Abstract
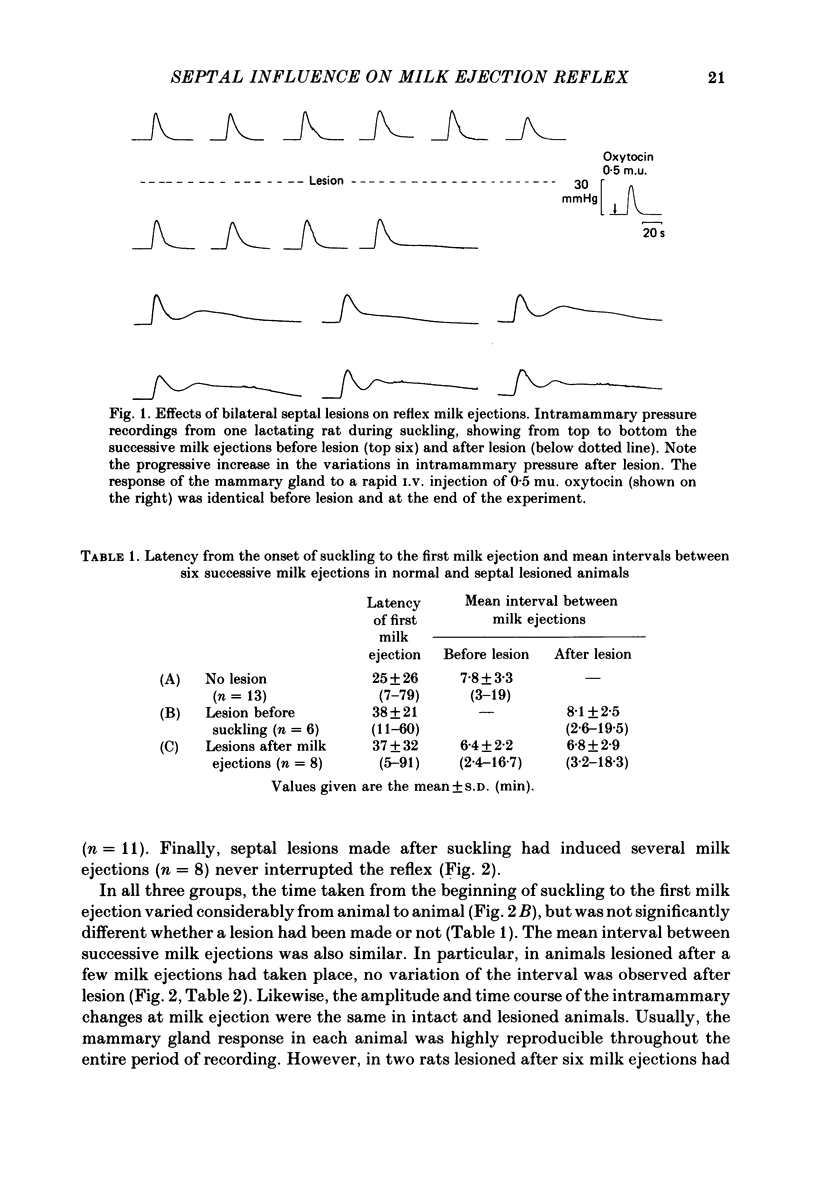
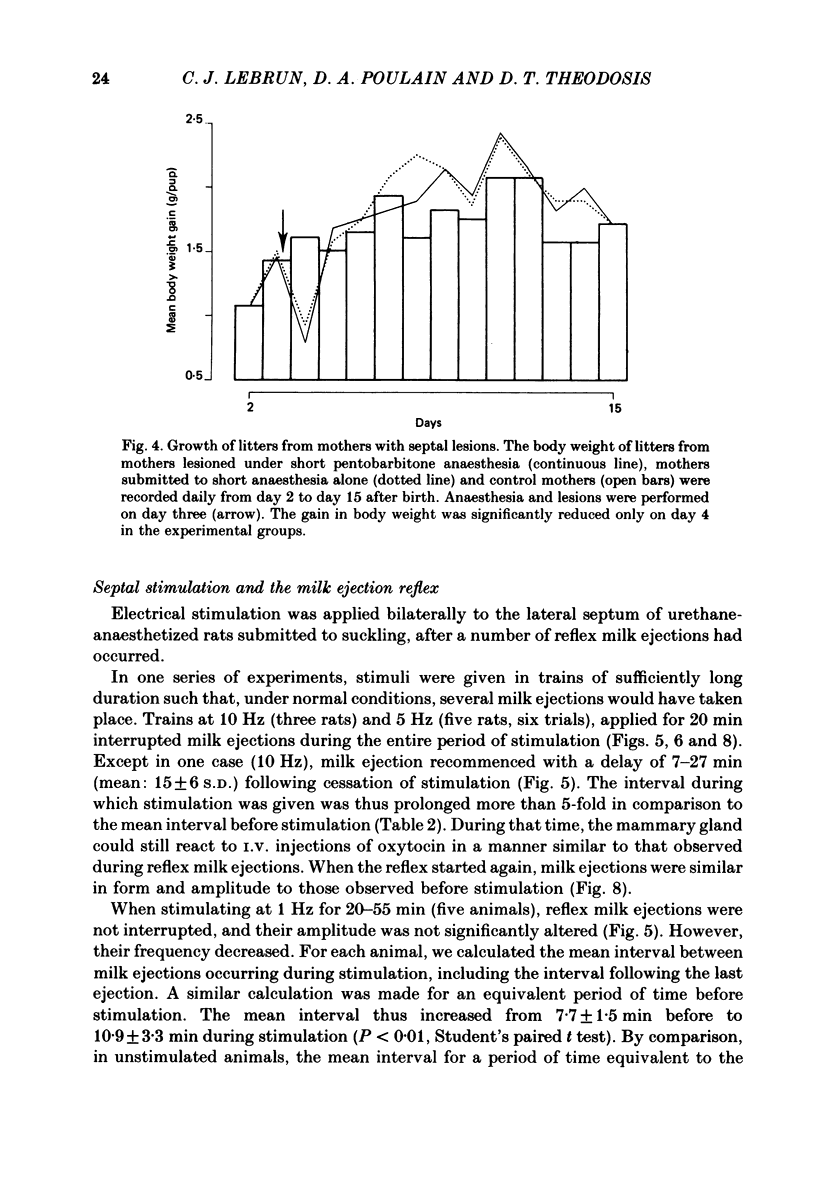
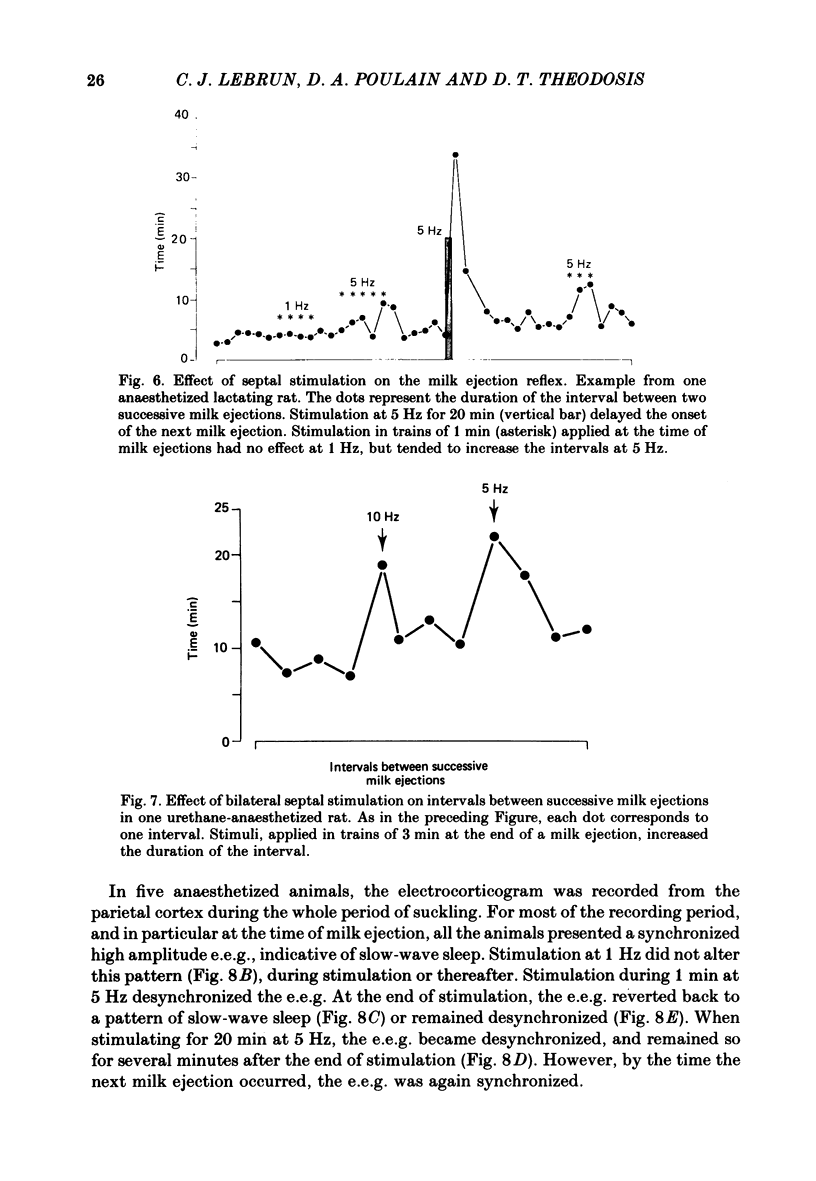
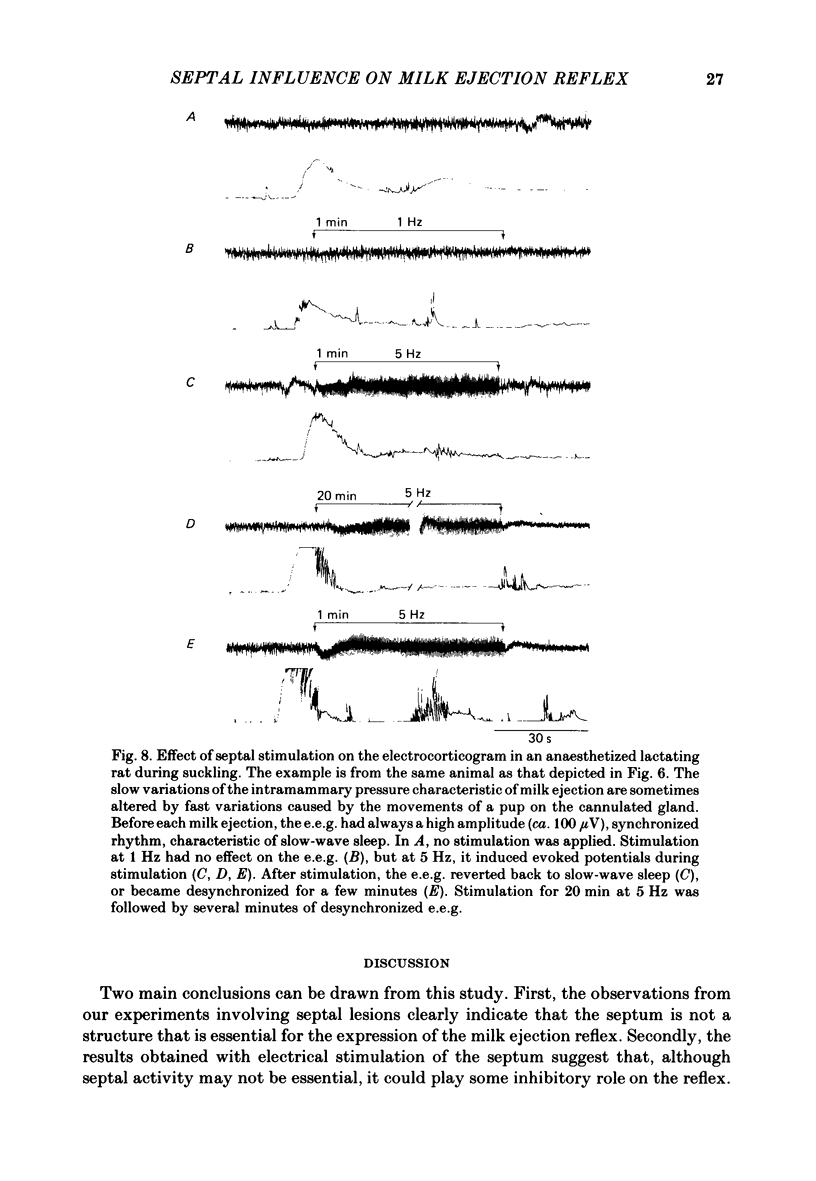
Experiments were undertaken to determine the role of the septum on the afferent control of the milk ejection reflex in lactating rats. Massive septal lesions were produced by passing radio-frequency current through lesioning electrodes. Intramammary pressure recordings during suckling showed no significant alterations either in the frequency of milk ejections or in their amplitude and time course. Electrophysiological recordings of identified oxytocin-secreting neurones in supraoptic nuclei of septal-lesioned rats engaged in suckling showed that the pattern of background electrical activity and of the high frequency discharges at milk ejection were normal. The weight of litters from rats lesioned on the third day post-partum increased in a way parallel to that of control litters up to the fifteenth post-natal day. Electrical stimulation was applied bilaterally to the lateral septum in trains of long duration (20-55 min) at varying frequencies. Frequencies of 5 and 10 Hz interrupted the reflex during the period of stimulation. At 1 Hz, milk ejections were not interrupted but the intervals between successive milk ejections were significantly increased in comparison to the intervals before stimulation. Electrical stimulation applied to the septum in short trains of 1 or 3 min at 5 and 10 Hz significantly delayed the appearance of the subsequent milk ejection. At 1 Hz, no effect was observed. Septal stimulation at 1 Hz for 20 min or more did not significantly alter the electrocorticogram during the period of stimulation. Stimulation at 5 Hz for the same period of time always desynchronized the e.e.g. for several minutes after the cessation of stimulation. It is concluded that the septum is not essential for the onset and the maintenance of reflex milk ejections during lactation. The results suggest, however, that in the normal non-anaesthetized animal, septal activation could modulate the frequency of milk ejections.