Abstract
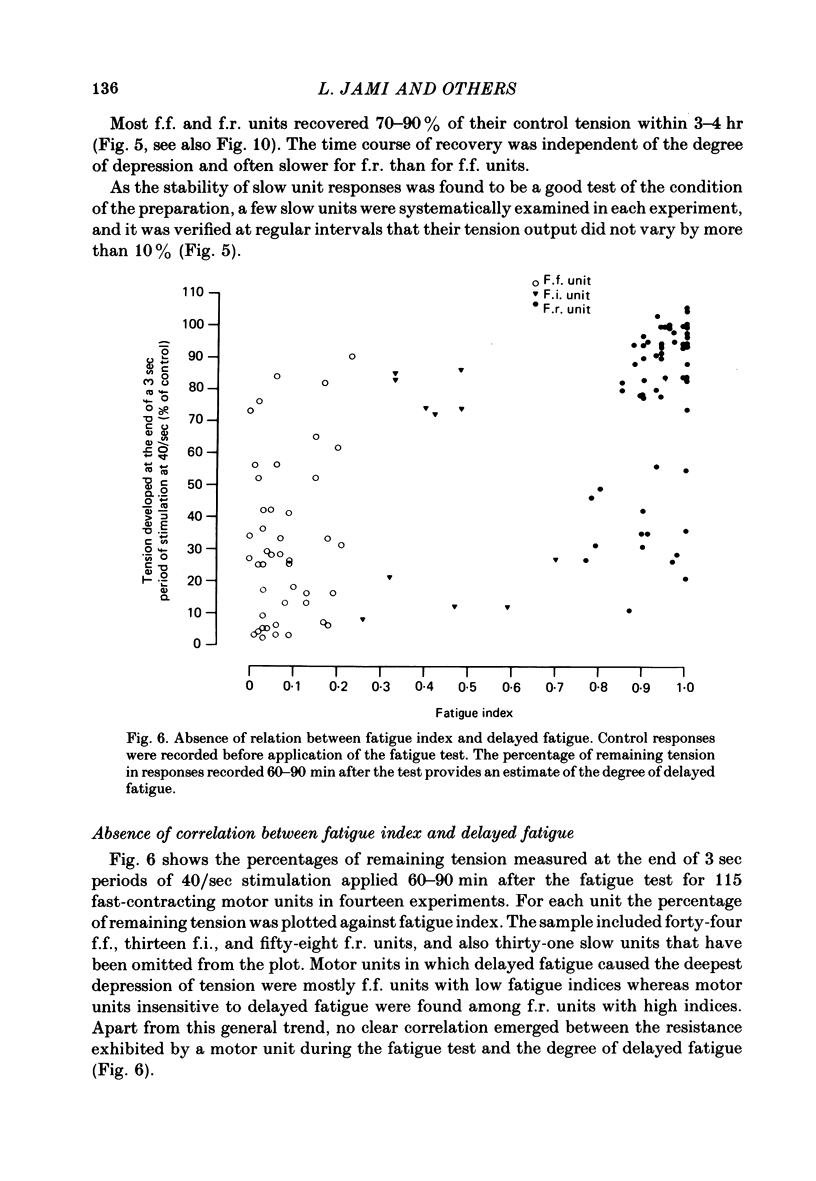
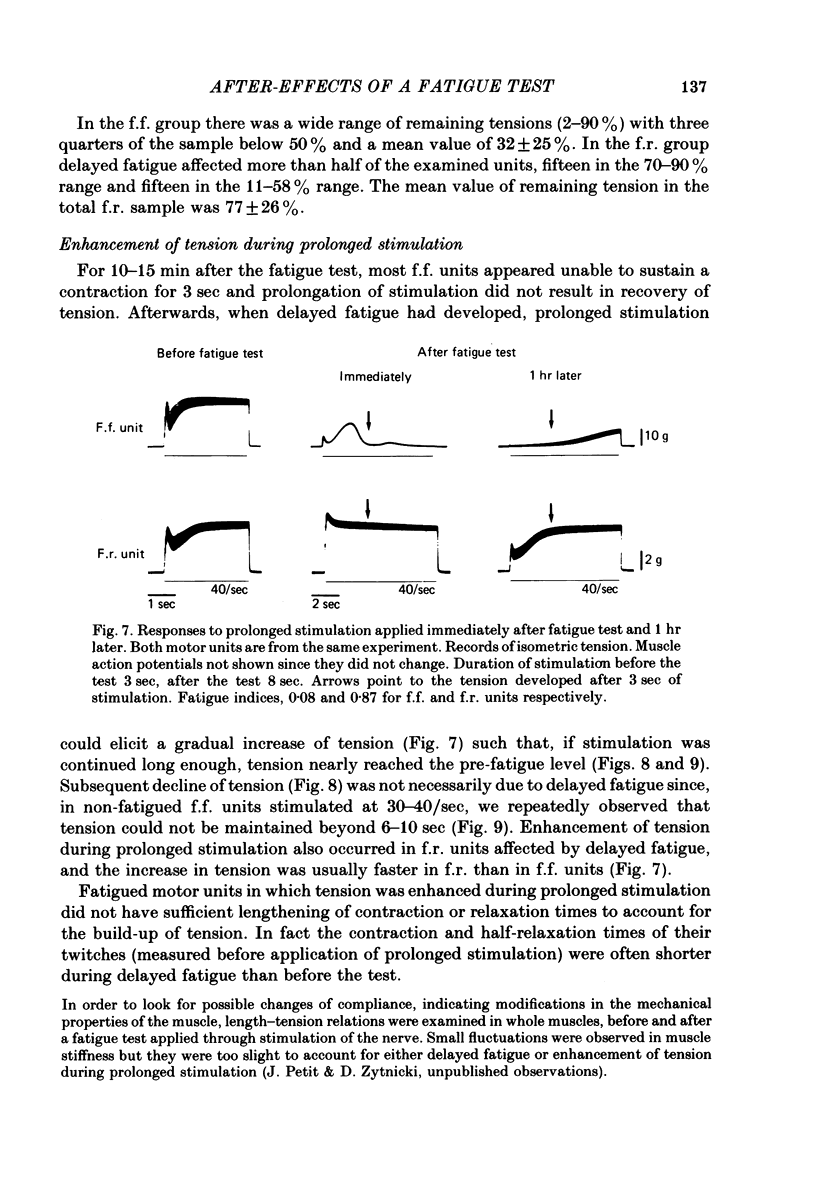
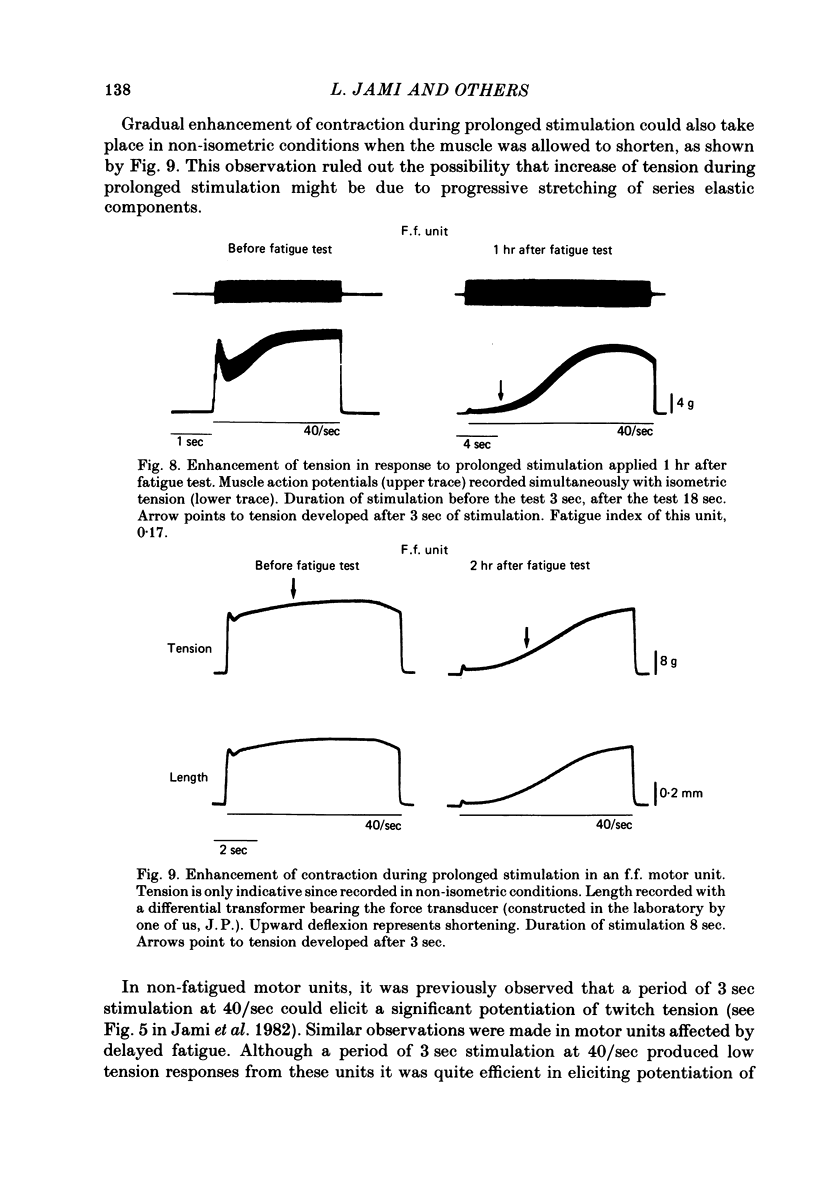
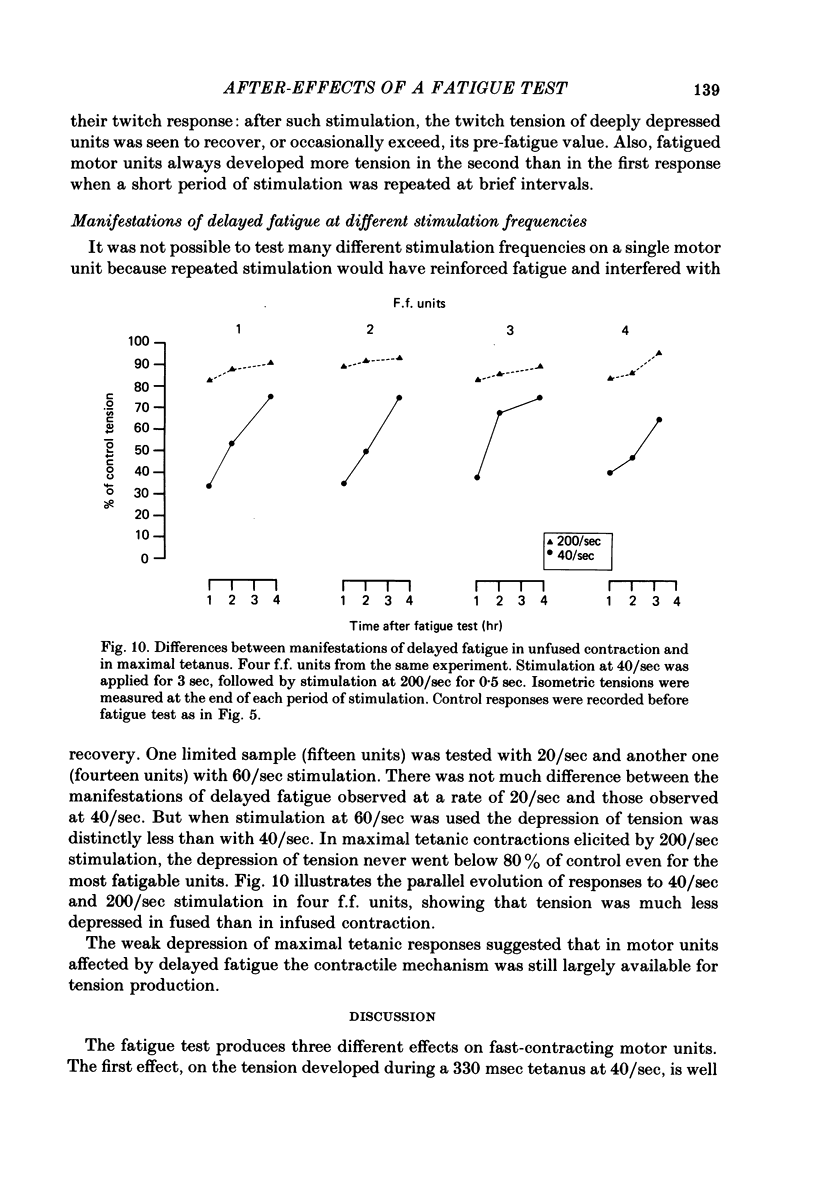
Twitch and tetanic contractions of single motor units of the cat peroneus tertius muscle were examined after application of a test allowing their identification as either fast fatigable (f.f.) or fast fatigue-resistant (f.r.) or fast intermediate (f.i.) or slow units as established by Burke, Levine, Tsairis & Zajac (1973). The test was found to leave two kinds of after-effects in f.f., f.r. and f.i. units whereas it did not affect slow units. The first after-effect was an early and brief potentiation of twitch tension occurring in all f.r. and f.i. units and in most f.f. units. The second after-effect, termed 'delayed fatigue', was a prolonged depression of tension output, that developed slowly following the early potentiation in all f.f. and f.i. units and more than half of the f.r. units. One hour after the test, unfused tetanic contractions elicited by 20-40/sec stimulation were deeply depressed in motor units that had been left without stimulation since the end of the test. Recovery took place in 3-5 hr. Motor units affected by delayed fatigue could nevertheless be made to develop nearly normal tension by gradual build-up upon prolonged stimulation at 30-40/sec. Maximal tetanic contractions elicited by 200/sec stimulation were much less depressed during delayed fatigue than unfused tetanic contractions. These observations suggest that contractile mechanism were not impaired by delayed fatigue. Since absence of change in muscle action potential indicated that excitation of muscle fibres was not affected either, delayed fatigue might be due to a temporary failure of excitation-contraction coupling.
