Abstract
1. Polyneuronal innervation of normal and reinnervated fourth deep lumbrical muscle fibres was studied with tension measurements and intracellular recordings. From the tenth day after a complete crush of the muscle nerve, some of the reinnervated muscles were completely paralysed for up to 15 days by local application of tetrodotoxin (TTX) to the sciatic nerve. Other animals received only systemic infusion of TTX during the muscle reinnervation.
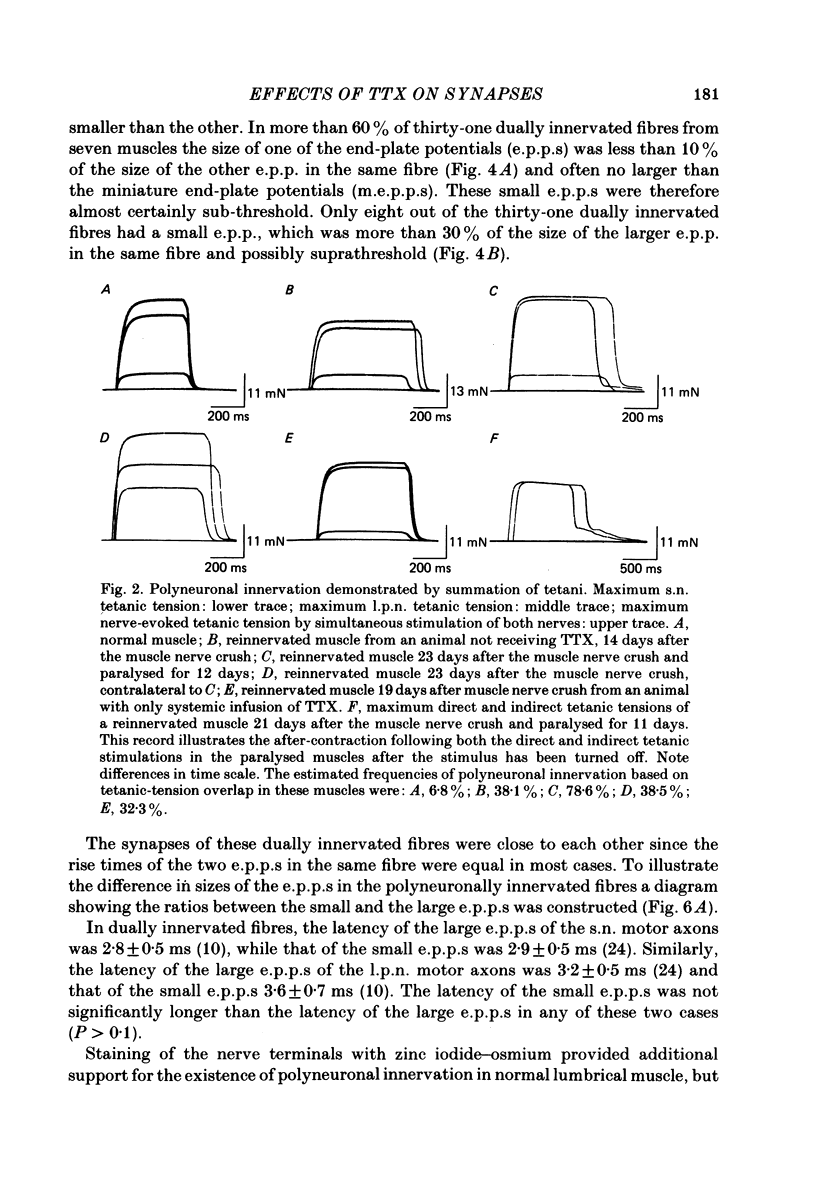
