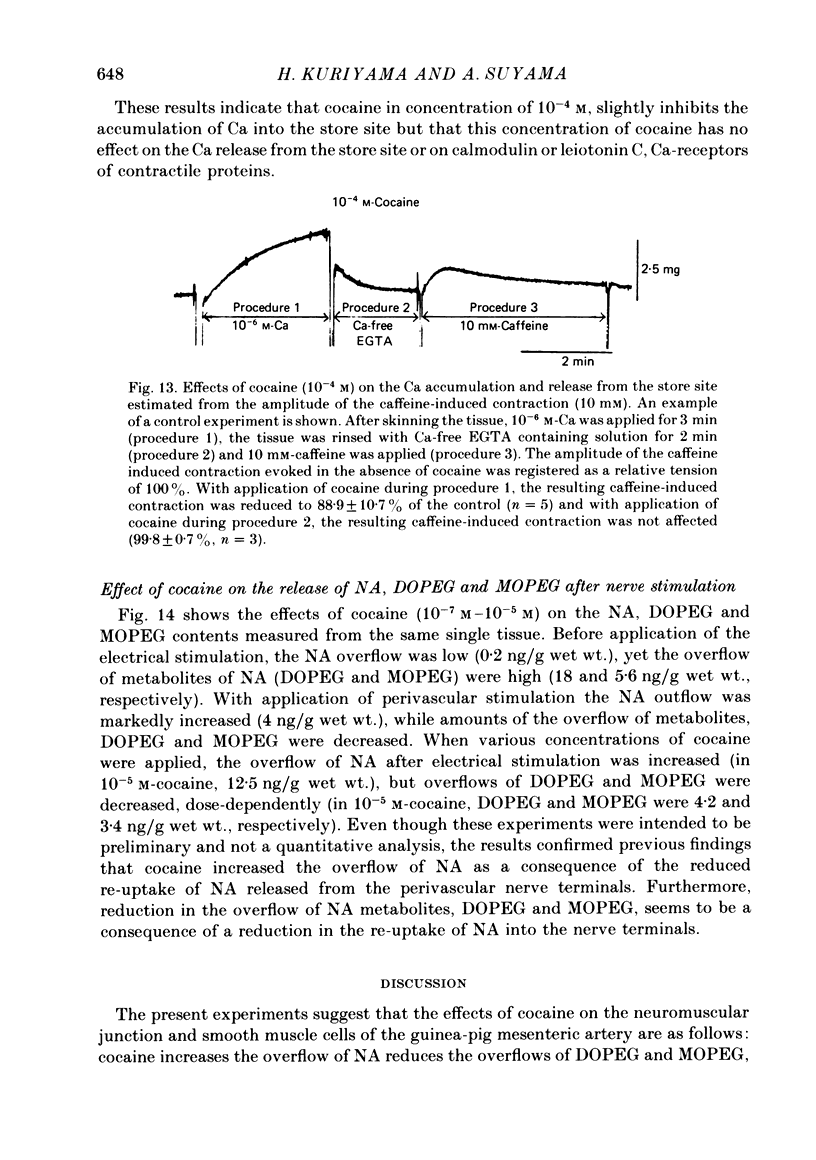
10. In saponin-treated skinned muscles, the pCa—tension relationship was not affected by application of 10-4 M-cocaine. The effects of cocaine on the Ca accumulation and release from the store site were estimated. It was found that cocaine (10-4 M) slightly inhibited the Ca accumulation (0·89 times the control) but did not modify the Ca-release mechanism.