Full text
PDF

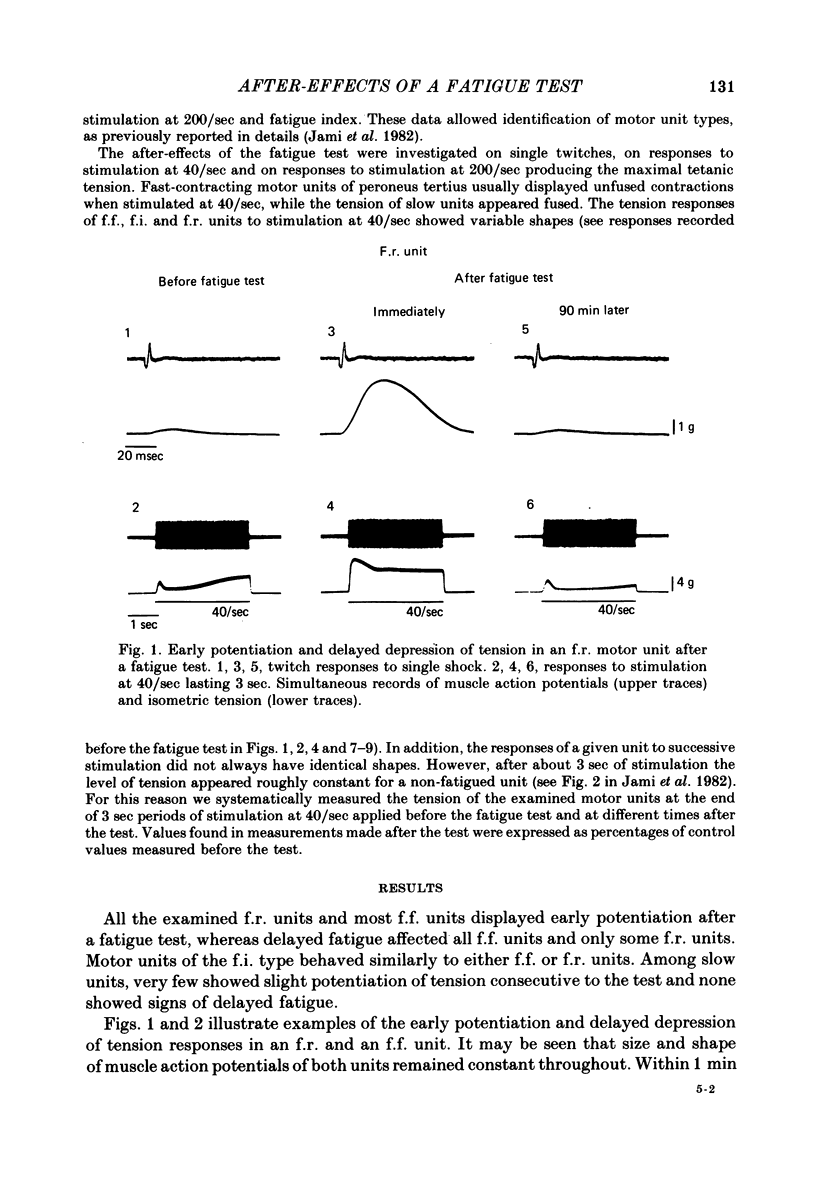
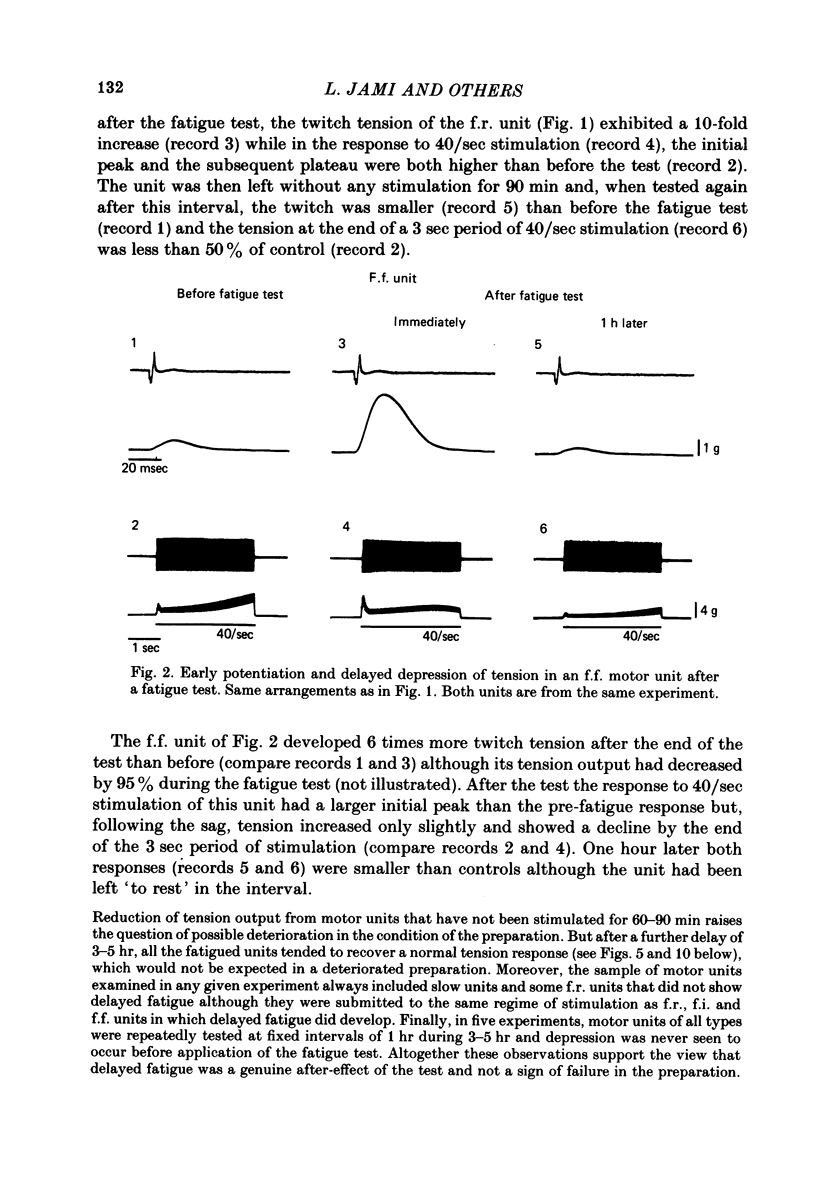
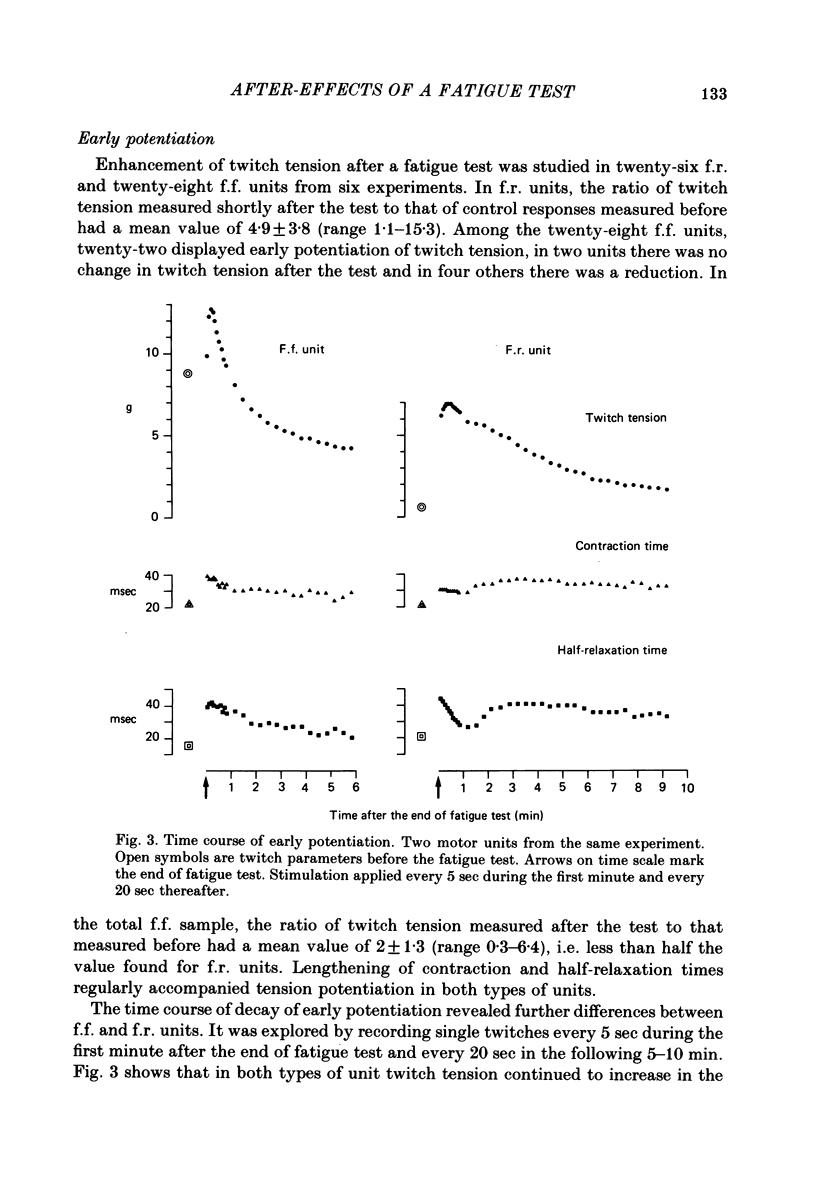

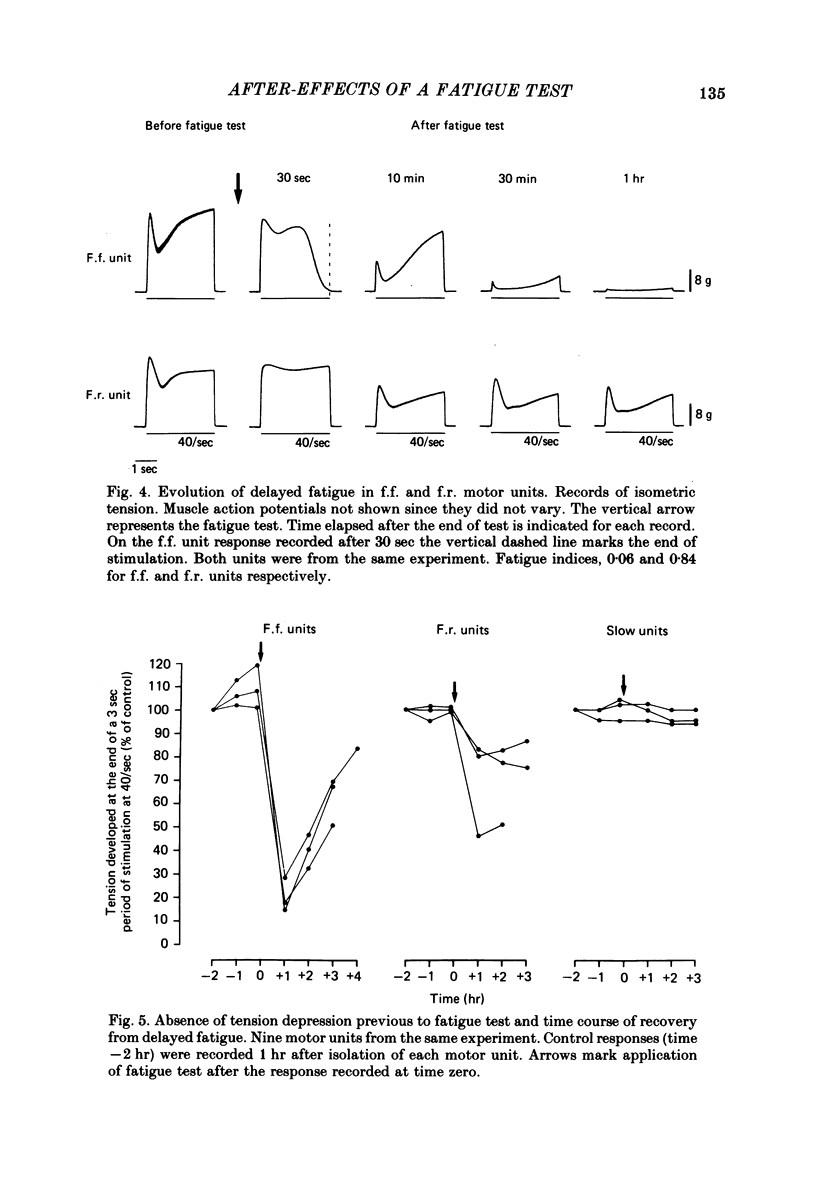




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Hill D. K., Jones D. A., Merton P. A. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977 Nov;272(3):769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards R. H., Hill D. K., Jones D. A. Metabolic changes associated with the slowing of relaxation in fatigued mouse muscle. J Physiol. 1975 Oct;251(2):287–301. doi: 10.1113/jphysiol.1975.sp011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski W., Lobsiger E. A., Lüttgau H. C. The effect of repetitive stimulation at low frequencies upon the electrical and mechanical activity