Abstract
1. The dorsal longitudinal flight muscles of Drosophila melanogaster contain three voltage-activated ion currents, two distinct potassium currents and a calcium current. The currents can be isolated from each other by exploiting the developmental properties of the system and genetic tools, as well as conventional pharmacology.
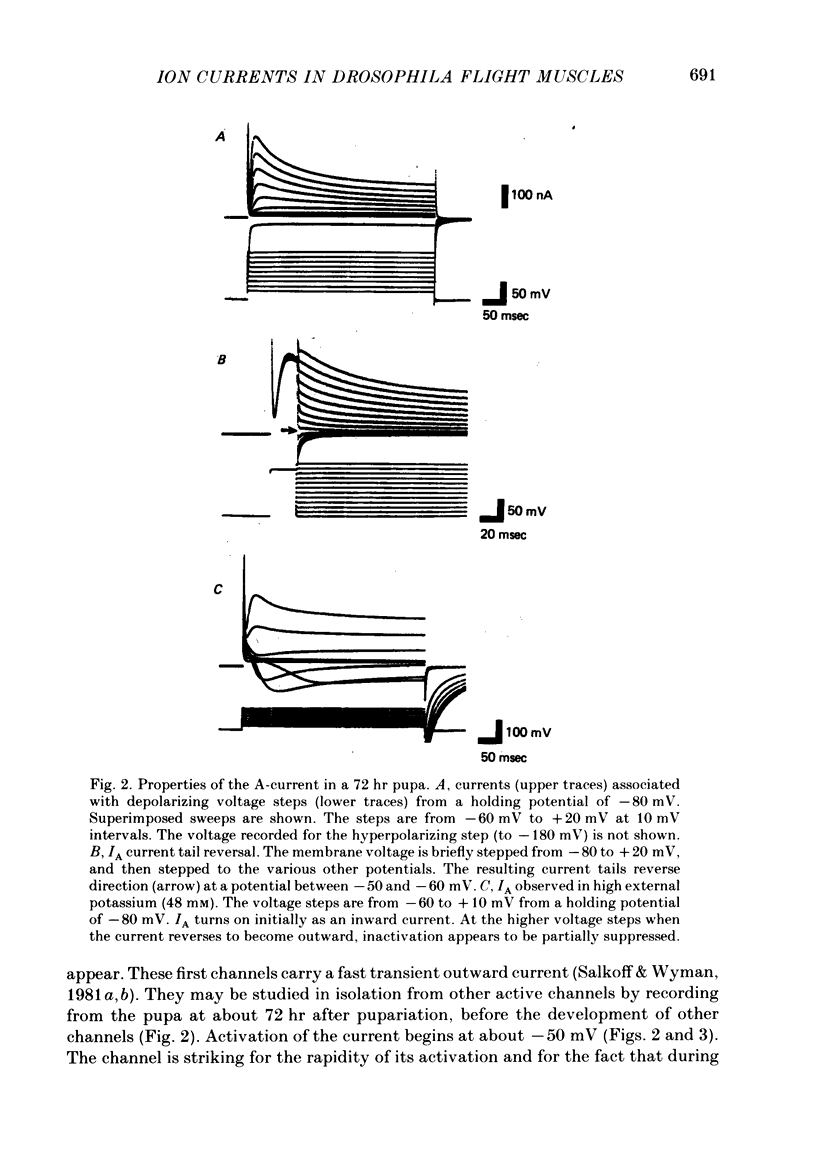
