Abstract
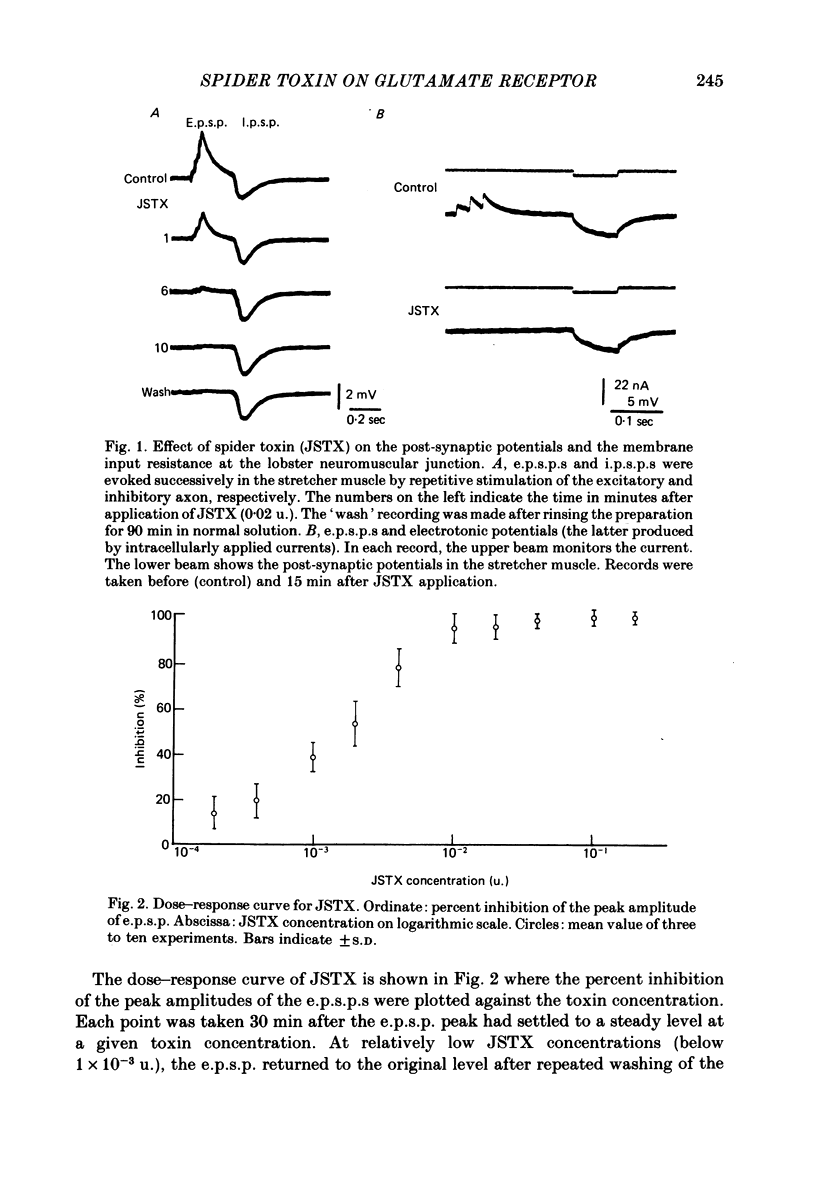
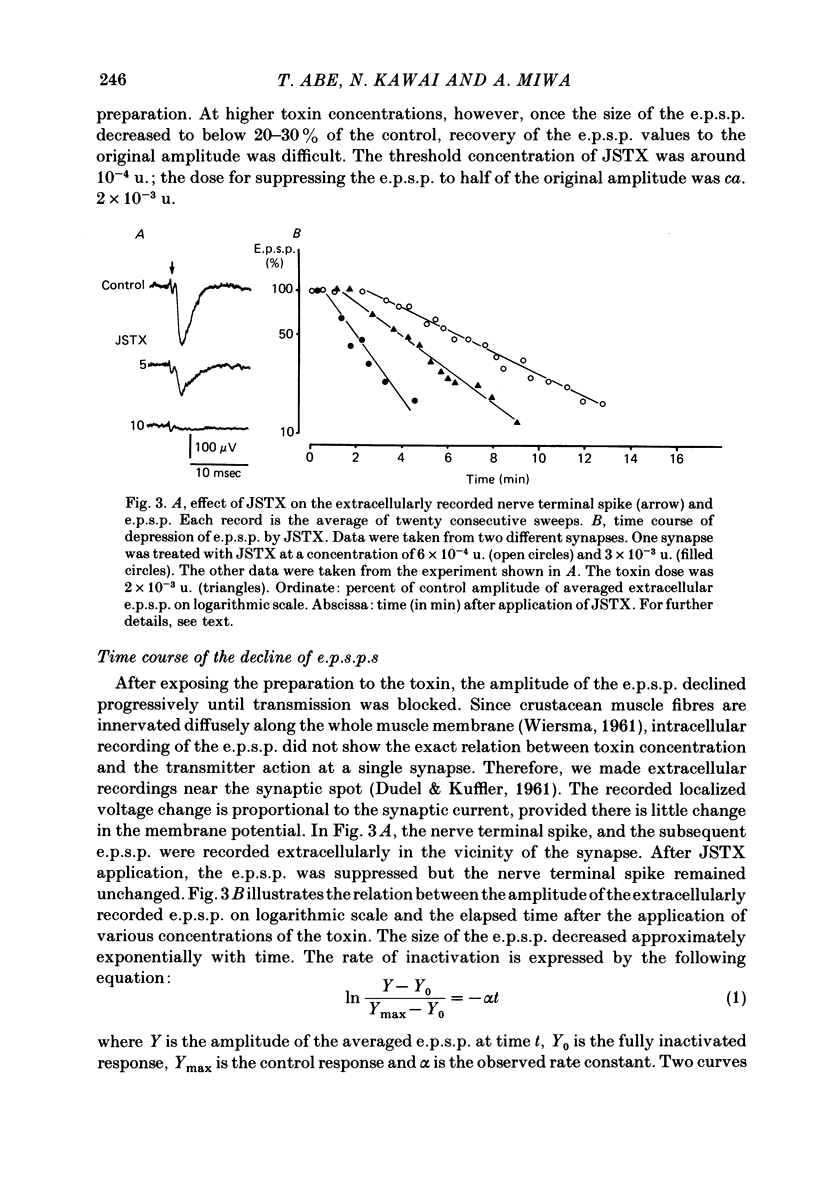
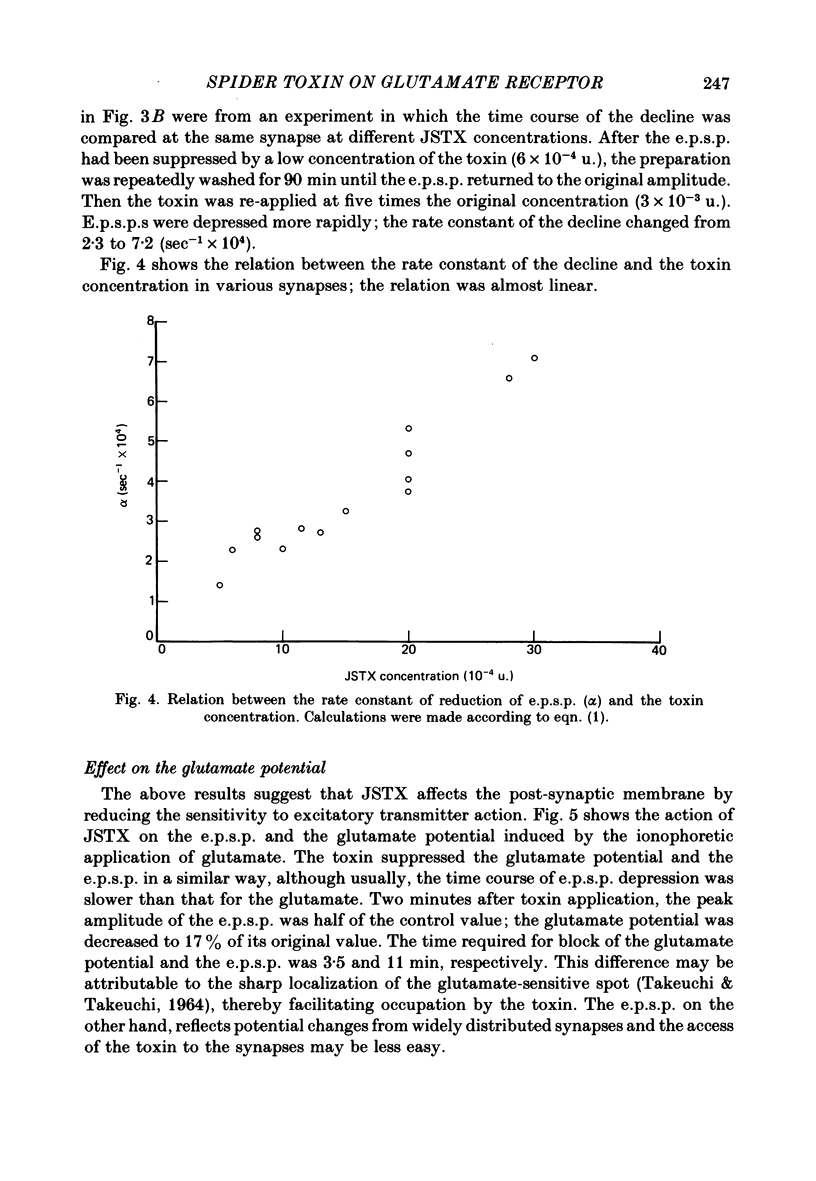
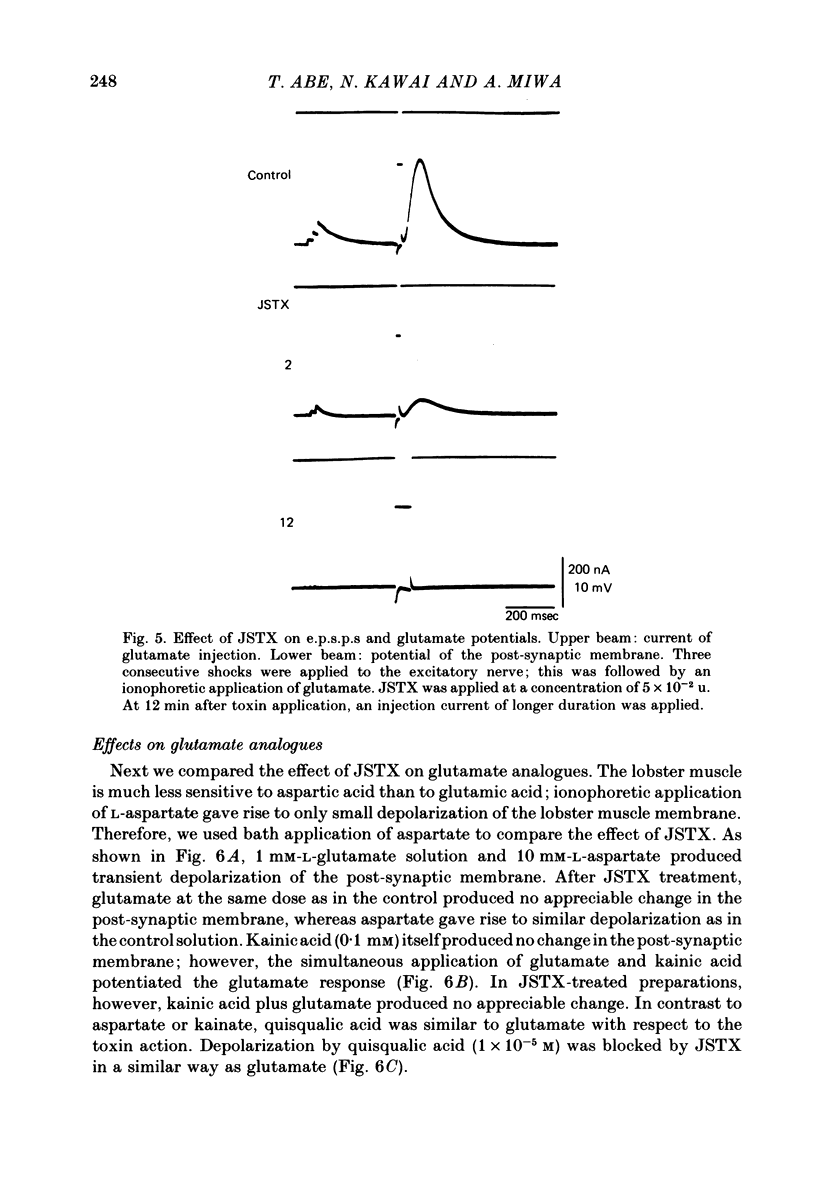
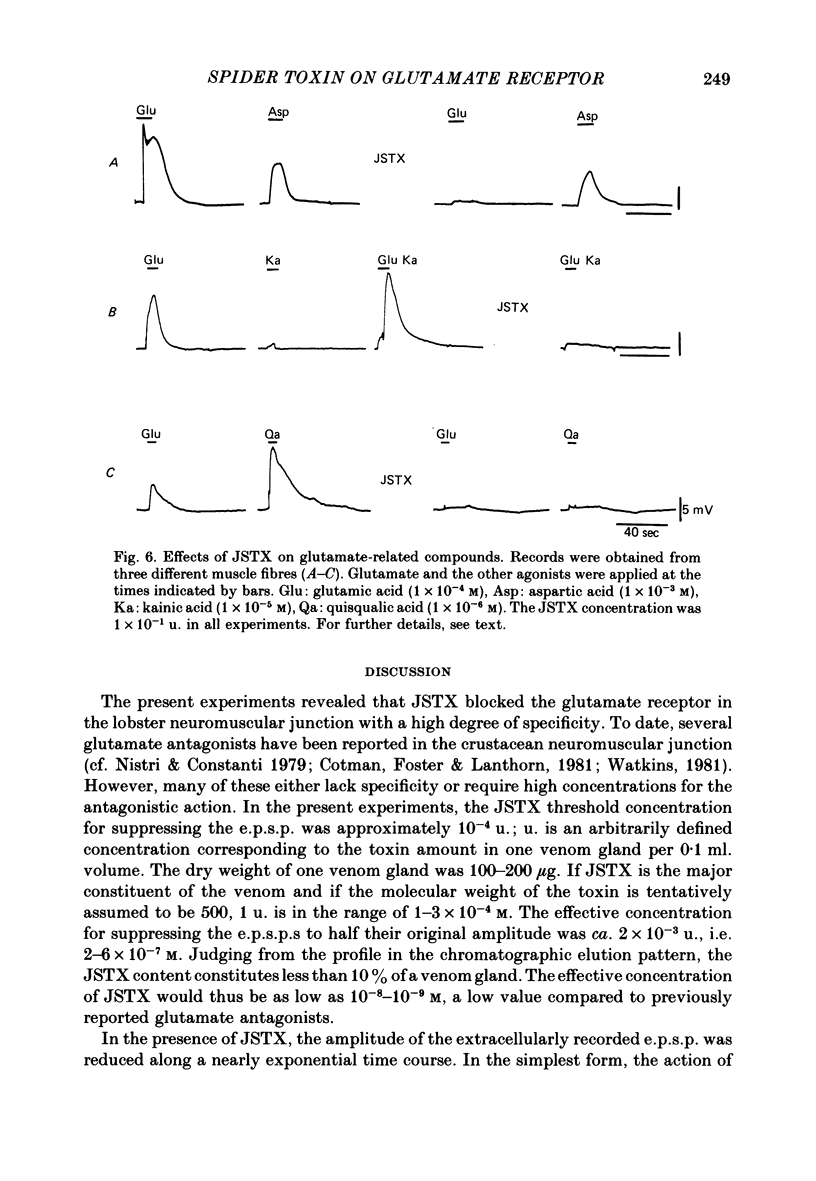
We studied the effect of neurotoxin (JSTX) separated from spider venom on the lobster neuromuscular junction. JSTX selectively suppressed excitatory post-synaptic potentials (e.p.s.p.s) without affecting the inhibitory post-synaptic potentials (i.p.s.p.s). The effect of JSTX was dose-dependent. The threshold dose for suppressing e.p.s.p.s corresponded to a small fraction of the toxin amount in a venom gland. At high concentration, JSTX irreversibly blocked e.p.s.p.s. The reduction in amplitude of extracellularly recorded e.p.s.p.s after JSTX application followed an exponential time course. The rate of suppression increased proportionally with the toxin concentration. JSTX blocked the glutamate potential in the post-synaptic membrane but it failed to affect the aspartate-induced depolarization. Kainic acid potentiated the glutamate-induced depolarization but it was without effect in the presence of JSTX. Depolarization produced by quisqualic acid is suppressed by the toxin. Our results suggest that the spider venom contains specific blockers of glutamate receptors in crustacean neuromuscular junctions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Kawai N., Niwa A. Purification and properties of a presynaptically acting neurotoxin, mandaratoxin, from hornet (Vespa mandarinia). Biochemistry. 1982 Mar 30;21(7):1693–1697. doi: 10.1021/bi00536a034. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Cull-Candy S. G., Miledi R. Glutamate current noise: post-synaptic channel kinetics investigated under voltage clamp. J Physiol. 1978 Sep;282:219–242. doi: 10.1113/jphysiol.1978.sp012459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. W., Mauro A., Longenecker H. E., Jr, Hurlbut W. P. Effects of black widow spider venom on the frog neuromuscular junction. Effects on the fine structure of the frog neuromuscular junction. Nature. 1970 Feb 21;225(5234):703–705. doi: 10.1038/225703a0. [DOI] [PubMed] [Google Scholar]
- Constanti A., Nistri A. A comparative study of the effects of glutamate and kainate on the lobster muscle fibre and the frog spinal cord. Br J Pharmacol. 1976 Jul;57(3):359–368. doi: 10.1111/j.1476-5381.1976.tb07674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman C. W., Foster A., Lanthorn T. An overview of glutamate as a neurotransmitter. Adv Biochem Psychopharmacol. 1981;27:1–27. [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. On the elementary conductance event produced by L-glutamate and quanta of the natural transmitter at the neuromuscular junctions of Maia squinado. J Physiol. 1976 Jun;258(1):205–225. doi: 10.1113/jphysiol.1976.sp011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. The synergistic action of L-glutamate and L-aspartate at crustacean excitatory neuromuscular junctions. J Physiol. 1977 Jul;268(3):697–709. doi: 10.1113/jphysiol.1977.sp011877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Neal H., Usherwood P. N. Action of black widow spider venom on an aminergic synapse. Nature. 