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aulsebrook L. H., Holland R. C. Central inhibition of oxytocin release. Am J Physiol. 1969 Apr;216(4):830–842. doi: 10.1152/ajplegacy.1969.216.4.830. [DOI] [PubMed] [Google Scholar]
- CROSS B. A. Neurohormonal mechanisms in emotional inhibition of milk ejection. J Endocrinol. 1955 Jan;12(1):29–37. doi: 10.1677/joe.0.0120029. [DOI] [PubMed] [Google Scholar]
- Carlson N. R., Thomas G. J. Maternal behavior of mice with limbic lesions. J Comp Physiol Psychol. 1968 Dec;66(3):731–737. doi: 10.1037/h0026513. [DOI] [PubMed] [Google Scholar]
- Cleverley J. D., Folley S. J. The blood levels of oxytocin during machine milking in cows with some observations on its half-life in the circulation. J Endocrinol. 1970 Mar;46(3):347–361. doi: 10.1677/joe.0.0460347. [DOI] [PubMed] [Google Scholar]
- Cruz M. L., Beyer C. Effects of septal lesions on maternal behavior and lactation in the rabbit. Physiol Behav. 1972 Sep;9(3):361–365. doi: 10.1016/0031-9384(72)90160-6. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Slotnick B. M. Disruption of maternal behavior in rats with lesions of the septal area. Physiol Behav. 1978 Aug;21(2):189–200. doi: 10.1016/0031-9384(78)90041-0. [DOI] [PubMed] [Google Scholar]
- GREEN J. D., ARDUINI A. A. Hippocampal electrical activity in arousal. J Neurophysiol. 1954 Nov;17(6):533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Garris D. R. Direct septo-hypothalamic projections in the rat. Neurosci Lett. 1979 Jun;13(1):83–90. doi: 10.1016/0304-3940(79)90080-6. [DOI] [PubMed] [Google Scholar]