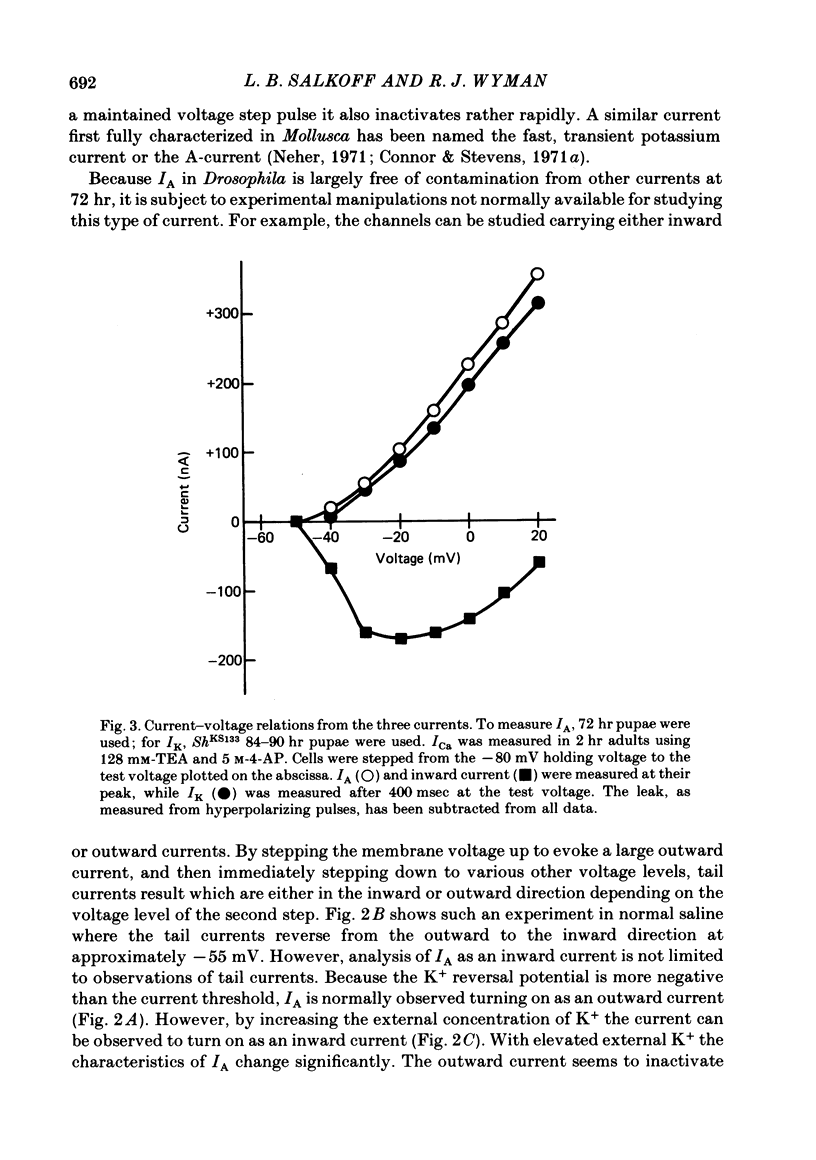
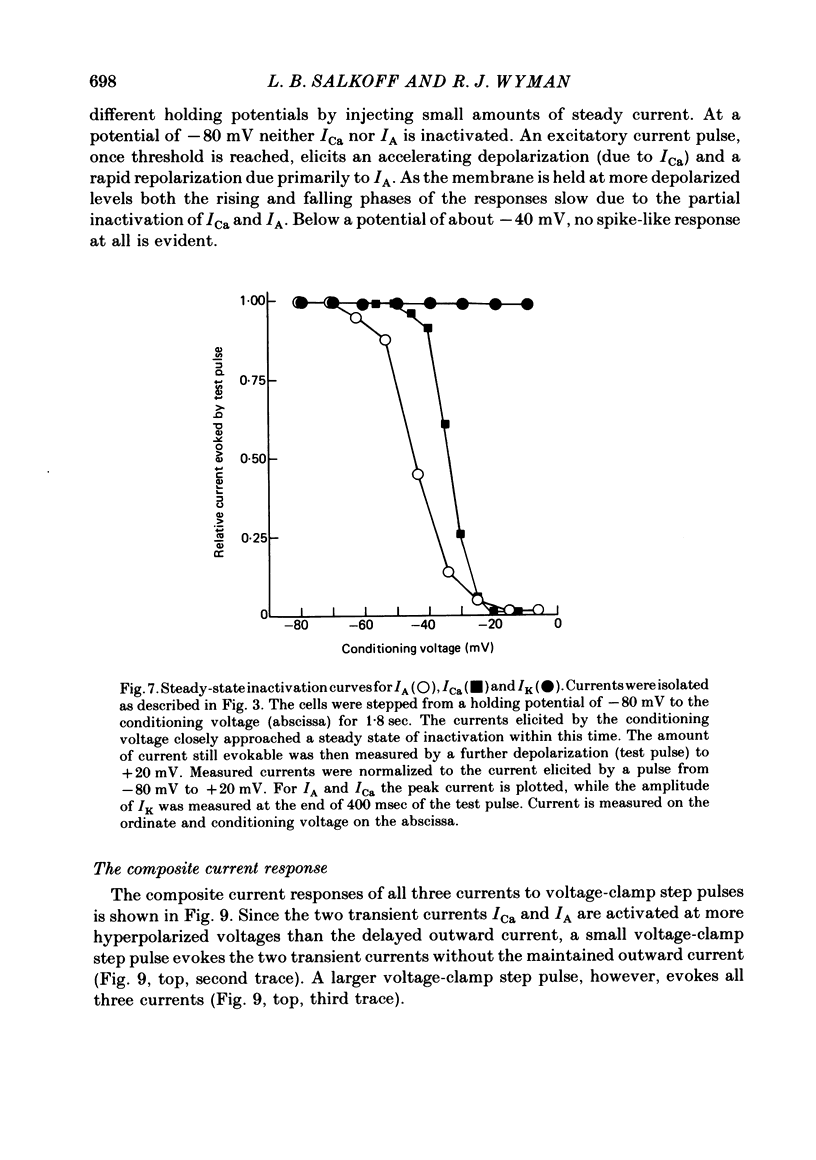
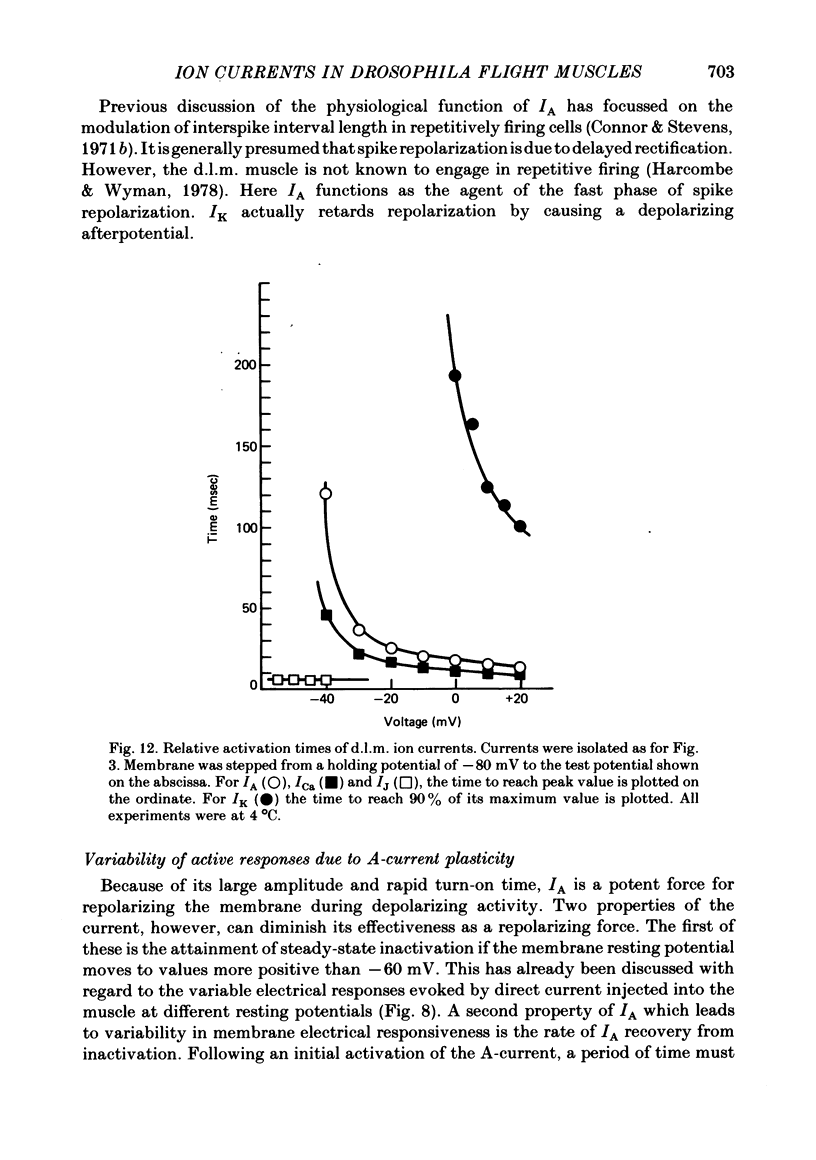
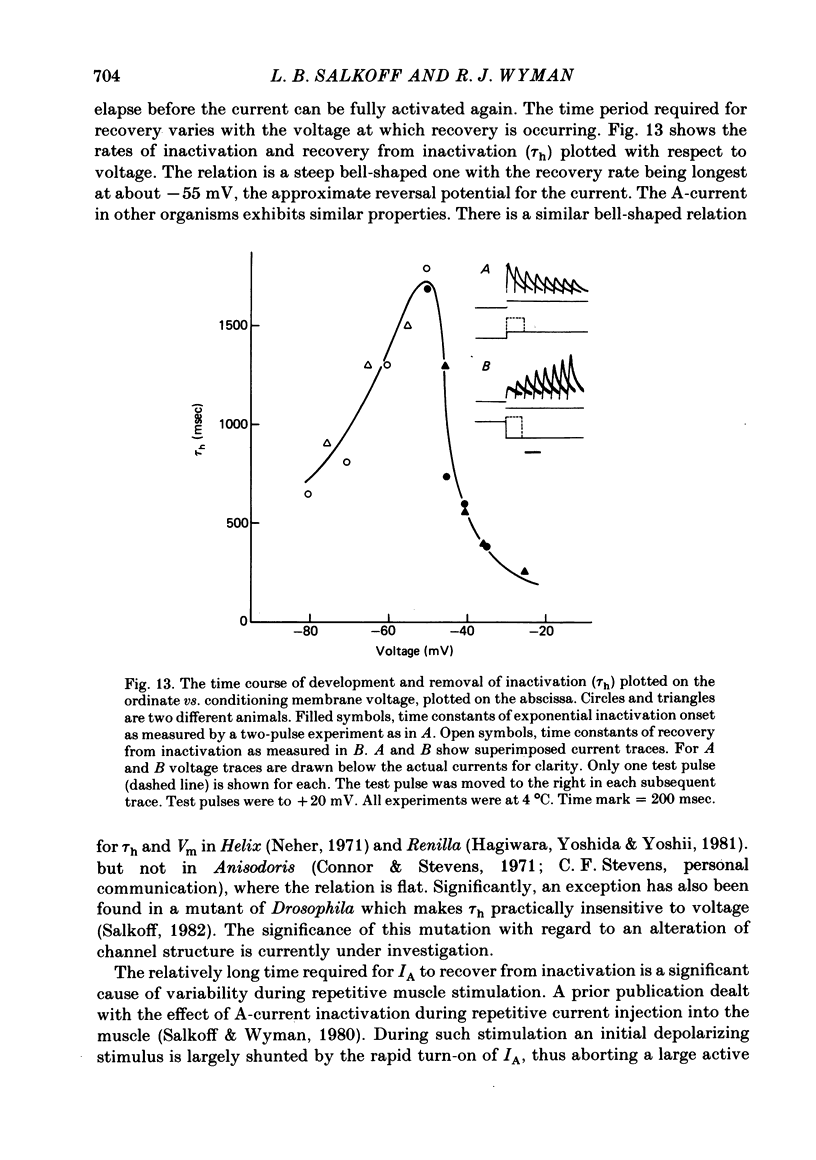
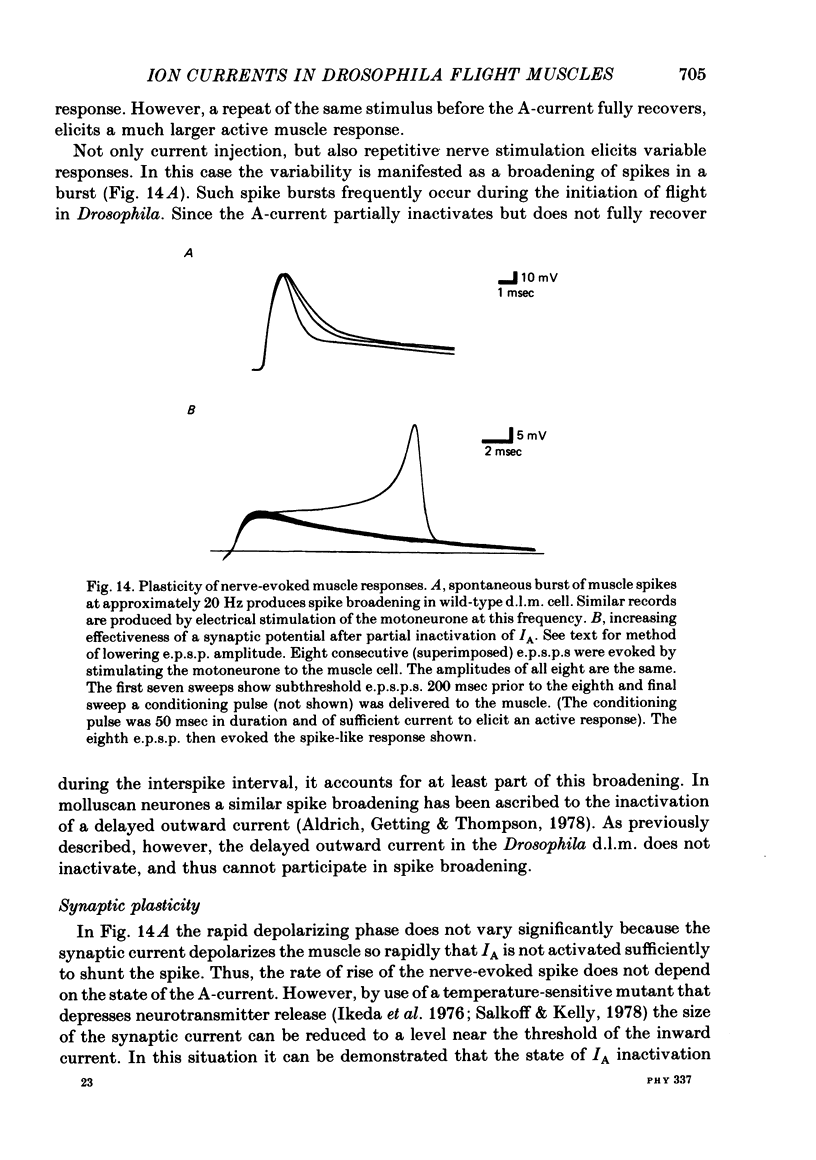
2. The fast transient potassium current (IA) is the first channel to appear in the developing muscle membrane. It can be studied in isolation between 60 and 70 hr of pupal development. The channels can be observed to carry both outward and inward currents depending on the external potassium concentration. IA is blocked by both tetraethylammonium ion (TEA) and 3- or 4-aminopyridine. The inactivation and recovery properties of IA are responsible for a facilitating effect on membrane excitability.