1973 Feb 2;241(5388):353–354. doi: 10.1038/241353a0. [DOI] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. The quantal nature of transmission and spontaneous miniature potentials at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:514–529. doi: 10.1113/jphysiol.1961.sp006644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud A., Usherwood P. N. Action of kainic acid on a glutamatergic synapse. Comp Biochem Physiol C. 1975 Oct 1;52(1):51–53. doi: 10.1016/0306-4492(75)90012-x. [DOI] [PubMed] [Google Scholar]
- Frontali N., Ceccarelli B., Gorio A., Mauro A., Siekevitz P., Tzeng M. C., Hurlbut W. P. Purification from black widow spider venom of a protein factor causing the depletion of synaptic vesicles at neuromuscular junctions. J Cell Biol. 1976 Mar;68(3):462–479. doi: 10.1083/jcb.68.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAVITZ E. A., KUFFLER S. W., POTTER D. D. GAMMA-AMINOBUTYRIC ACID AND OTHER BLOCKING COMPOUNDS IN CRUSTACEA. III. THEIR RELATIVE CONCENTRATIONS IN SEPARATED MOTOR AND INHIBITORY AXONS. J Neurophysiol. 1963 Sep;26:739–751. doi: 10.1152/jn.1963.26.5.739. [DOI] [PubMed] [Google Scholar]
- KRAVITZ E. A., KUFFLER S. W., POTTER D. D., VANGELDER N. M. GAMMA-AMINOBUTYRIC ACID AND OTHER BLOCKING COMPOUNDS IN CRUSTACEA. II. PERIPHERAL NERVOUS SYSTEM. J Neurophysiol. 1963 Sep;26:729–738. doi: 10.1152/jn.1963.26.5.729. [DOI] [PubMed] [Google Scholar]