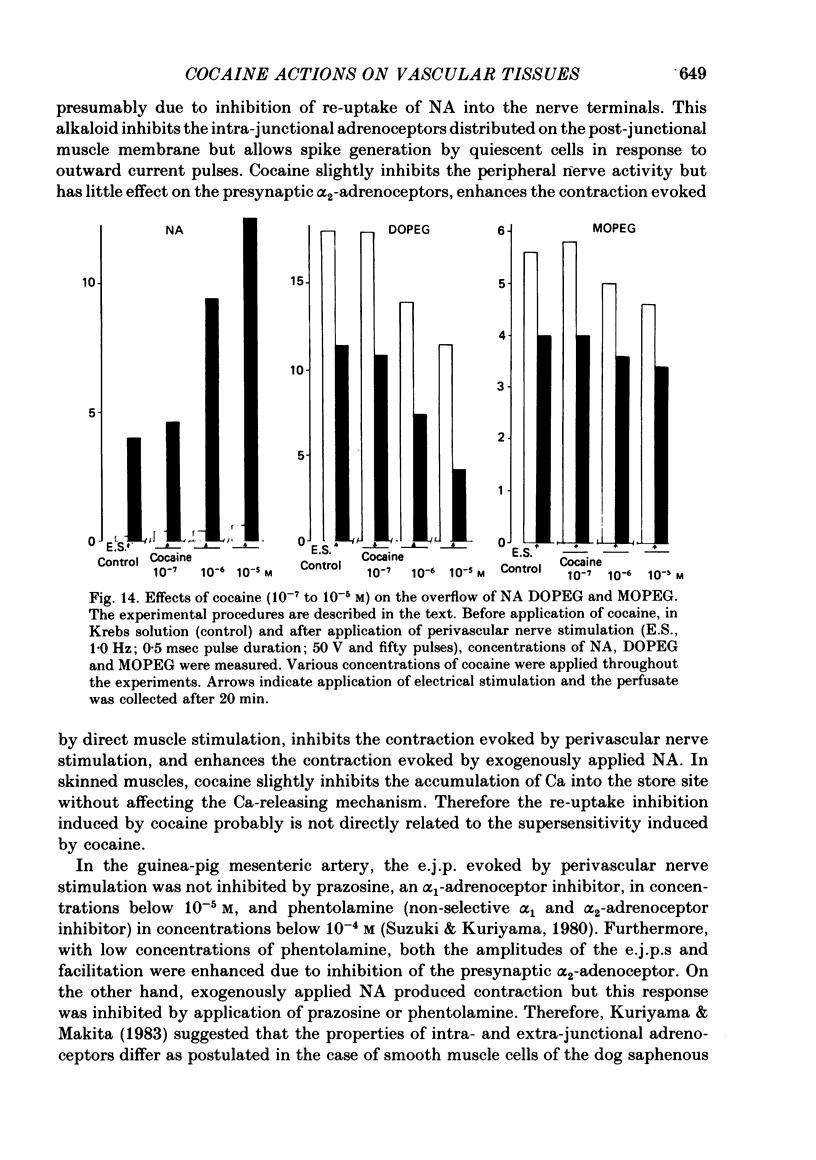
11. The overflow of NA, 3,4-dihydroxyphenylglycol (DOPEG) and 3-methoxy-4-hydroxyphenylglycol (MOPEG) were measured in the same tissue before and after application of perivascular nerve stimulation in the presence or absence of cocaine (10-7 to 10-5 M). Cocaine induced a concentration-dependent increase in the overflow of NA and a reduction in the amounts of DOPEG and MOPEG.
12. We conclude from these studies that cocaine mainly inhibits the sensitivity of the intra-junctional adrenoceptor, but increases the sensitivity of the extrajunctional adrenoceptor distributed on the post-junctional muscle membrane, with increase in the overflow of NA. The enhancement of mechanical response in the presence of cocaine is probably due to an increased sensitivity of the extra-junctional adrenoceptor and changes in the post-junctional muscle membrane, without any marked effect on the prejunctional mechanism.