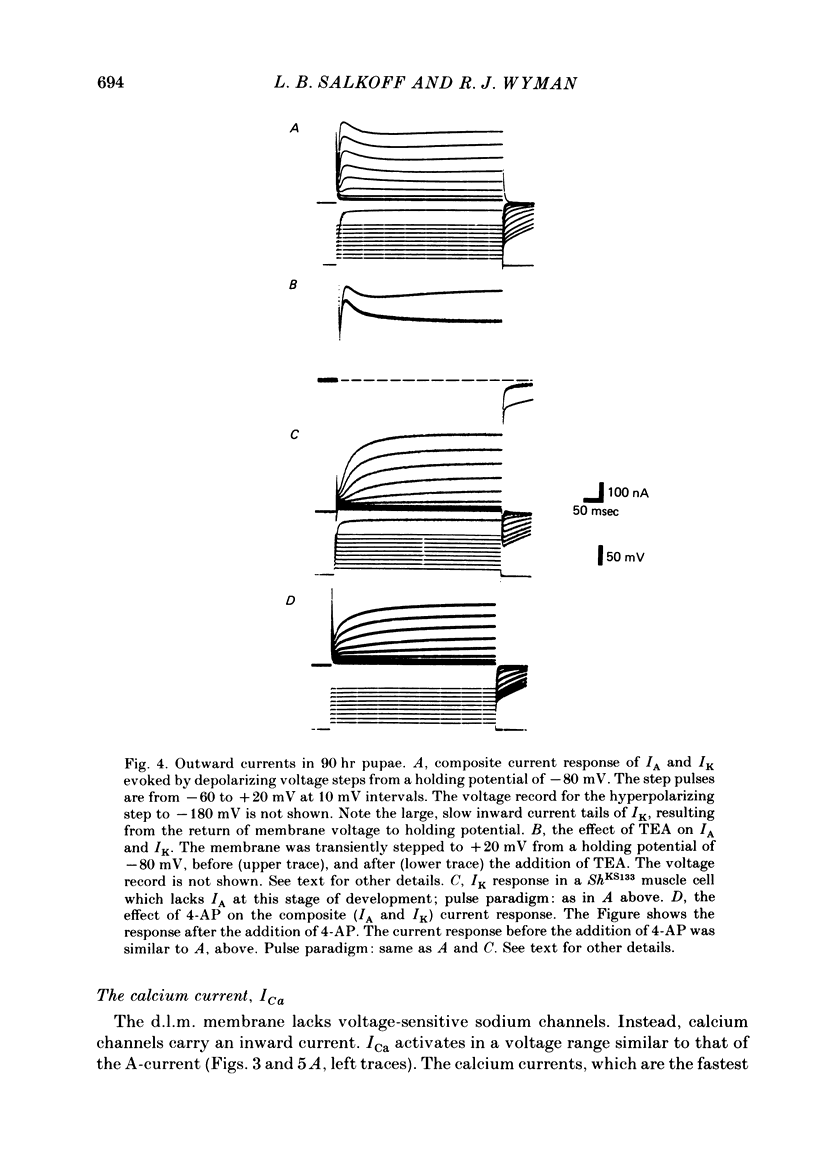
3. The delayed outward current (IK) develops after maturation of the IA system. IK can be isolated from IA by use of a mutation that removes IA from the membrane current response and can be studied before the development of Ca2+ channels. IK shows no inactivation. The channels are more sensitive to blockage by TEA than IA channels, but are not substantially blocked by 3- or 4-aminopyridine.
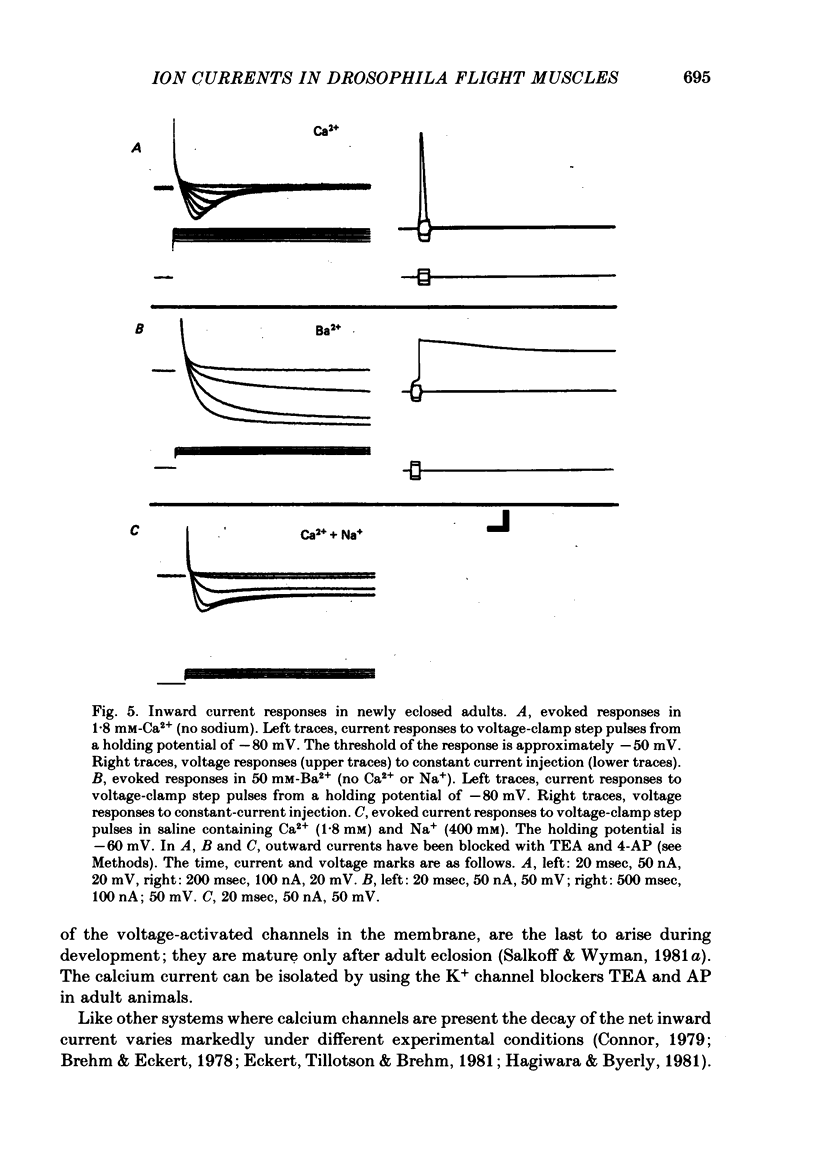
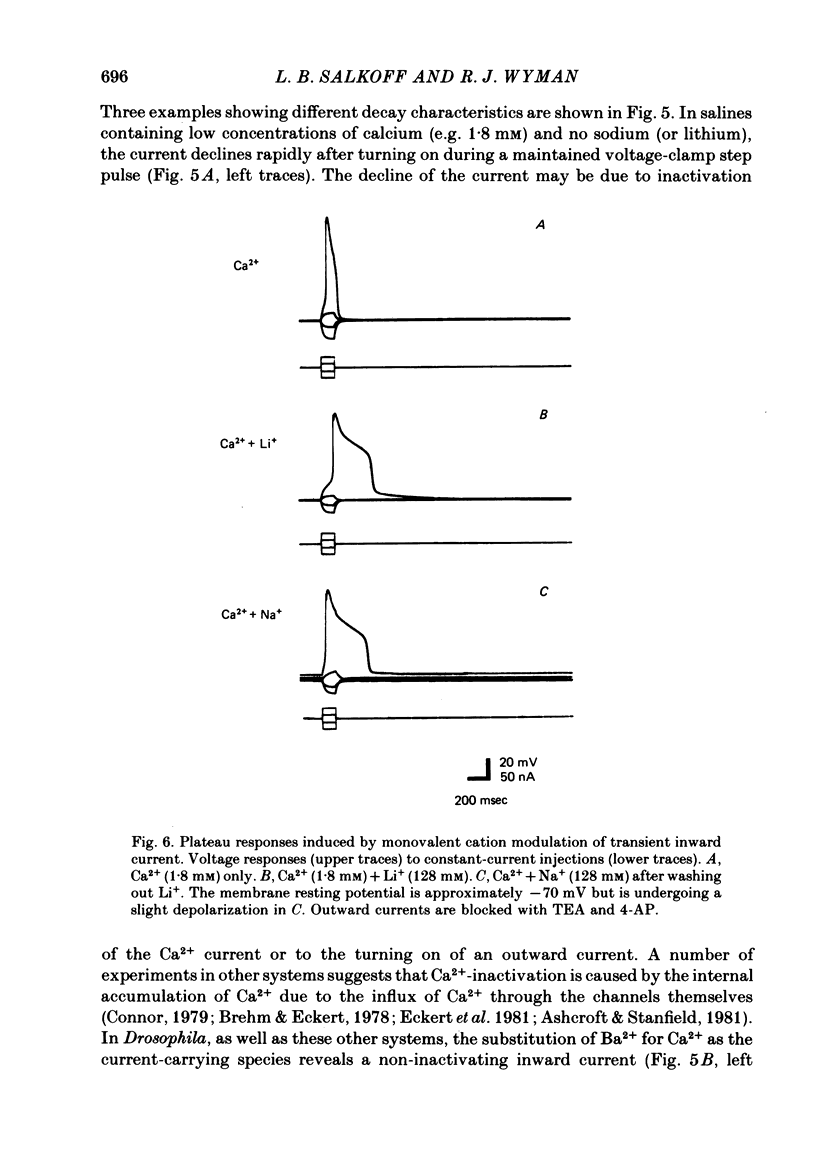
4. The calcium current (ICa) is the last of the major currents to develop and must be isolated pharmacologically with potassium-blocking agents. ICa shows inactivation when Ca2+ is present but not when Ba2+ is the sole current carrier. When Ca2+ is the current carrier, the addition of Na+ or Li+ retards the inactivation of the net inward current. When the membrane voltage is not clamped, Ba2+ alone, or Ca2+ with Na+ (or Li+), produces a plateau response of extended duration.
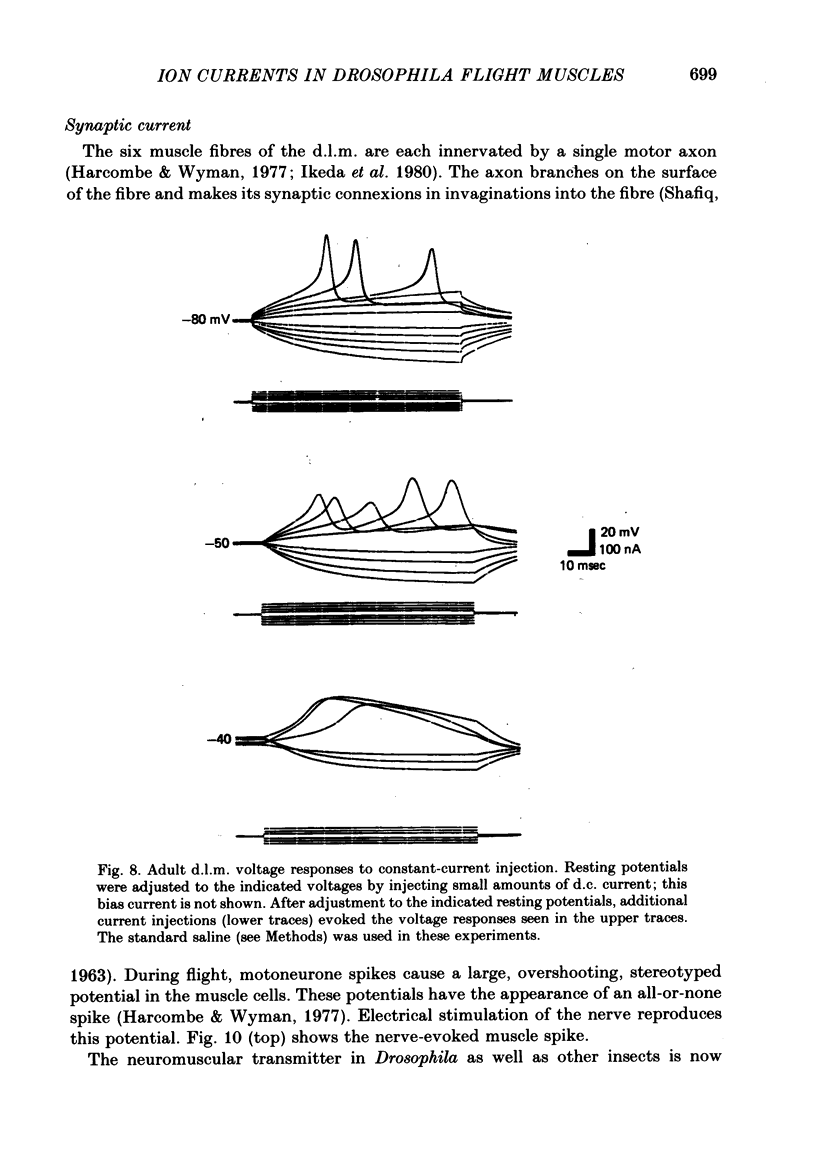
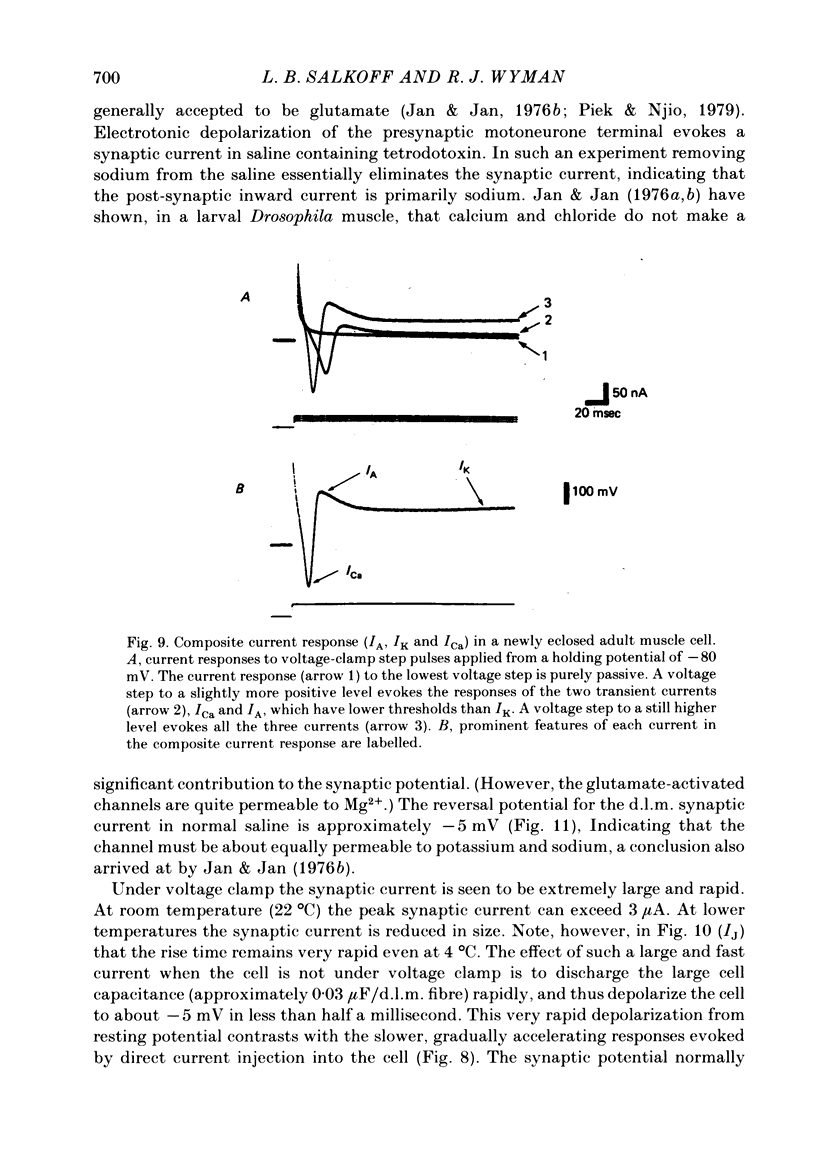
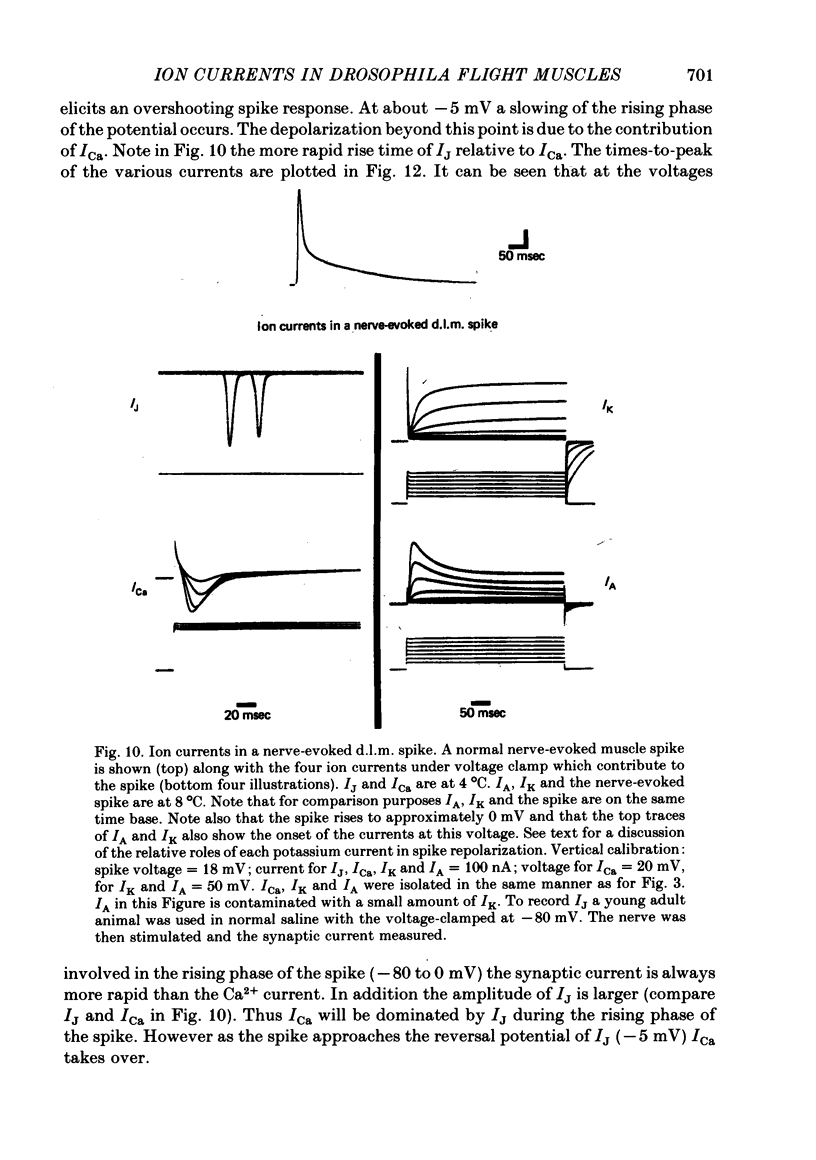
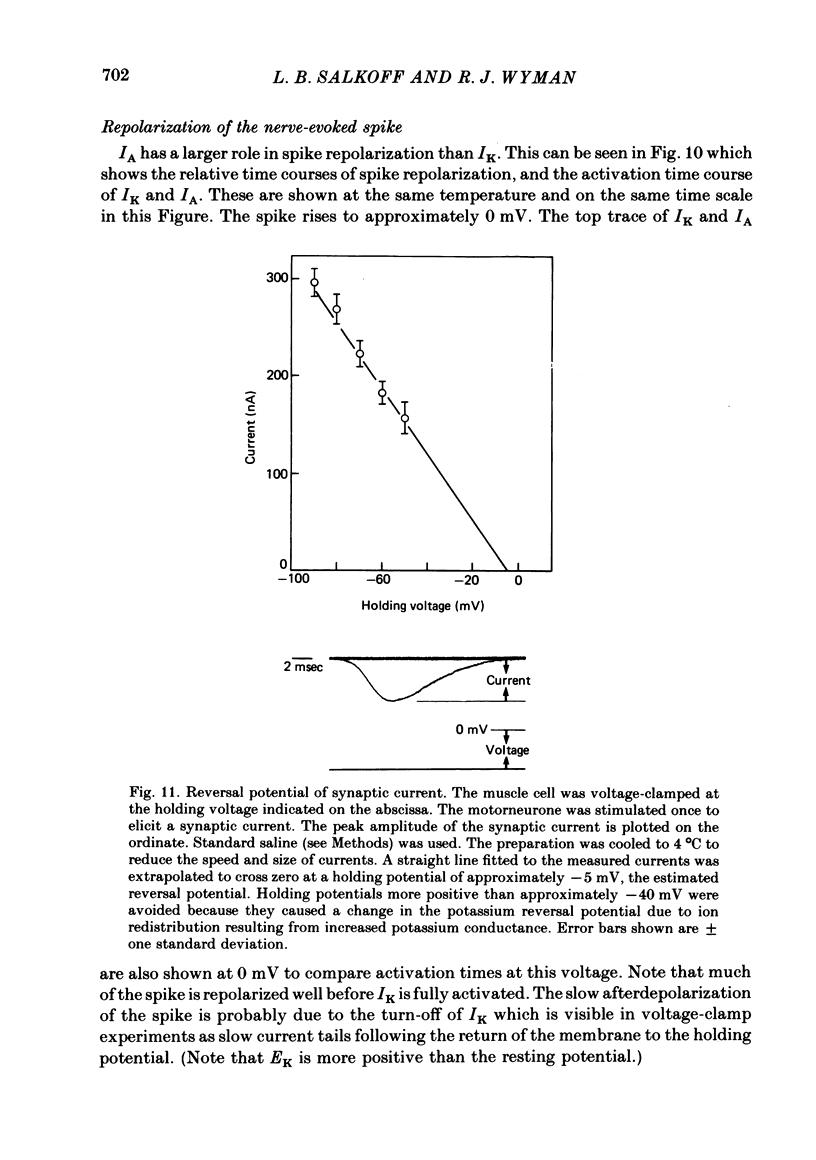
5. The synaptic current (IJ) evoked by motoneurone stimulation is the fastest and largest of the current systems. It has a reversal potential of approximately -5 mV, indicating roughly equal permeabilities of Na+ and K+. During a nerve-driven muscle spike, IJ is the major inward current, causing a very rapid depolarization away from resting potential. An exceptionally large synaptic current is necessary to rapidly discharge the high membrane capacitance (0·03 μF/cell) in these large (0·05 × 0·1 × 0·8 mm) isopotential cells.
