Abstract
1. To define the neural volleys responsible for the Achilles tendon jerk and the H reflex, muscle afferent activity was recorded using micro-electrodes inserted percutaneously into appropriate fascicles of the tibial nerve in the popliteal fossa.
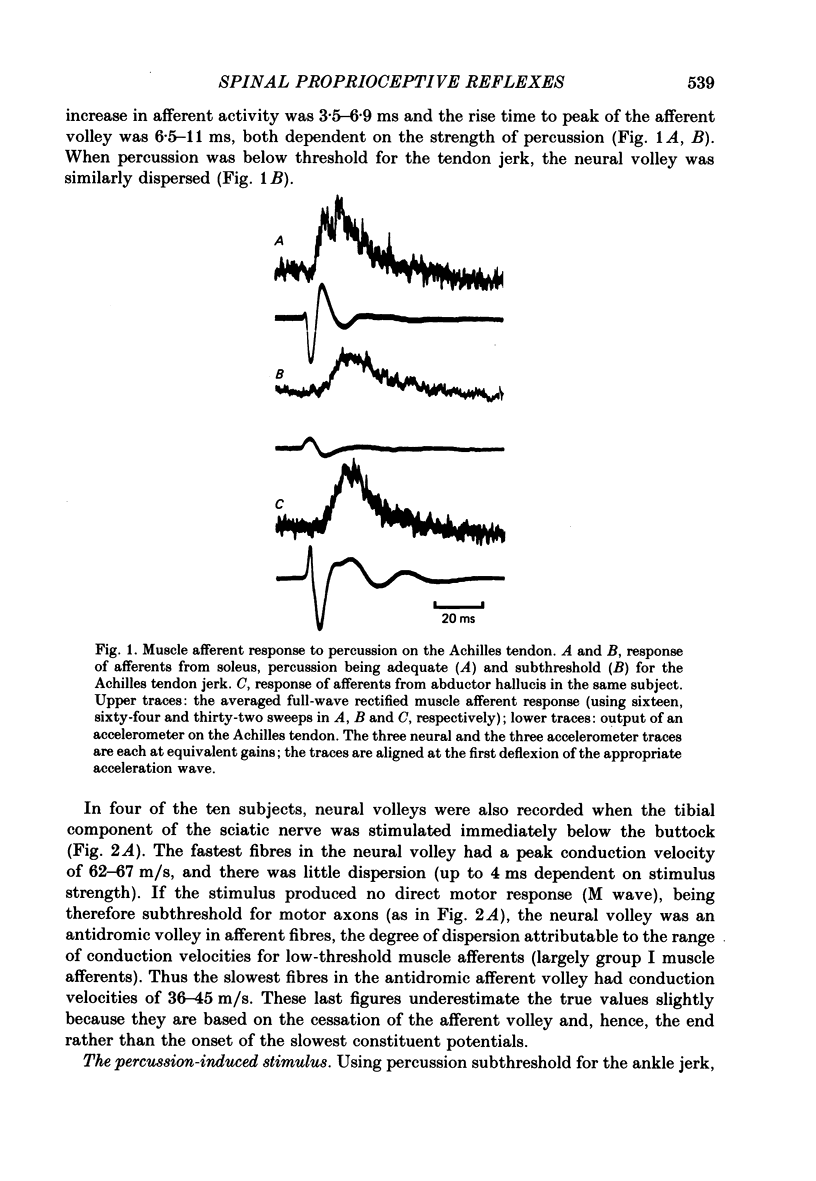
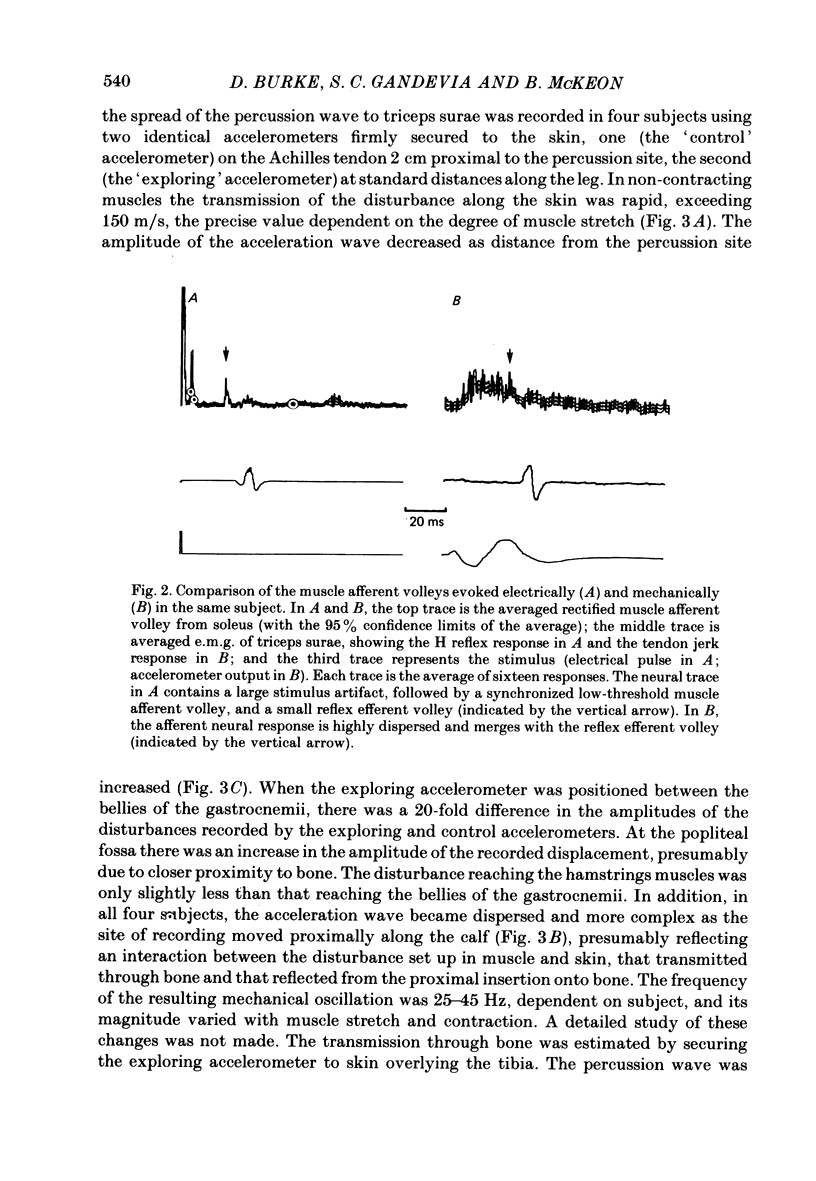
2. The response of soleus muscle afferents to tendon percussion consisted of a dispersed