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Membrane potentials of smooth muscle fibres of the taenia coli of the guinea-pig. J Physiol. 1954 Aug 27;125(2):302–315. doi: 10.1113/jphysiol.1954.sp005159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T. An electrophysiological analysis of the effect of Ca ions on neuromuscular transmission in the mouse vas deferens. Br J Pharmacol. 1975 Sep;55(1):97–104. doi: 10.1111/j.1476-5381.1975.tb07616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A., Waldeck B. Different mechanisms of drug-induced release of noradrenaline and its congeners alpha-methylnoradrenaline and metaraminol. Eur J Pharmacol. 1968 Sep;4(2):165–168. doi: 10.1016/0014-2999(68)90172-6. [DOI] [PubMed] [Google Scholar]
- Davidson W. J., Innes I. R. Dissociation of potentiation of isoprenaline by cocaine from inhibition of uptake in cat spleen. Br J Pharmacol. 1970 May;39(1):175–181. doi: 10.1111/j.1476-5381.1970.tb09567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R. F., KIRPEKAR S. M., RIEKER M., SCHWAB A. ACTIONS AND INTERACTIONS OF NOREPINEPHRINE, TYRAMINE AND COCAINE ON AORTIC STRIPS OF RABBIT AND LEFT ATRIA OF GUINEA PIG AND CAT. J Pharmacol Exp Ther. 1963 Oct;142:39–58. [PubMed] [Google Scholar]
- Granata A. R., Langer S. Z. Effects of cocaine or denervation on responses of isolated strips of cat spleen to (-)-noradrenaline and (-)-isoprenaline. Br J Pharmacol. 1973 Aug;48(4):667–675. doi: 10.1111/j.1476-5381.1973.tb08255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R. D., 3rd, Fleming W. W. Analysis of supersensitivity in the isolated spleen of the cat. J Pharmacol Exp Ther. 1968 Aug;162(2):254–262. [PubMed] [Google Scholar]
- Greenberg R., Innes I. R. The role of bound calcium in supersensitivity induced by cocaine. Br J Pharmacol. 1976 Jul;57(3):329–334. doi: 10.1111/j.1476-5381.1976.tb07670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S., Long J. P. The effects of cocaine, norepinephrine and ionic stimulants on the isolated, superfused rat vas deferens: antagonism by "adrenergic neuron blockers" and reserpine. J Pharmacol Exp Ther. 1971 Apr;177(1):136–145. [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Hashiguchi T., Ito Y., Kuriyama H. Effects of cocaine on a hypogastric nerve-vas deferens preparation of the guinea pig. Jpn J Physiol. 1974 Dec;24(6):633–647. doi: 10.2170/jjphysiol.24.633. [DOI] [PubMed] [Google Scholar]
- Hirata M., Mikawa T., Nonomura Y., Ebashi S. Ca2+ regulation in vascular smooth muscle. II. Ca2+ binding of aorta leiotonin. J Biochem. 1980 Feb;87(2):369–378. doi: 10.1093/oxfordjournals.jbchem.a132757. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Localization of specialized noradrenaline receptors at neuromuscular junctions on arterioles of the guinea-pig. J Physiol. 1981;313:343–350. doi: 10.1113/jphysiol.1981.sp013669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M. Tension responses of chemically skinned fibre bundles of the guinea-pig taenia caeci under varied ionic environments. J Physiol. 1981 Nov;320:449–467. doi: 10.1113/jphysiol.1981.sp013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes I. R., Karr G. W. Protection against induction of supersensitivity to catecholamines by cocaine. Br J Pharmacol. 1971 Aug;42(4):603–610. doi: 10.1111/j.1476-5381.1971.tb07144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes I. R., Mailhot R. Effect of cocaine on the affinity of -adrenoceptors for noradrenaline. Br J Pharmacol. 1973 May;48(1):139–143. doi: 10.1111/j.1476-5381.1973.tb08231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Tajima K. Action of morphine on the neuro-effector transmission in the guinea-pig ileum and in the mouse vas deferens. J Physiol. 1980 Oct;307:367–383. doi: 10.1113/jphysiol.1980.sp013440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Excitation--contraction coupling in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1981 Dec;321:513–535. doi: 10.1113/jphysiol.1981.sp014000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRPEKAR S. M., CERVONI P. EFFECT OF COCAINE, PHENOXYBENZAMINE AND PHENTOLAMINE ON THE CATECHOLAMINE OUTPUT FROM SPLEEN AND ADRENAL MEDULLA. J Pharmacol Exp Ther. 1963 Oct;142:59–70. [PubMed] [Google Scholar]