- Gogolák G., Stumpf C., Petsche H., Sterc J. The firing pattern of septal neurons and the form of the hippocampal theta wave. Brain Res. 1968 Feb;7(2):201–207. doi: 10.1016/0006-8993(68)90098-x. [DOI] [PubMed] [Google Scholar]
- Lebrun C. J., Poulain D. A. Electrical activity of septal neurones during suckling and the milk ejection reflex in the lactating rat. Exp Brain Res. 1982;47(2):203–208. doi: 10.1007/BF00239379. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Hentzen K., Hin T., van der Schoot P., Clarke G., Summerlee A. J. Sleep: a prerequisite for reflex milk ejection in the rat. Exp Brain Res. 1980 Jan;38(2):151–162. doi: 10.1007/BF00236736. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Wakerley J. B. Electrophysiological evidence for the activation of supraoptic neurones during the release of oxytocin. J Physiol. 1974 Oct;242(2):533–554. doi: 10.1113/jphysiol.1974.sp010722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln D. W., Wakerley J. B. Factors governing the periodic activation of supraoptic and paraventricular neurosecretory cells during suckling in the rat. J Physiol. 1975 Sep;250(2):443–461. doi: 10.1113/jphysiol.1975.sp011064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan H., Miller J. J. The hippocampal control of neuronal discharges in the septum of the rat. J Physiol. 1974 Mar;237(3):607–624. doi: 10.1113/jphysiol.1974.sp010500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales F. R., Roig J. A., Monti J. M., Macadar O., Budelli R. Septal unit activity and hippocampal EEG during the sleep-wakefulness cycle of the rat. Physiol Behav. 1971 May;6(5):563–567. doi: 10.1016/0031-9384(71)90206-x. [DOI] [PubMed] [Google Scholar]
- PEETERS G., STORMORKEN H., VANSCHOUBROEK F. The effect of different stimuli on milk ejection and diuresis in the lactating cow. J Endocrinol. 1960 Apr;20:163–172. doi: 10.1677/joe.0.0200163. [DOI] [PubMed] [Google Scholar]