volley, starting 3·5-7·0 ms after percussion, increasing to a peak over 6·5-11·0 ms, and lasting 25-30 ms, depending on the strength of percussion. Electrical stimuli to the sciatic nerve at a level adequate to evoke an H reflex but subthreshold for the M wave produced a more synchronized volley, the fastest fibres of which had conduction velocities of 62-67 m/s, and the slowest 36-45 m/s.
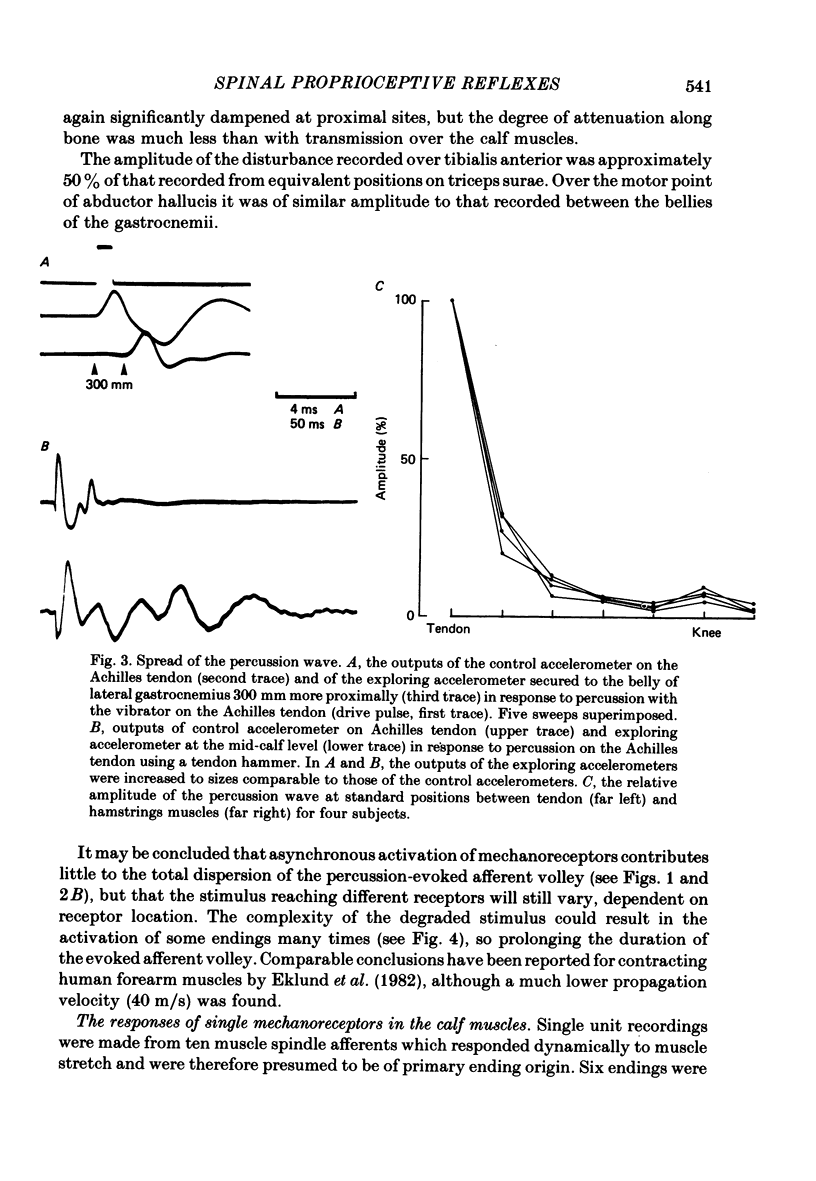