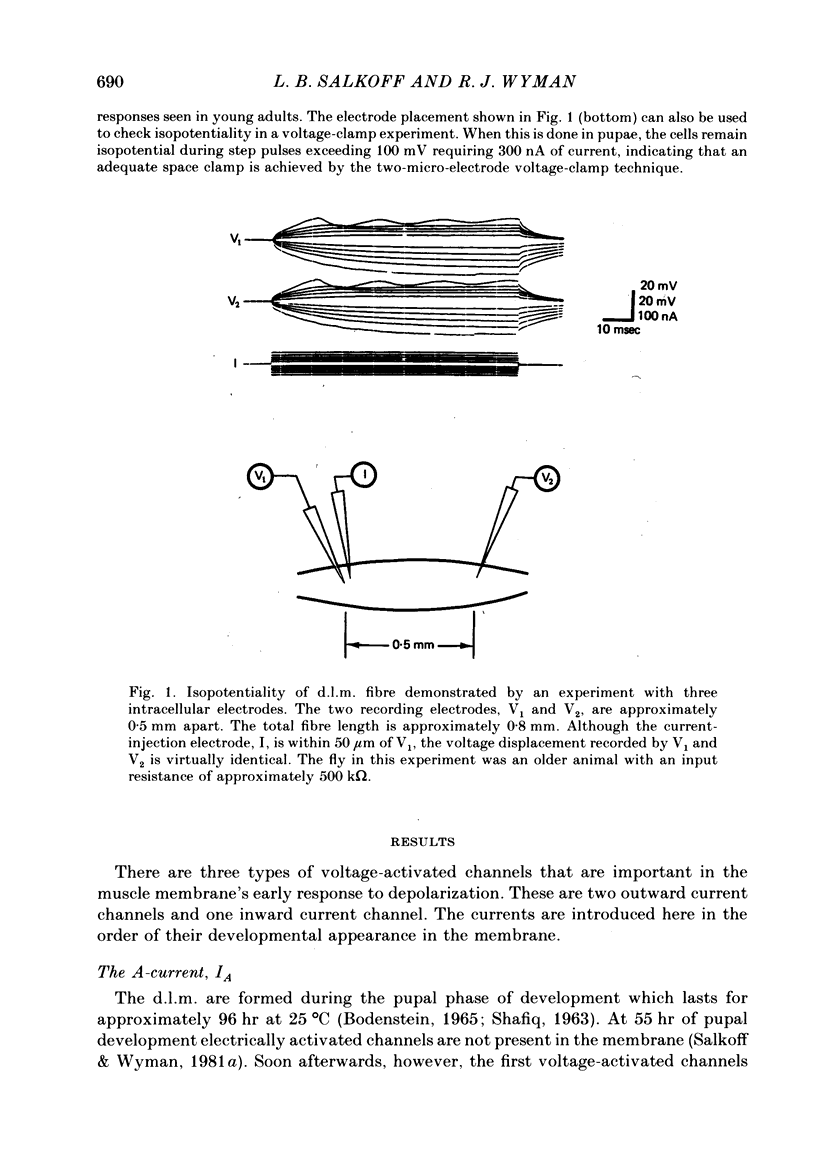
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Jr, Getting P. A., Thompson S. H. Mechanism of frequency-dependent broadening of molluscan neurone soma spikes. J Physiol. 1979 Jun;291:531–544. doi: 10.1113/jphysiol.1979.sp012829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Stanfield P. R. Calcium and potassium currents in muscle fibres of an insect (Carausius morosus). J Physiol. 1982 Feb;323:93–115. doi: 10.1113/jphysiol.1982.sp014063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft F. M., Stanfield P. R. Calcium dependence of the inactivation of calcium currents in skeletal muscle fibers of an insect. Science. 1981 Jul 10;213(4504):224–226. doi: 10.1126/science.213.4504.224. [DOI] [PubMed] [Google Scholar]
- Brehm P., Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978 Dec 15;202(4373):1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Byrne J. H. Analysis of ionic conductance mechanisms in motor cells mediating inking behavior in Aplysia californica. J Neurophysiol. 1980 Mar;43(3):630–650. doi: 10.1152/jn.1980.43.3.630. [DOI] [PubMed] [Google Scholar]
- CERF J. A., GRUNDFEST H., HOYLE G., McCANN F. V. The mechanism of dual responsiveness in muscle fibers of the grasshopper Romalea microptera. J Gen Physiol. 1959 Nov;43:377–395. doi: 10.1085/jgp.43.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Connor J. A. Calcium current in molluscan neurones: measurement under conditions which maximize its visibility. J Physiol. 1979 Jan;286:41–60. doi: 10.1113/jphysiol.1979.sp012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Prediction of repetitive firing behaviour from voltage clamp data on an isolated neurone soma. J Physiol. 