- KOPIN I. J. STORAGE AND METABOLISM OF CATECHOLAMINES: THE ROLE OF MONOAMINE OXIDASE. Pharmacol Rev. 1964 Jun;16:179–191. [PubMed] [Google Scholar]
- Kasuya Y., Goto K. The mechanism of supersensitivity to norepinephrine induced by cocaine in rat isolated vas deferens. Eur J Pharmacol. 1968 Nov;4(4):355–362. doi: 10.1016/0014-2999(68)90019-8. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Makita Y. Modulation of noradrenergic transmission in the guinea-pig mesenteric artery: an electrophysiological study. J Physiol. 1983 Feb;335:609–627. doi: 10.1113/jphysiol.1983.sp014554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Suzuki H. Adrenergic transmissions in the guinea-pig mesenteric artery and their cholinergic modulations. J Physiol. 1981 Aug;317:383–396. doi: 10.1113/jphysiol.1981.sp013831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z., Dubocovich M. L. Cocaine and amphetamine antagonize the decrease of noradrenergic neurotransmission elicited by oxymetazoline but potentiate the inhibition by alpha-methylnorepinephrine in the perfused cat spleen. J Pharmacol Exp Ther. 1981 Jan;216(1):162–171. [PubMed] [Google Scholar]
- MUSCHOLL E. Effect of cocaine and related drugs on the uptake of noradrenaline by heart and spleen. Br J Pharmacol Chemother. 1961 Jun;16:352–359. doi: 10.1111/j.1476-5381.1961.tb01095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell R. A., Wastila W. B., Eckhardt S. B. Some factors determining the response of rabbit aortic strips to dl-norepinephrine-7-H3 hydrochloride and the influence of cocaine, guanethidine and methylphenidate on these factors. J Pharmacol Exp Ther. 1966 Feb;151(2):253–261. [PubMed] [Google Scholar]
- Reiffenstein R. J. Effects of cocaine on the rate of contraction to noradrenaline in the cat spleen strip: mode of action of cocaine. Br J Pharmacol Chemother. 1968 Mar;32(3):591–597. doi: 10.1111/j.1476-5381.1968.tb00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saida K., Nonomura Y. Characteristics of Ca2+- and Mg2+-induced tension development in chemically skinned smooth muscle fibers. J Gen Physiol. 1978 Jul;72(1):1–14. doi: 10.1085/jgp.72.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saida K., Suzuki A. Mode of action of prilocaine on sarcoplasmic reticulum in skinned skeletal muscle fibers. J Pharmacol Exp Ther. 1981 Dec;219(3):815–820. [PubMed] [Google Scholar]
- Shibata S., Hattori K., Sakurai I., Mori J., Fujiwara M. Adrenergic innervation and cocaine-induced potentiation of adrenergic responses of aortic strips from young and old rabbits. J Pharmacol Exp Ther. 1971 Jun;177(3):621–632. [PubMed] [Google Scholar]
- Suzuki H. Effects of endogenous and exogenous noradrenaline on the smooth muscle of guinea-pig mesenteric vein. J Physiol. 1981 Dec;321:495–512. doi: 10.1113/jphysiol.1981.sp013999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Kuriyama H. Observation of quantal release of noradrenaline from vascular smooth muscles in potassium-free solution. Jpn J Physiol. 1980;30(4):665–670. doi: 10.2170/jjphysiol.30.665. [DOI] [PubMed] [Google Scholar]
- TRENDELENBURG U. Supersensitivity and subsensitivity to sympathomimetic amines. Pharmacol Rev. 1963 Jun;15:225–276. [PubMed] [Google Scholar]
- Trendelenburg U., Graefe K. H., Eckert E. The prejunctional effect of cocaine on the isolated nictitating membrane of the cat. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(1):69–82. doi: 10.1007/BF00505068. [DOI] [PubMed] [Google Scholar]