3. The wave of acceleration produced by percussion subthreshold for the ankle jerk spread along the skin at over 150 m/s. Midway between the bellies of the gastrocnemii it consisted of a damped oscillation with four to five separate phases and maximum amplitude approximately one-twentieth of that recorded on the Achilles tendon.
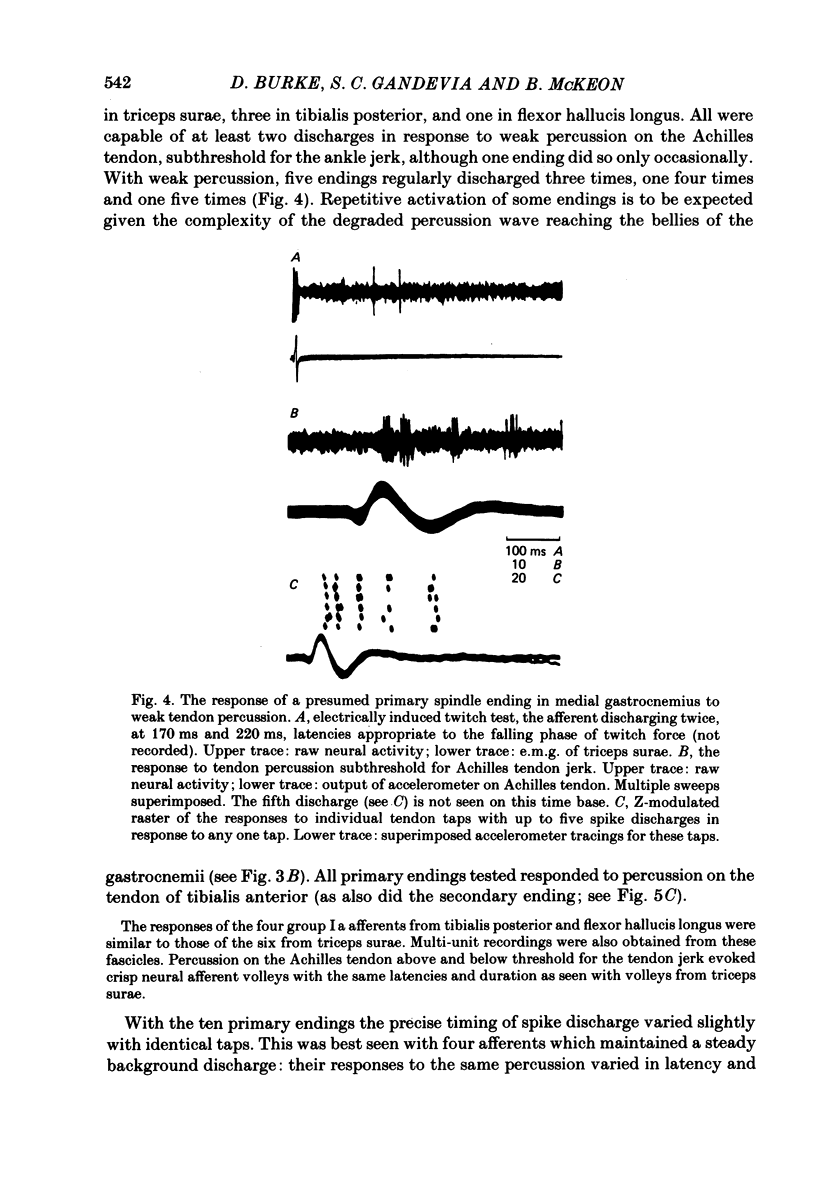
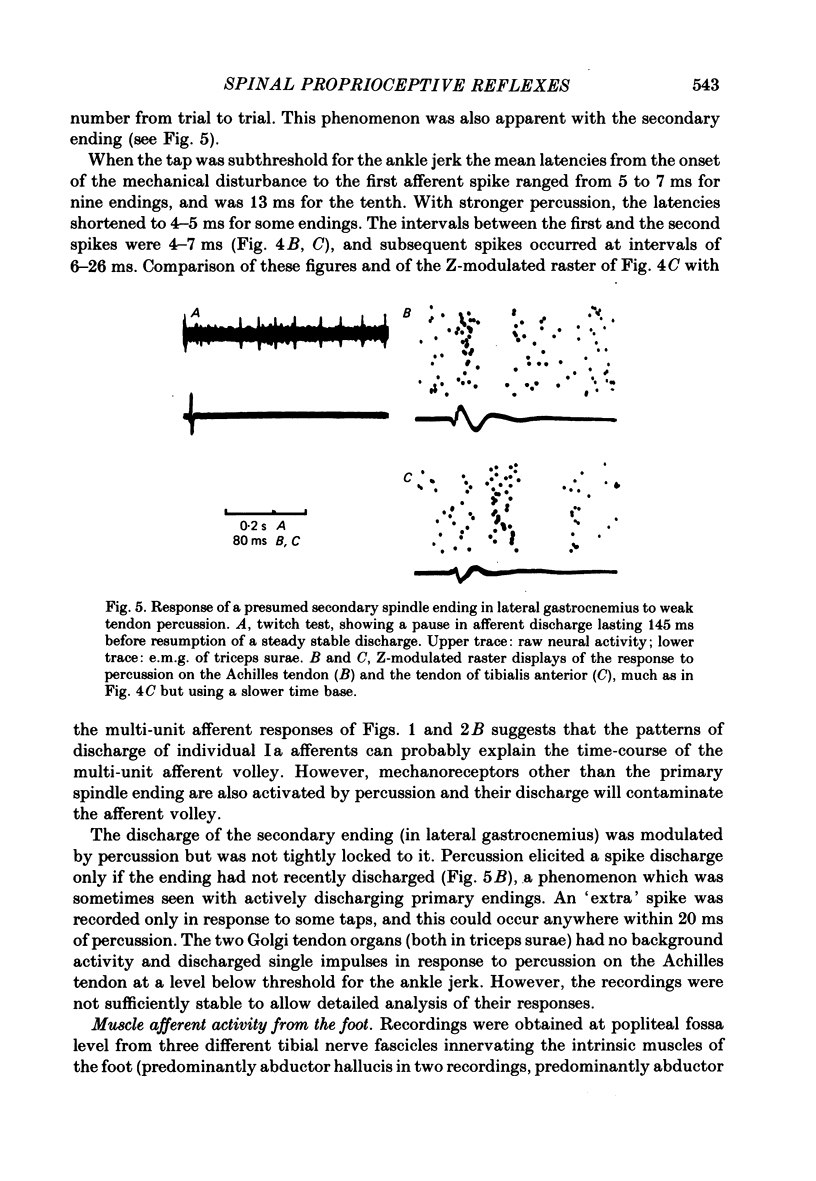
4. With ten primary spindle endings, tendon percussion subthreshold for the ankle jerk elicited two to five spike discharges per tap, the shortest interspike intervals being 4-7 ms. Tendon percussion elicited single discharges from two Golgi tendon organs, and altered the discharge pattern of a single secondary spindle ending. The degree of dispersion of the multi-unit muscle afferent volley can be explained by the pattern of discharge of primary spindle endings.