- Kawagoe R., Onodera K., Takeuchi A. On the quantal release of endogenous glutamate from the crayfish neuromuscular junction. J Physiol. 1982 Jan;322:529–539. doi: 10.1113/jphysiol.1982.sp014053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagoe R., Onodera K., Takeuchi A. Release of glutamate from the crayfish neuromuscular junction. J Physiol. 1981 Mar;312:225–236. doi: 10.1113/jphysiol.1981.sp013625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai N., Mauro A., Grundfest H. Effect of black widow spider venom on the lobster neuromuscular junctions. J Gen Physiol. 1972 Dec;60(6):650–664. doi: 10.1085/jgp.60.6.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai N., Niwa A., Abe T. Spider venom contains specific receptor blocker of glutaminergic synapses. Brain Res. 1982 Sep 9;247(1):169–171. doi: 10.1016/0006-8993(82)91044-7. [DOI] [PubMed] [Google Scholar]
- Kawai N., Niwa A. Hyperpolarization of the excitatory nerve terminals by inhibitory nerve stimulation in lobser. Brain Res. 1977 Dec 2;137(2):365–368. doi: 10.1016/0006-8993(77)90348-1. [DOI] [PubMed] [Google Scholar]
- Kawai N., Niwa A. Neuromuscular transmission without sodium activation of the presynaptic nerve terminal in the lobster. J Physiol. 1980 Aug;305:73–85. doi: 10.1113/jphysiol.1980.sp013350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowagie C., Gerschenfeld H. M. Glutamate antagonists at a crayfish neuromuscular junction. Nature. 1974 Apr 5;248(448):533–535. doi: 10.1038/248533a0. [DOI] [PubMed] [Google Scholar]
- McBride W. J., Shank R. P., Freeman A. R., Aprison M. H. Levels of free amino acids in excitatory, inhibitory and sensory axons of the walking limbs of the lobster. Life Sci. 1974 Mar 16;14(6):1109–1120. doi: 10.1016/0024-3205(74)90235-5. [DOI] [PubMed] [Google Scholar]