of single muscle fibres. Pflugers Arch. 1972;334(3):222–239. doi: 10.1007/BF00626225. [DOI] [PubMed] [Google Scholar]
- Jami L., Murthy K. S., Petit J., Zytnicki D. Action of dantrolene sodium on single motor units of cat muscle in vivo. Brain Res. 1983 Feb 21;261(2):285–294. doi: 10.1016/0006-8993(83)90631-5. [DOI] [PubMed] [Google Scholar]
- Jami L., Murthy K. S., Petit J., Zytnicki D. Distribution of physiological types of motor units in the cat peroneus tertius muscle. Exp Brain Res. 1982;48(2):177–184. doi: 10.1007/BF00237213. [DOI] [PubMed] [Google Scholar]
- Krarup C. Enhancement and diminution of mechanical tension evoked by staircase and by tetanus in rat muscle. J Physiol. 1981 Feb;311:355–372. doi: 10.1113/jphysiol.1981.sp013589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelberg E., Lindegren B. Transmission and contraction fatigue of rat motor units in relation to succinate dehydrogenase activity of motor unit fibres. J Physiol. 1979 Mar;288:285–300. [PMC free article] [PubMed] [Google Scholar]
- MERTON P. A. Voluntary strength and fatigue. J Physiol. 1954 Mar 29;123(3):553–564. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassar-Gentina V., Passonneau J. V., Vergara J. L., Rapoport S. I. Metabolic correlates of fatigue and of recovery from fatigue in single frog muscle fibers. J Gen Physiol. 1978 Nov;72(5):593–606. doi: 10.1085/jgp.72.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U., Waite P. M. Properties of types of motor units in the medial gastrochemius muscle of the cat. Brain Res. 1974 Feb 15;67(1):89–101. doi: 10.1016/0006-8993(74)90300-x. [DOI] [PubMed] [Google Scholar]
- Proske U., Waite P. M. The relation between tension and axonal conduction velocity for motor units in the medial gastrocnemius muscle of the cat. Exp Brain Res. 1976 Oct 28;26(3):325–328. doi: 10.1007/BF00234936. [DOI] [PubMed] [Google Scholar]
- Stephens J. A., Taylor A. Fatigue of maintained voluntary muscle contraction in man. J Physiol. 1972 Jan;220(1):1–18. doi: 10.1113/jphysiol.1972.sp009691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara J. L., Rapoprot S. I., Nassar-Gentina V. Fatigue and posttetanic potentiation in single muscle fibers of the frog. Am J Physiol. 1977 May;232(5):C185–C190. doi: 10.1152/ajpcell.1977.232.5.C185. [DOI] [PubMed] [Google Scholar]