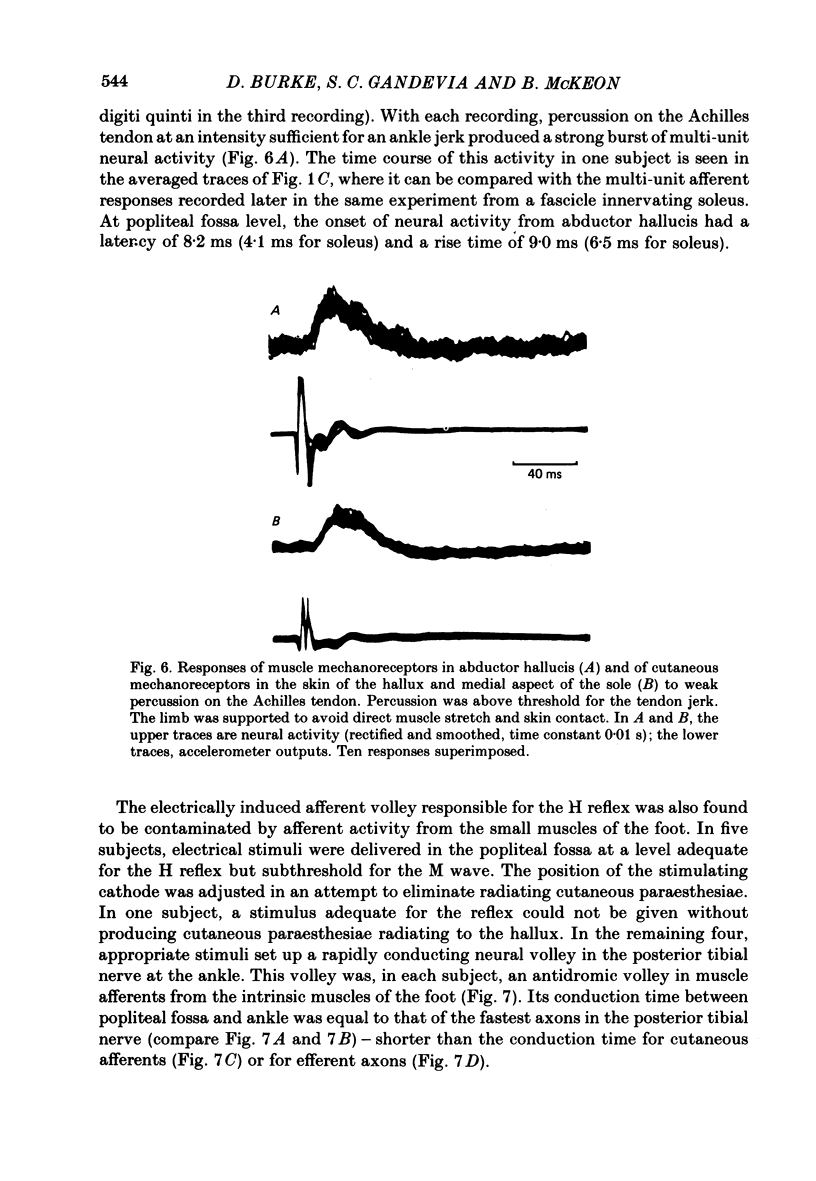
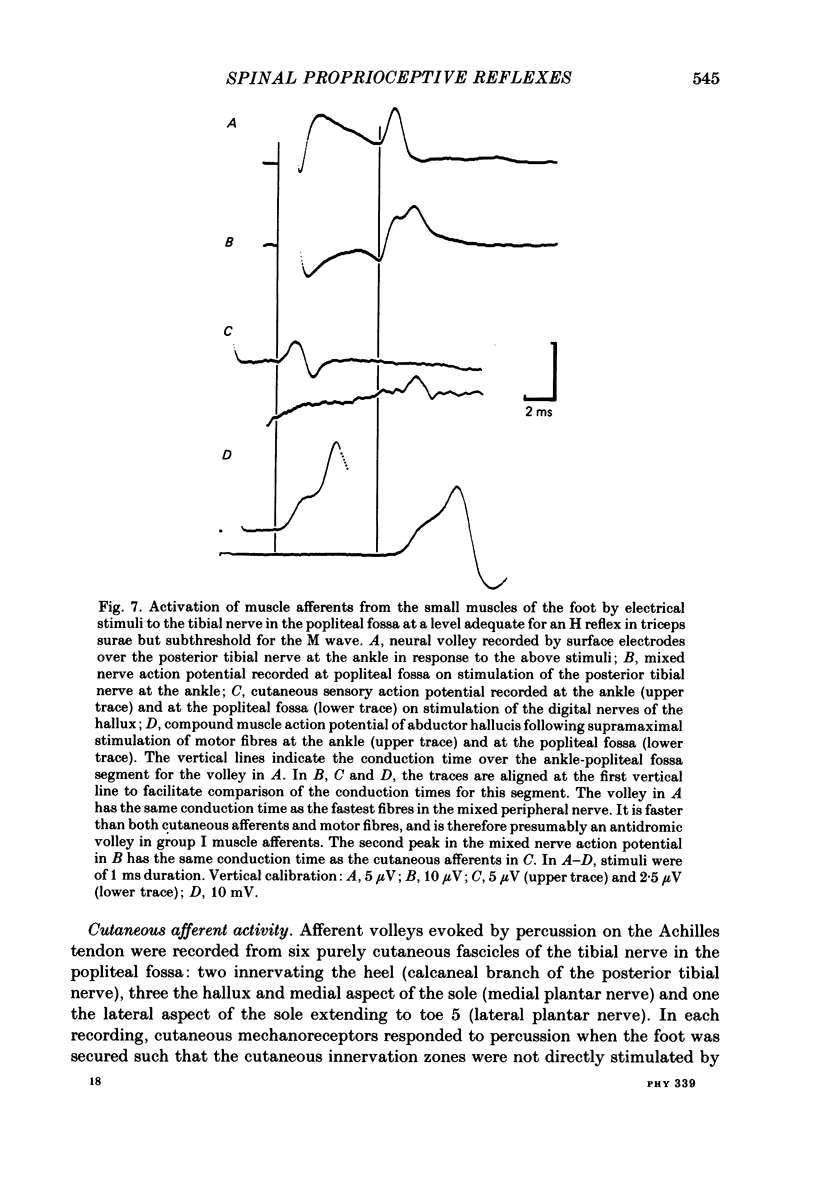
5. Percussion on the Achilles tendon evoked crisp afferent volleys in recordings from nerve fascicles innervating flexor hallucis longus, tibialis posterior, the intrinsic muscles of the foot and the skin of the foot. Electrical stimuli delivered to the tibial nerve in the popliteal fossa at a level sufficient for the H reflex of soleus produced either a volley in muscle afferents from the intrinsic muscles of the foot or a volley in cutaneous afferents from the foot.
6. For comparable stimuli in the two positions, the H reflex was inhibited but the Achilles tendon jerk enhanced when the ankle was dorsiflexed from 105° to 90°.
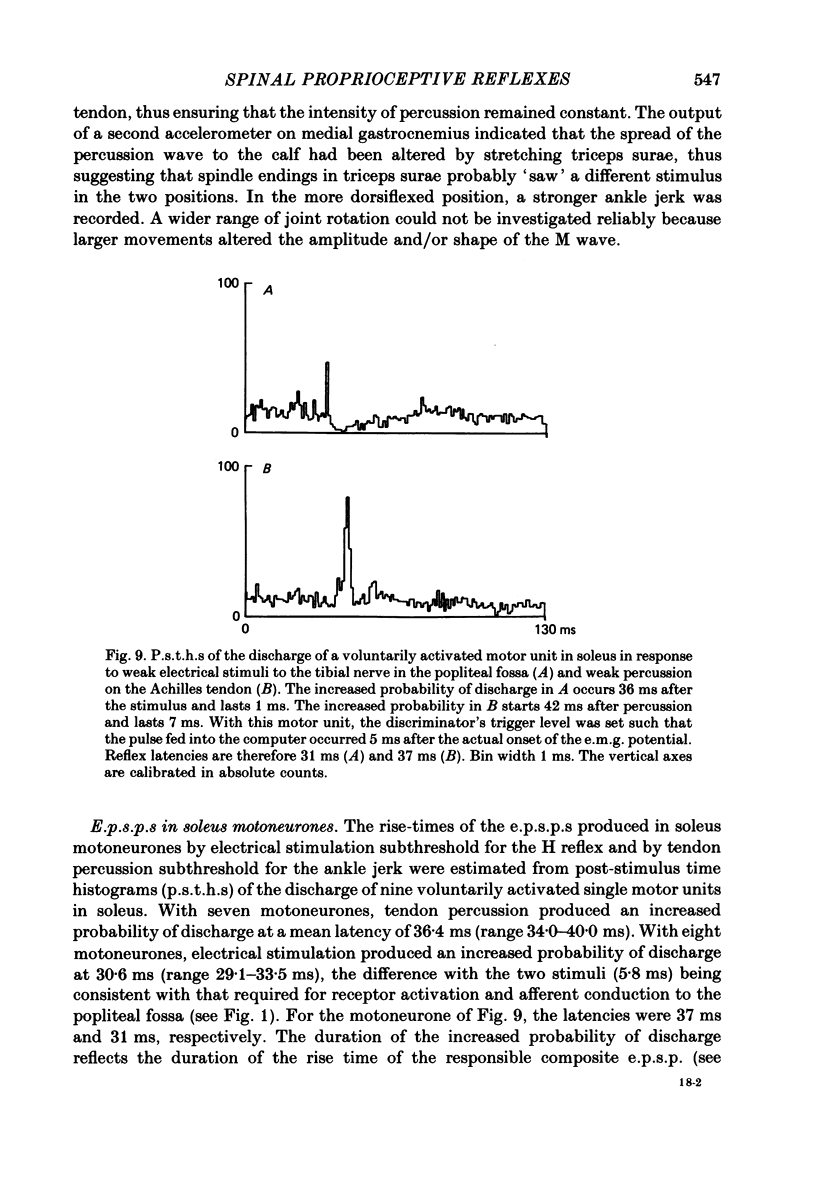
7. The duration of the rise times of the excitatory post-synaptic potentials (e.p.s.p.s) produced in soleus motoneurones by electrical stimulation, and by tendon percussion subthreshold for the H reflex and the ankle jerk respectively, was estimated from post-stimulus time histograms of the discharge of voluntarily activated single motor units in soleus. The mean e.p.s.p. rise times were 1·9 ms for electrical stimulation and 6·6 ms for tendon percussion. There was evidence that the duration of the electrically evoked e.p.s.p. was curtailed by an inhibitory post-synaptic potential (i.p.s.p.) of only slightly longer latency than the e.p.s.p.
8. The mechanically induced and electrically induced afferent volleys are not homogeneous volleys in group I a afferents from triceps surae. The afferent volleys differ in so many respects that it is probably invalid to compare the H reflex and tendon jerk as a measure of fusimotor activity. It is suggested that neither reflex can be considered a purely monosynaptic reflex.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby P., Labelle K. Effects of extensor and flexor group I afferent volleys on the excitability of individual soleus motoneurones in man. J Neurol Neurosurg Psychiatry. 1977 Sep;40(9):910–919. doi: 10.1136/jnnp.40.9.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLER A. J., DORNHORST A. C. The reinforcement of tendon-reflexes. Lancet. 1957 Dec 21;273(7008):1260–1262. doi: 10.1016/s0140-6736(57)91542-8. [DOI] [PubMed] [Google Scholar]