- Nistri A., Constanti A. Pharmacological characterization of different types of GABA and glutamate receptors in vertebrates and invertebrates. Prog Neurobiol. 1979;13(2):117–235. doi: 10.1016/0301-0082(79)90016-9. [DOI] [PubMed] [Google Scholar]
- Niwa A., Kawai N. Tetrodotoxin-resistant propagating action potentials in presynaptic axon of the lobster. J Neurophysiol. 1982 Mar;47(3):353–361. doi: 10.1152/jn.1982.47.3.353. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Distribution and pharmacological properties of synaptic and extrasynaptic glutamate receptors on crayfish muscle. J Physiol. 1980 Sep;306:233–250. doi: 10.1113/jphysiol.1980.sp013394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Inhibitory effect of streptomycin and related antibiotics on the glutamate receptor of the crayfish neuromuscular junction. Neuropharmacology. 1977 Mar;16(3):171–177. doi: 10.1016/0028-3908(77)90092-2. [DOI] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Ionic mechanism of the excitatory synaptic membrane of the crayfish neuromuscular junction. J Physiol. 1975 Oct;252(1):295–318. doi: 10.1113/jphysiol.1975.sp011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera K., Takeuchi A. Permeability changes produced by L-glutamate at the excitatory post-synaptic membrane of the crayfish muscle. J Physiol. 1976 Mar;255(3):669–685. doi: 10.1113/jphysiol.1976.sp011302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki H., Shibuya I. A new potent excitant, quisqualic acid: effects on crayfish neuromuscular junction. Neuropharmacology. 1974 Jul;13(7):665–672. doi: 10.1016/0028-3908(74)90056-2. [DOI] [PubMed] [Google Scholar]