1971 Feb;213(1):31–53. doi: 10.1113/jphysiol.1971.sp009366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J. Modulation of the excitatory synaptic response by fast transient K+ current in snail neurones. Nat New Biol. 1973 Dec 19;246(155):193–196. doi: 10.1038/newbio246193a0. [DOI] [PubMed] [Google Scholar]
- Dionne V. E., Stevens C. F. Voltage dependence of agonist effectiveness at the frog neuromuscular junction: resolution of a paradox. J Physiol. 1975 Oct;251(2):245–270. doi: 10.1113/jphysiol.1975.sp011090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L., Brehm P. Calcium-mediated control of Ca and K currents. Fed Proc. 1981 Jun;40(8):2226–2232. [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Membrane biophysics of calcium currents. Fed Proc. 1981 Jun;40(8):2220–2225. [PubMed] [Google Scholar]
- Hagiwara S., Yoshida S., Yoshii M. Transient and delayed potassium currents in the egg cell membrane of the coelenterate, Renilla koellikeri. J Physiol. 1981 Sep;318:123–141. doi: 10.1113/jphysiol.1981.sp013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcombe E. S., Wyman R. J. Output pattern generation by Drosophila flight motoneurons. J Neurophysiol. 1977 Sep;40(5):1066–1077. doi: 10.1152/jn.1977.40.5.1066. [DOI] [PubMed] [Google Scholar]
- Huddart H., Wood D. W. The effect of DNP on the resting potential and ionic content of some insect skeletal muscle fibres. Comp Biochem Physiol. 1966 Aug;18(4):681–688. doi: 10.1016/0010-406x(66)90204-0. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Koenig J. H., Tsuruhara T. Organization of identified axons innervating the dorsal longitudinal flight muscle of Drosophila melanogaster. J Neurocytol. 