- BULLER A. J. The ankle-jerk in early hemiplegia. Lancet. 1957 Dec 21;273(7008):1262–1263. doi: 10.1016/s0140-6736(57)91543-x. [DOI] [PubMed] [Google Scholar]
- Bishop B., Machover S., Johnston R., Anderson M. A quantitative assessment of gamma-motoneuron contribution to the achilles tendon reflex in normal subjects. Arch Phys Med Rehabil. 1968 Mar;49(3):145–154. [PubMed] [Google Scholar]
- Burke D., Andrews C., Ashby P. autogenic effects of static muscle stretch in spastic man. Arch Neurol. 1971 Oct;25(4):367–372. doi: 10.1001/archneur.1971.00490040093011. [DOI] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L., Wallin B. G. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976 Oct;261(3):695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D. The activity of human muscle spindle endings in normal motor behavior. Int Rev Physiol. 1981;25:91–126. [PubMed] [Google Scholar]
- Delwaide P. J., Crenna P., Fleron M. H. Cutaneous nerve stimulation and motoneuronal excitability: I, soleus and tibialis anterior excitability after ipsilateral and contralateral sural nerve stimulation. J Neurol Neurosurg Psychiatry. 1981 Aug;44(8):699–707. doi: 10.1136/jnnp.44.8.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamantopoulos E., Gassel M. M. Electrically induced monosynaptic reflexes in man. J Neurol Neurosurg Psychiatry. 1965 Dec;28(6):496–502. doi: 10.1136/jnnp.28.6.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrichson P. Phasic ankle reflex in spasticity and Parkinsonian rigidity. The role of the fusimotor system. Acta Neurol Scand. 1971;47(1):22–51. doi: 10.1111/j.1600-0404.1971.tb07462.x. [DOI] [PubMed] [Google Scholar]