- Shinozaki H., Shibuya I. Potentiation of glutamate-induced depolarization by kainic acid in the crayfish opener muscle. Neuropharmacology. 1974 Nov;13(10-11):1057–1065. doi: 10.1016/0028-3908(74)90096-3. [DOI] [PubMed] [Google Scholar]
- Sorenson M. M. The free amino acids in peripheral nerves and in isolated inhibitory and excitatory nerve fibres of Cancer magister. J Neurochem. 1973 Apr;20(4):1231–1245. doi: 10.1111/j.1471-4159.1973.tb00092.x. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. THE EFFECT ON CRAYFISH MUSCLE OF IONTOPHORETICALLY APPLIED GLUTAMATE. J Physiol. 1964 Mar;170:296–317. doi: 10.1113/jphysiol.1964.sp007332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harreveld A. L-proline as a glutamate antagonist at a crustacean neuromuscular junction. J Neurobiol. 1980 Nov;11(6):519–529. doi: 10.1002/neu.480110603. [DOI] [PubMed] [Google Scholar]
- Wheal H. V., Kerkut G. A. Structure activity studies on the excitatory receptor of the crustacean neuromuscular junction. Comp Biochem Physiol C. 1976;53(1):51–55. doi: 10.1016/0306-4492(76)90050-2. [DOI] [PubMed] [Google Scholar]
- Wheale H. V., Kerkut G. A. The antagonistic action of L-glutamate diethyl ester on the excitatory postsynaptic membrane of the crab neuromuscular junction. Comp Biochem Physiol C. 1975 Jun 1;51(1):79–81. doi: 10.1016/0306-4492(75)90042-8. [DOI] [PubMed] [Google Scholar]