1980 Dec;9(6):799–823. doi: 10.1007/BF01205020. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Ozawa S., Hagiwara S. Synaptic transmission reversibly conditioned by single-gene mutation in Drosophila melanogaster. Nature. 1976 Feb 12;259(5543):489–491. doi: 10.1038/259489a0. [DOI] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol. 1976 Oct;262(1):215–236. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol. 1976 Oct;262(1):189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Dennis M. J. Two mutations of synaptic transmission in Drosophila. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):87–108. doi: 10.1098/rspb.1977.0087. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheuben M. B. The resting potential of moth muscle fibre. J Physiol. 1972 Sep;225(3):529–554. doi: 10.1113/jphysiol.1972.sp009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAFIQ S. A. Electron microscopic studies on the indirect flight muscles of Drosophila melanogaster. I. Structure of the myofibrils. J Cell Biol. 1963 May;17:351–362. doi: 10.1083/jcb.17.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L., Kelly L. Temperature-induced seizure and frequency-dependent neuromuscular block in a ts mutant of Drosophila. Nature. 1978 May 11;273(5658):156–158. doi: 10.1038/273156a0. [DOI] [PubMed] [Google Scholar]
- Salkoff L., Wyman R. Facilitation of membrane electrical excitability in Drosophila. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6216–6220. doi: 10.1073/pnas.77.10.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salkoff L., Wyman R. Outward currents in developing Drosophila flight muscle. Science. 1981 Apr 24;212(4493):461–463. doi: 10.1126/science.6259736. [DOI] [PubMed] [Google Scholar]