- Eklund G., Hagbarth K. E., Hägglund J. V., Wallin E. U. Mechanical oscillations contributing to the segmentation of the reflex electromyogram response to stretching human muscles. J Physiol. 1982 May;326:65–77. doi: 10.1113/jphysiol.1982.sp014177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E., Jankowska E., Johannisson T., Lipski J. Autogenetic inhibition of motoneurones by impulses in group Ia muscle spindle afferents. J Physiol. 1979 Aug;293:173–195. doi: 10.1113/jphysiol.1979.sp012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassel M. M., Diamantopoulos E. Mechanically and electrically elicited monosynaptic reflexes in man. J Appl Physiol. 1966 May;21(3):1053–1058. doi: 10.1152/jappl.1966.21.3.1053. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Hägglund J. V., Wallin E. U., Young R. R. Grouped spindle and electromyographic responses to abrupt wrist extension movements in man. J Physiol. 1981 Mar;312:81–96. doi: 10.1113/jphysiol.1981.sp013617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R. Relationship between the H reflex and the tendon jerk response. Electromyography. 1969 Nov-Dec;9(4):359–370. [PubMed] [Google Scholar]
- Iles J. F. Responses in human pretibial muscles to sudden stretch and to nerve stimulation. Exp Brain Res. 1977 Dec 19;30(4):451–470. doi: 10.1007/BF00237637. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The effects of single afferent impulses on the probability of firing of external intercostal motoneurones in the cat. J Physiol. 1982 Jan;322:315–336. doi: 10.1113/jphysiol.1982.sp014039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCE J. W., DEGAIL P. SPREAD OF PHASIC MUSCLE REFLEXES IN NORMAL AND SPASTIC SUBJECTS. J Neurol Neurosurg Psychiatry. 1965 Aug;28:328–334. doi: 10.1136/jnnp.28.4.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Cutaneous facilitation of transmission in reflex pathways from Ib afferents to motoneurones. J Physiol. 1977 Mar;265(3):763–780. doi: 10.1113/jphysiol.1977.sp011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGLADERY J. W., McDOUGAL D. B., Jr Electrophysiological studies of nerve and reflex activity in normal man. I. Identification of certain reflexes in the electromyogram and the conduction velocity of peripheral nerve fibers. Bull Johns Hopkins Hosp. 1950 May;86(5):265–290. [PubMed] [Google Scholar]
