Abstract
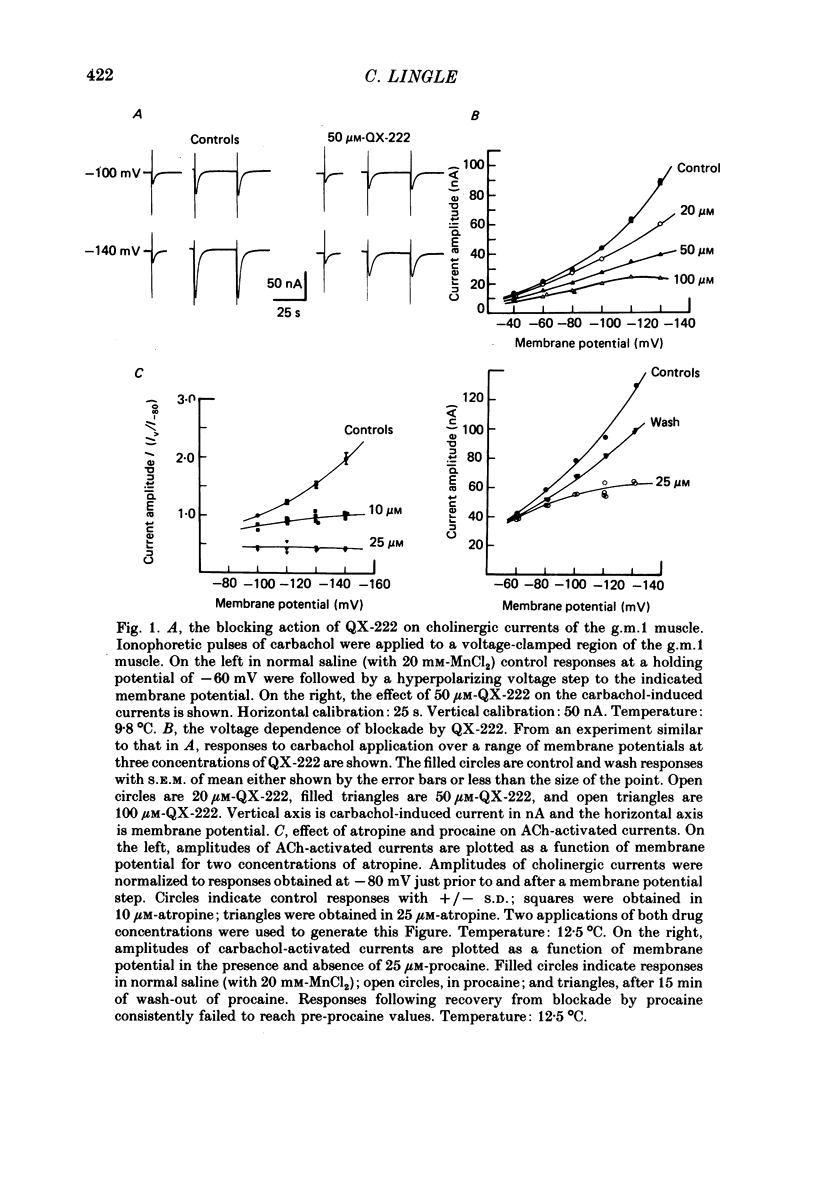
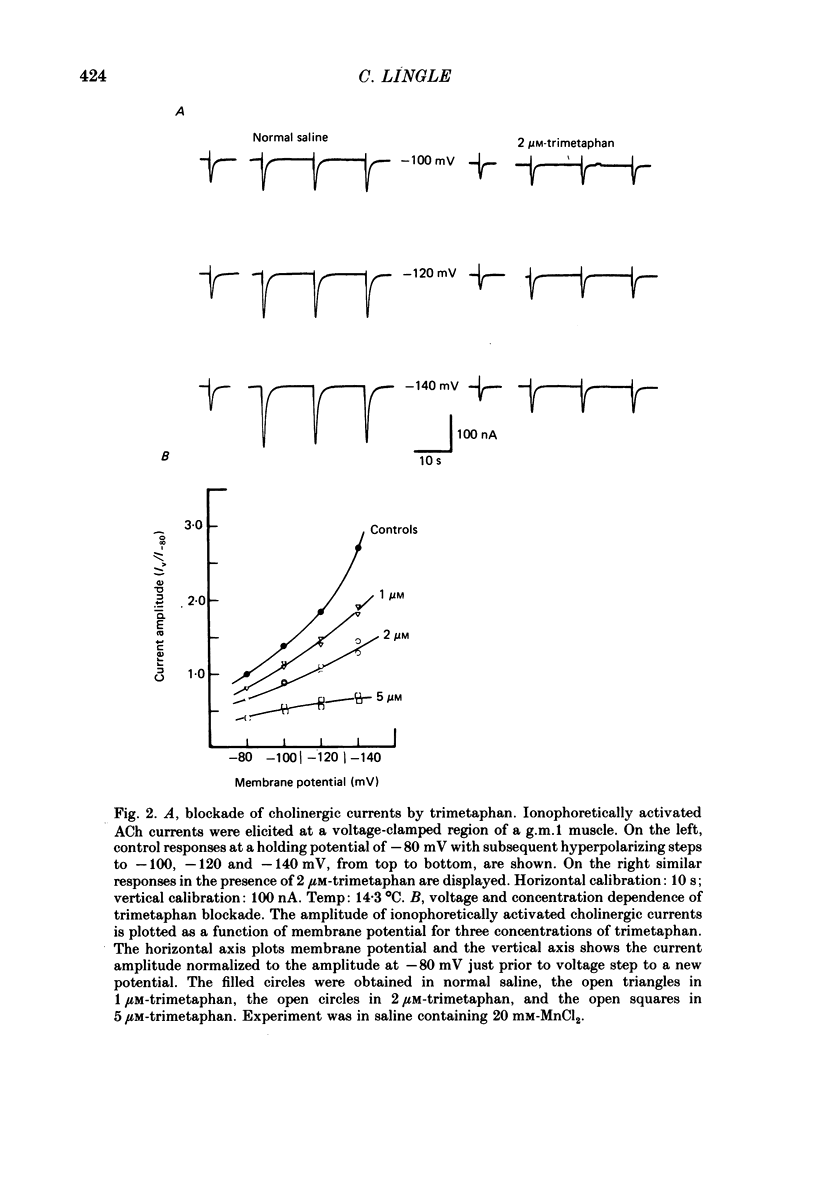
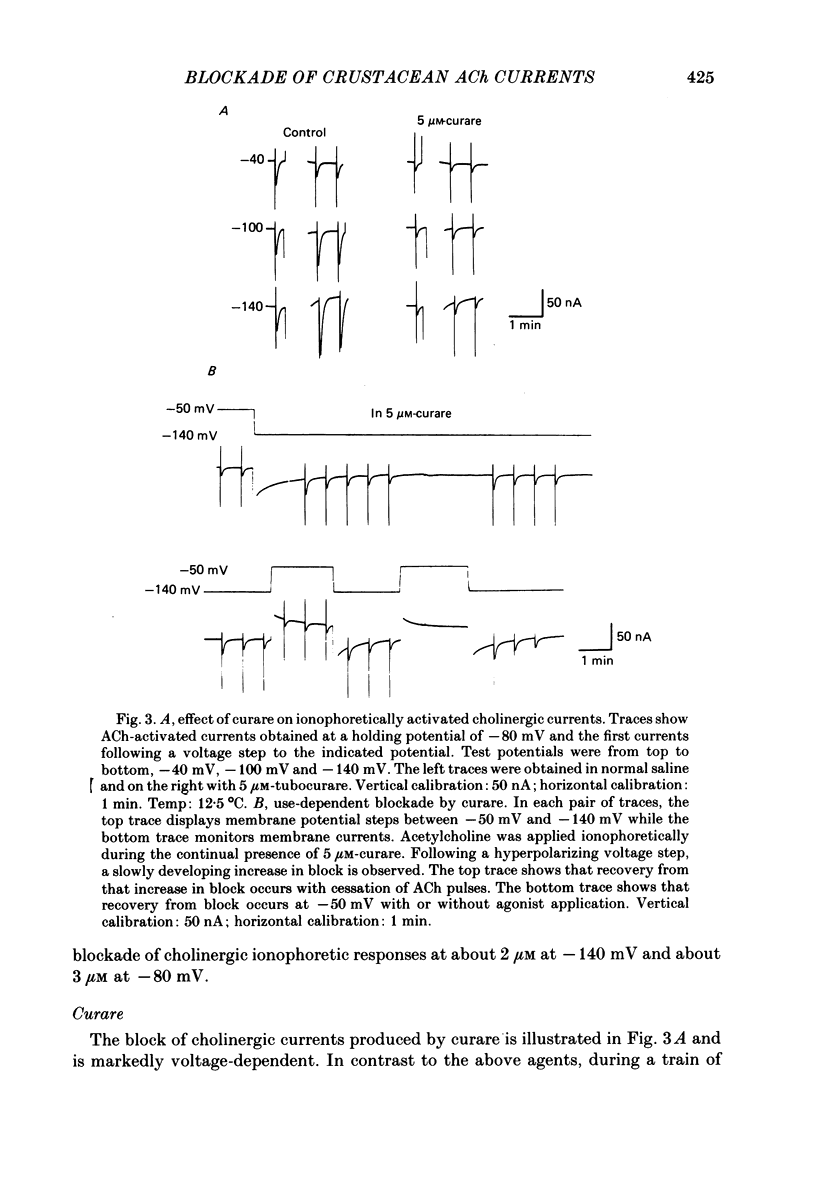
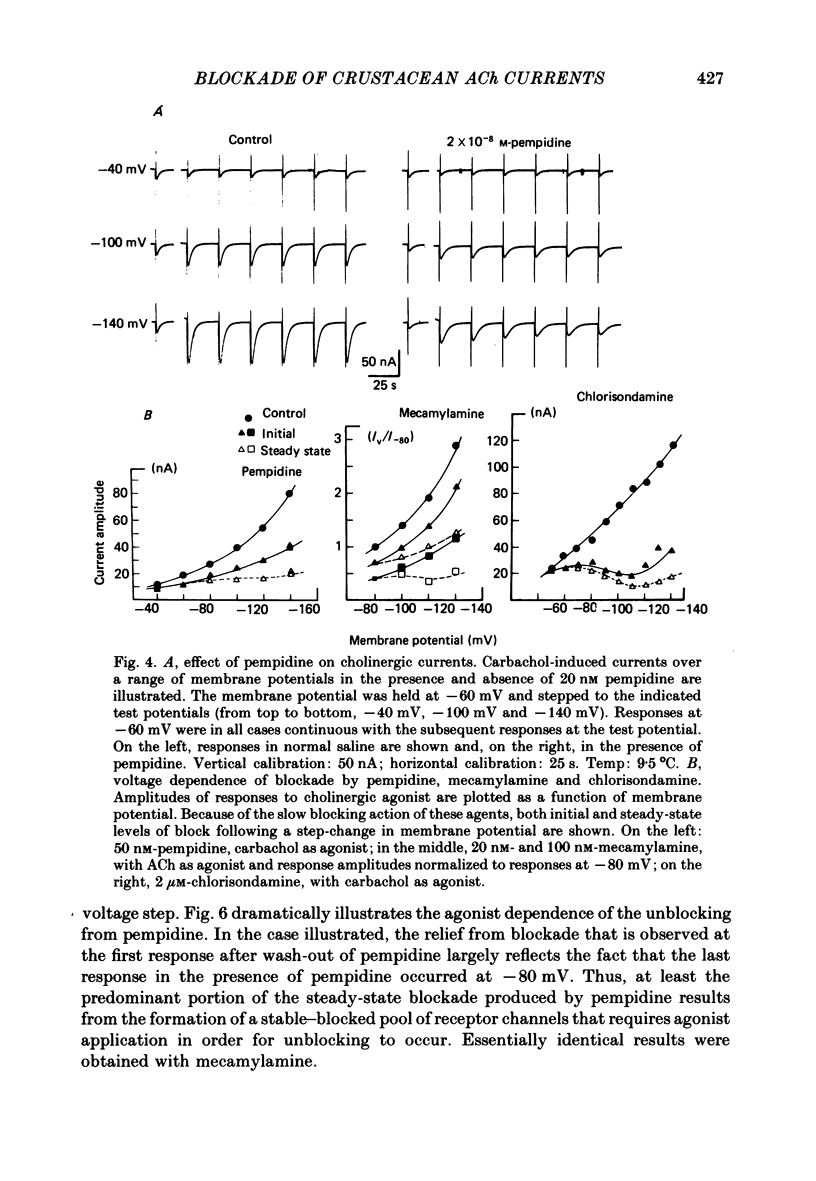
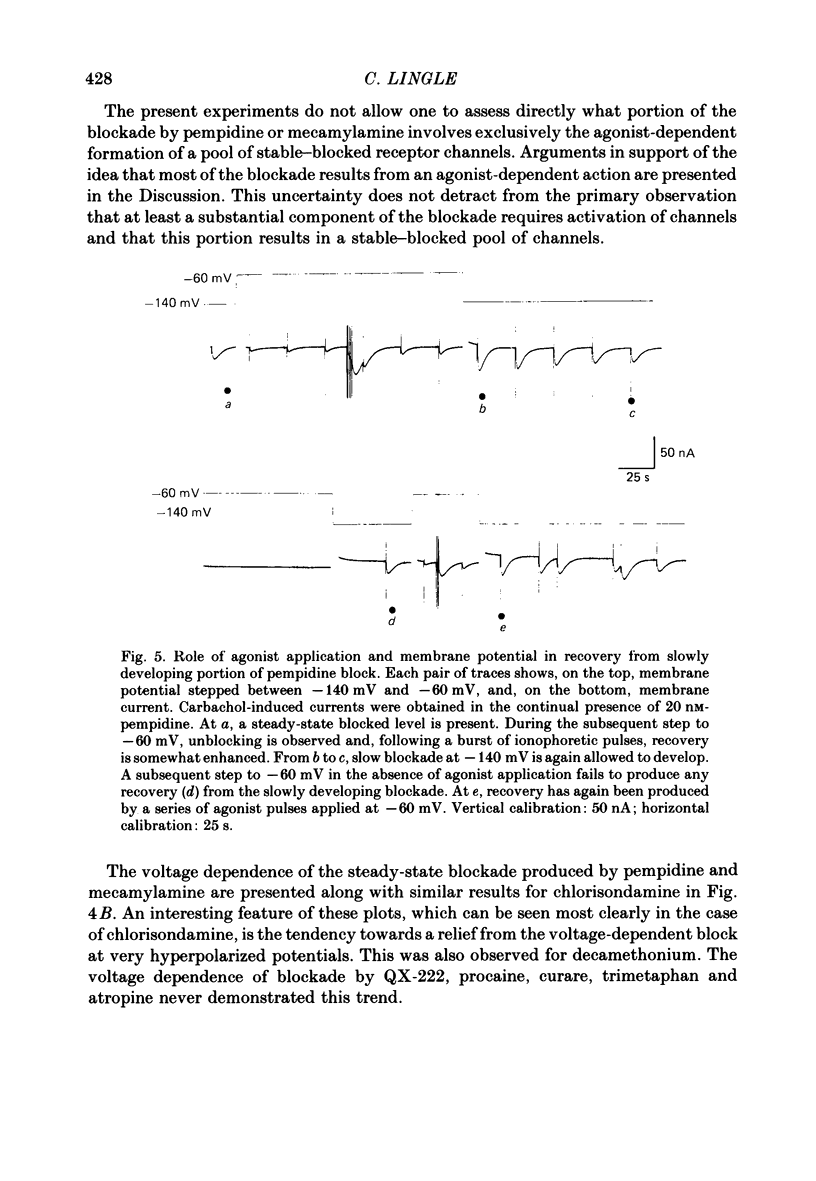
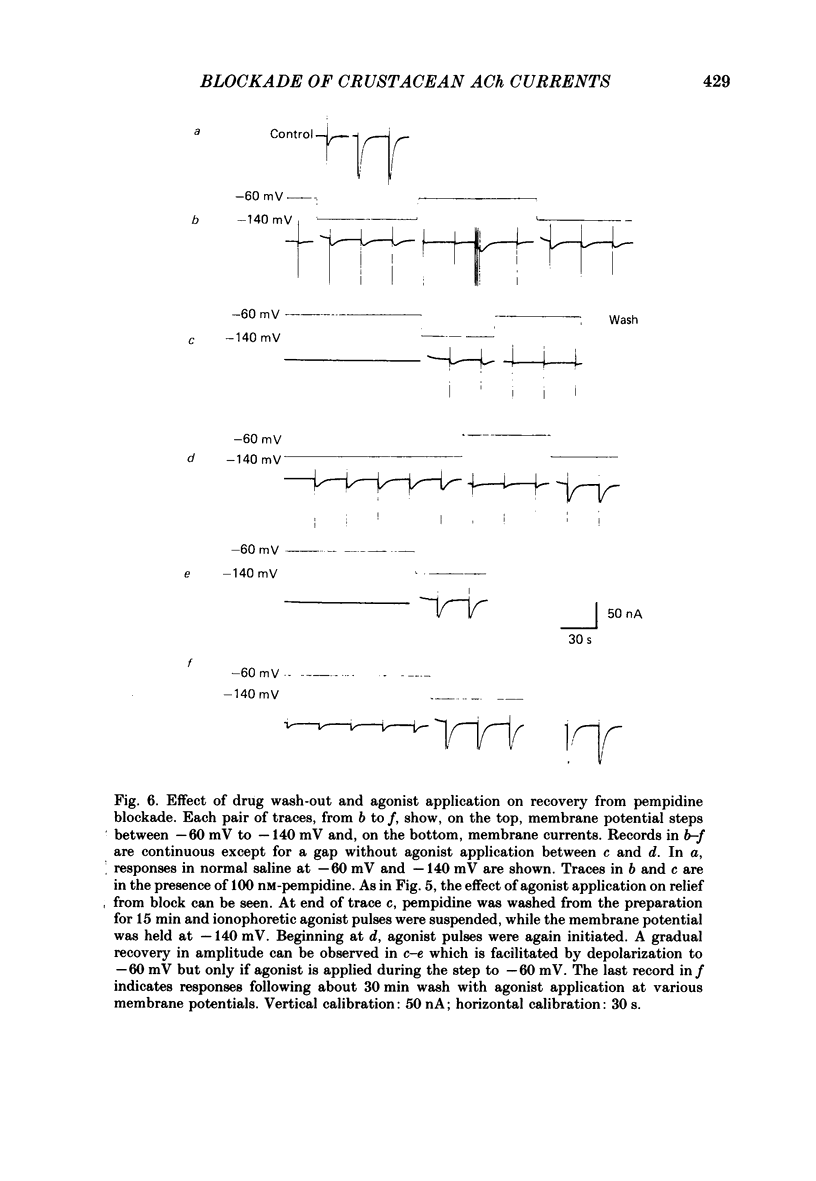
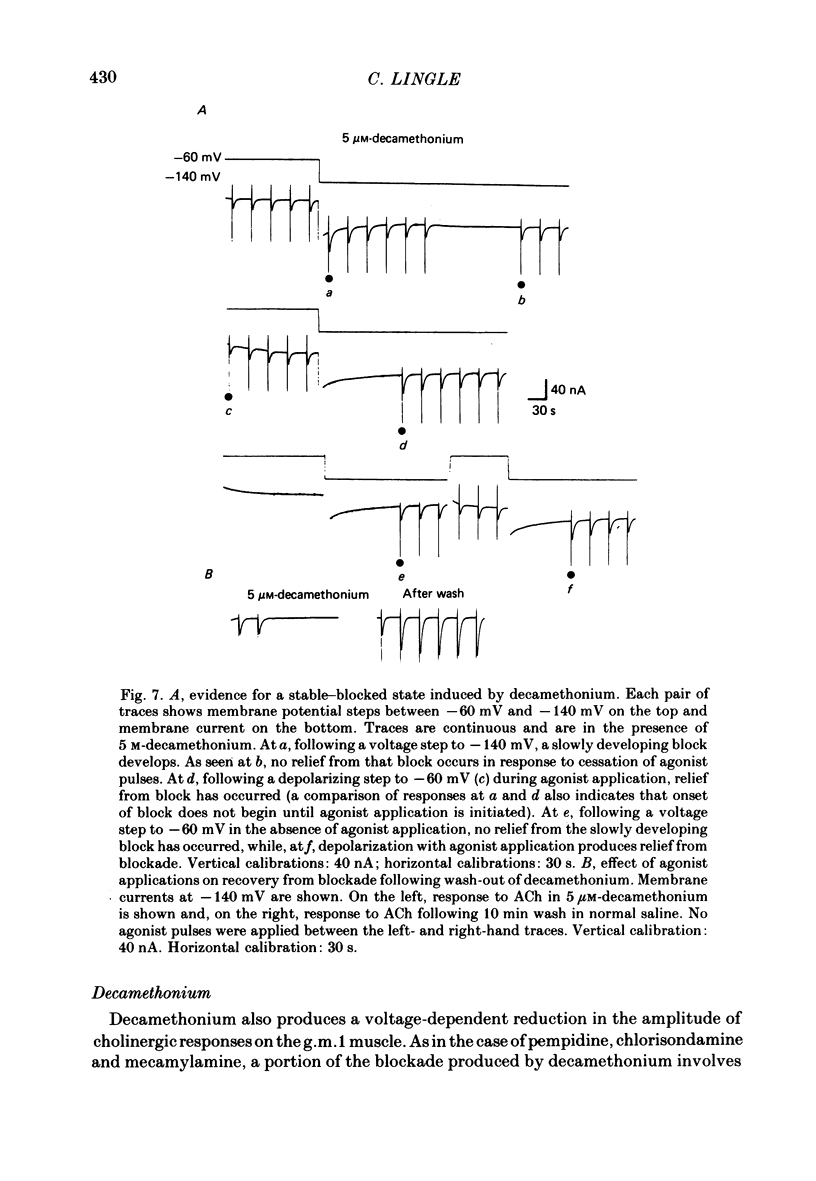
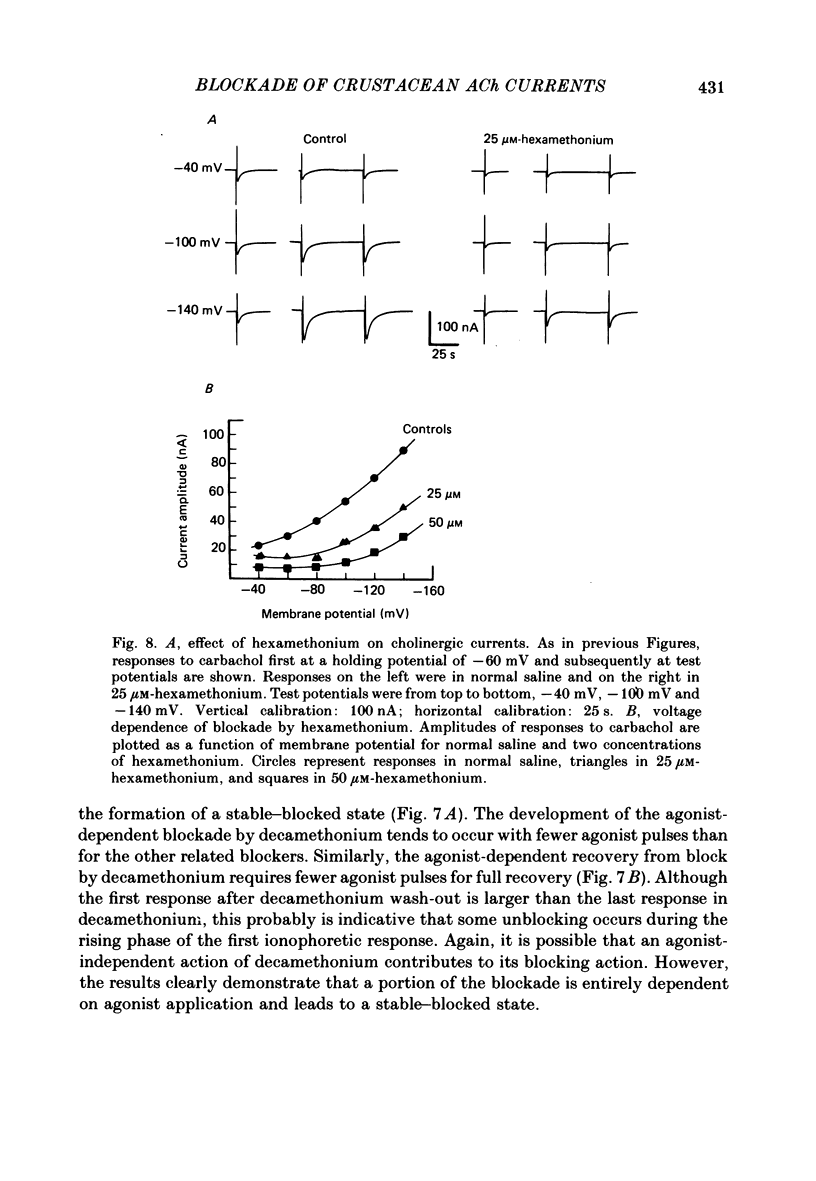
The voltage dependence, concentration dependence, and agonist dependence of blocking and unblocking produced by anticholinergic agents on the ionophoretically activated cholinergic currents of the lobster gastric mill 1 (g.m.1) muscle were examined. Although the ionophoretic technique provides only