- PETSCHE H., STUMPF C., GOGOLAK G. [The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells]. Electroencephalogr Clin Neurophysiol. 1962 Apr;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Ellendorff F., Vincent J. D. Septal connections with identified oxytocin and vasopressin neurones in the supraoptic nucleus of the rat. An electrophysiological investigation. Neuroscience. 1980;5(2):379–387. doi: 10.1016/0306-4522(80)90113-x. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Lebrun C. J., Vincent J. D. Electrophysiological evidence for connections between septal neurones and the supraoptic nucleus of the hypothalamus of the rat. Exp Brain Res. 1981;42(3-4):260–268. doi: 10.1007/BF00237493. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B., Dyball R. E. Electrophysiological differentiation of oxytocin- and vasopressin-secreting neurones. Proc R Soc Lond B Biol Sci. 1977 Apr;196(1125):367–384. doi: 10.1098/rspb.1977.0046. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Powell E. W., Rorie D. K. Septal projections to nuclei functioning in oxytocin release. Am J Anat. 1967 May;120(3):605–610. doi: 10.1002/aja.1001200310. [DOI] [PubMed] [Google Scholar]
- Raisman G. The connexions of the septum. Brain. 1966 Jun;89(2):317–348. doi: 10.1093/brain/89.2.317. [DOI] [PubMed] [Google Scholar]
- STUTINSKY F., TERMINN Y. EFFETS DES L'ESIONS DU COMPLEXE AMYGDALIEN SUR LE R'EFLEXE D'EJECTION DE LAIT CHEZ LA RATTE. J Physiol (Paris) 1965 Jan-Feb;57:279–280. [PubMed] [Google Scholar]
- Slotnick B. M. Disturbances of maternal behavior in the rat following lesions of the cingulate cortex. Behaviour. 1967;29(2):204–236. doi: 10.1163/156853967x00127. [DOI] [PubMed] [Google Scholar]
- Slotnick B. M., Nigrosh B. J. Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. J Comp Physiol Psychol. 1975 Jan;88(1):118–127. doi: 10.1037/h0076200. [DOI] [PubMed] [Google Scholar]