- Mark R. F., Coquery J. M., Paillard J. Autogenetic reflex effects of slow or steady stretch of the calf muscles in man. Exp Brain Res. 1968;6(2):130–145. doi: 10.1007/BF00239167. [DOI] [PubMed] [Google Scholar]
- McKeon B., Burke D. Identification of muscle spindle afferents during in vivo recordings in man. Electroencephalogr Clin Neurophysiol. 1980 May;48(5):606–608. doi: 10.1016/0013-4694(80)90297-7. [DOI] [PubMed] [Google Scholar]
- Morelli M., Nicotra L., Barnes C. D., Cangiano A., Cook W. A., Jr, Pompeiano O. An apparatus for producing small-amplitude high-frequency sinusoidal stretching of the muscle. Arch Ital Biol. 1970 Apr;108(2):222–232. [PubMed] [Google Scholar]
- Noguchi T., Homma S., Nakajima Y. Measurements of excitatory postsynaptic potentials in the stretch reflex of normal subjects and spastic patients. J Neurol Neurosurg Psychiatry. 1979 Dec;42(12):1100–1105. doi: 10.1136/jnnp.42.12.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAILLARD J. Functional organization of afferent innervation of muscle studied in man by monosynaptic testing. Am J Phys Med. 1959 Dec;38:239–247. [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Bergego C., Katz R., Morin C. Cutaneous depression of Ib reflex pathways to motoneurones in man. Exp Brain Res. 1981;42(3-4):351–361. doi: 10.1007/BF00237500. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E., Morin C., Bergego C., Tankov N. Pattern of group I fibre projections from ankle flexor and extensor muscles in man. Exp Brain Res. 1981;42(3-4):337–350. doi: 10.1007/BF00237499. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B., Hagbarth K. E., Torebjörk H. E., Wallin B. G. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979 Oct;59(4):919–957. doi: 10.1152/physrev.1979.59.4.919. [DOI] [PubMed] [Google Scholar]
- WEAVER R. A., LANDAU W. M., HIGGINS J. F. FUSIMOTOR FUNCTION. II. EVIDENCE OF FUSIMOTOR DEPRESSION IN HUMAN SPINAL SHOCK. Arch Neurol. 1963 Aug;9:127–132. doi: 10.1001/archneur.1963.00460080037004. [DOI] [PubMed] [Google Scholar]