- Shapiro E., Castellucci V. F., Kandel E. R. Presynaptic membrane potential affects transmitter release in an identified neuron in Aplysia by modulating the Ca2+ and K+ currents. Proc Natl Acad Sci U S A. 1980 Jan;77(1):629–633. doi: 10.1073/pnas.77.1.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi O., Benzer S. Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3253–3257. doi: 10.1073/pnas.73.9.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. G., Jr, Barker J. L., Gainer H. Requirements for bursting pacemaker potential activity in molluscan neurones. Nature. 1975 Feb 6;253(5491):450–452. doi: 10.1038/253450a0. [DOI] [PubMed] [Google Scholar]
- Stanfield P. R., Ashcroft F. M., Plant T. D. Gating of a muscle K+ channel and its dependence on the permeating ion species. Nature. 1981 Feb 5;289(5797):509–511. doi: 10.1038/289509a0. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Jr, Armstrong C. M. K+ channels close more slowly in the presence of external K+ and Rb+. Nature. 1981 Jun 4;291(5814):427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]
- Tanouye M. A., Ferrus A., Fujita S. C. Abnormal action potentials associated with the Shaker complex locus of Drosophila. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6548–6552. doi: 10.1073/pnas.78.10.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. F., Ganetzky B., Jan L. Y., Jan Y. N., Benzer S. A Drosophila mutant with a temperature-sensitive block in nerve conduction. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4047–4051. doi: 10.1073/pnas.75.8.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]