- TALEISNIK S., DEIS R. P. INFLUENCE OF CEREBRAL CORTEX IN INHIBITION OF OXYTOCIN RELEASE INDUCED BY STRESSFUL STIMULI. Am J Physiol. 1964 Dec;207:1394–1398. doi: 10.1152/ajplegacy.1964.207.6.1394. [DOI] [PubMed] [Google Scholar]
- Tangapregassom A. M., Tangapregassom M. J., Soulairac A., Soulairac M. L. Effets des lésions septales sur l'ultrastructure du noyau supra-optique. Ann Endocrinol (Paris) 1974 Mar-Apr;35(2):149–152. [PubMed] [Google Scholar]
- Tribollet E., Clarke G., Dreifuss J. J., Lincoln D. W. The role of central adrenergic receptors in the reflex release of oxytocin. Brain Res. 1978 Feb 17;142(1):69–84. doi: 10.1016/0006-8993(78)90177-4. [DOI] [PubMed] [Google Scholar]
- Tribollet E., Dreifuss J. J. Localization of neurones projecting to the hypothalamic paraventricular nucleus area of the rat: a horseradish peroxidase study. Neuroscience. 1981;6(7):1315–1328. doi: 10.1016/0306-4522(81)90190-1. [DOI] [PubMed] [Google Scholar]
- Uhlir I., Seggie J., Brown G. M. The effect of septal lesions on the threshold of adrenal stress response. Neuroendocrinology. 1974;14(6):351–355. doi: 10.1159/000122279. [DOI] [PubMed] [Google Scholar]
- Voloschin L. M., Tramezzani J. H. The neural input of the milk ejection reflex in the hypothalamus. Endocrinology. 1973 Apr;92(4):973–983. doi: 10.1210/endo-92-4-973. [DOI] [PubMed] [Google Scholar]
- Wakerley J. B., Lincoln D. W. The milk-ejection reflex of the rat: a 20- to 40-fold acceleration in the firing of paraventricular neurones during oxytocin release. J Endocrinol. 1973 Jun;57(3):477–493. doi: 10.1677/joe.0.0570477. [DOI] [PubMed] [Google Scholar]
- Záborszky L., Léránth C., Makara G. B., Palkovits M. Quantitative studies on the supraoptic nucleus in the rat. II. Afferent fiber connections. Exp Brain Res. 1975 May 22;22(5):525–540. doi: 10.1007/BF00237352. [DOI] [PubMed] [Google Scholar]