qualitative information as to blocking mechanisms it is useful in revealing slow components of the blocking action of some drugs. At least two qualitatively different types of voltage-dependent block of the crustacean cholinergic currents were observed. For pempidine, mecamylamine and decamethonium (also chlorisondamine: Lingle, 1983), a slowly developing voltage-dependent block was produced that led to the formation of a stable-blocked state. Recovery from this stable-blocked state is largely dependent on subsequent application of agonist. In contrast, recovery from the voltage-dependent block produced by QX-222, atropine, procaine and curare either proceeds independently of agonist application or occurs too rapidly to be observed by the present methods. Blockade by hexamethonium reveals anomalous voltage dependence, being enhanced over some voltages and relieved with additional hyperpolarization. Blockade by trimetaphan is largely independent of membrane potential except at higher concentrations.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Feltz A. End-plate channel opening and the kinetics of quinacrine (mepacrine) block. J Physiol. 1980 Sep;306:283–306. doi: 10.1113/jphysiol.1980.sp013397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Feltz A. Quinacrine (mepacrine) action at frog end-plate. J Physiol. 1980 Sep;306:261–281. doi: 10.1113/jphysiol.1980.sp013396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R., Sakmann B. Decamethonium both opens and blocks endplate channels. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2994–2998. doi: 10.1073/pnas.75.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams P. R. Voltage jump analysis of procaine action at frog end-plate. J Physiol. 1977 Jun;268(2):291–318. doi: 10.1113/jphysiol.1977.sp011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Large W. A., Rang H. P. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol. 1979 Oct;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. The mode of action of antagonists of the excitatory response to acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:207–235. doi: 10.1113/jphysiol.1978.sp012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese R., Maclagan J. Entry of decamethonium in rat muscle studied by autoradiography. J Physiol. 1970 Sep;210(2):363–386. doi: 10.1113/jphysiol.1970.sp009215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., Van Helden D. Effects of permeant monovalent cations on end-plate channels. J Physiol. 1979 Mar;288:509–528. [PMC free article] [PubMed] [Google Scholar]
- Lingle C. Blockade of cholinergic channels by chlorisondamine on a crustacean muscle. J Physiol. 1983 Jun;339:395–417. doi: 10.1113/jphysiol.1983.sp014723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchais D., Marty A. Action of glucosamine on acetylcholine-sensitive channels. J Membr Biol. 1980 Aug 21;56(1):43–48. doi: 10.1007/BF01869350. [DOI] [PubMed] [Google Scholar]
- Marchais D., Marty A. Interaction of permeant ions with channels activated by acetylcholine in Aplysia neurones. J Physiol. 1979 Dec;297(0):9–45. doi: 10.1113/jphysiol.1979.sp013025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E. Cholinergic motor neurones in the stomatogastric system of the lobster. J Physiol. 1976 May;257(1):63–86. doi: 10.1113/jphysiol.1976.sp011356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E., Paupardin-Tritsch D. The pharmacological profile of the acetylcholine response of a crustacean muscle. J Exp Biol. 1980 Oct;88:147–159. doi: 10.1242/jeb.88.1.147. [DOI] [PubMed] [Google Scholar]
- Rang H. P., Colquhoun D., Rang H. P. The action of ganglionic blocking drugs on the synaptic responses of rat submandibular ganglion cells. Br J Pharmacol. 1982 Jan;75(1):151–168. doi: 10.1111/j.1476-5381.1982.tb08768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