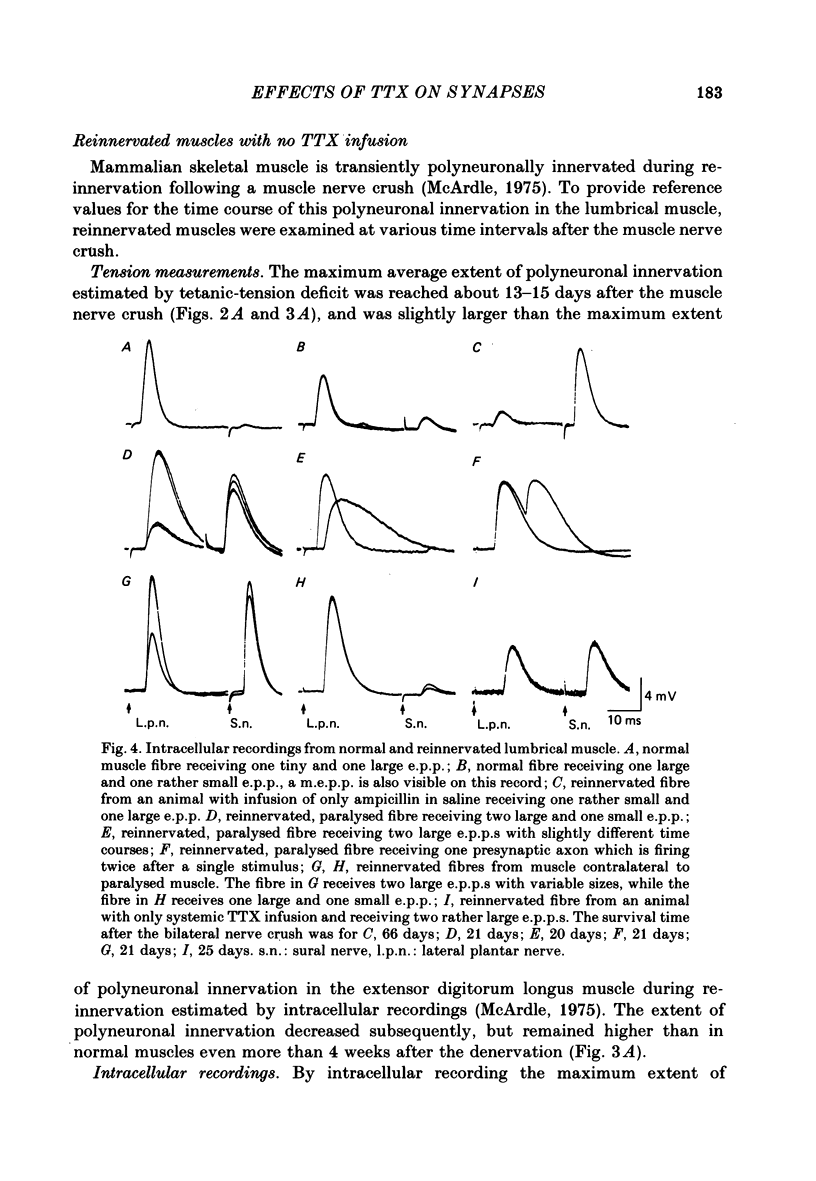
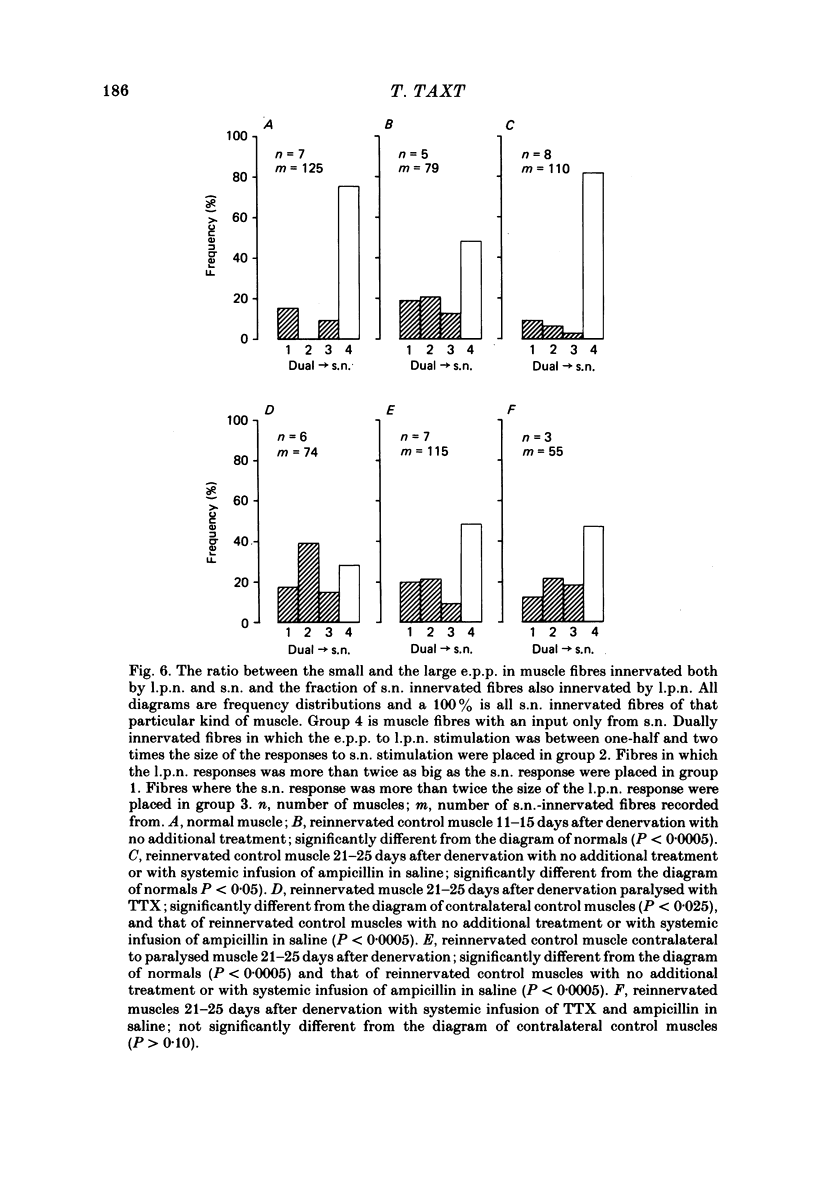
2. Measurements of tetanic-tension overlap suggested that about 6% of the muscle fibres in the normal lumbrical muscle were polyneuronally innervated, while intracellular recordings suggested that the percentage was as high as 25%. This discrepancy was mainly due to the presence of one small, sub-threshold end-plate potential (e.p.p.) and one large, suprathreshold e.p.p. in almost all polyneuronally innervated muscle fibres.
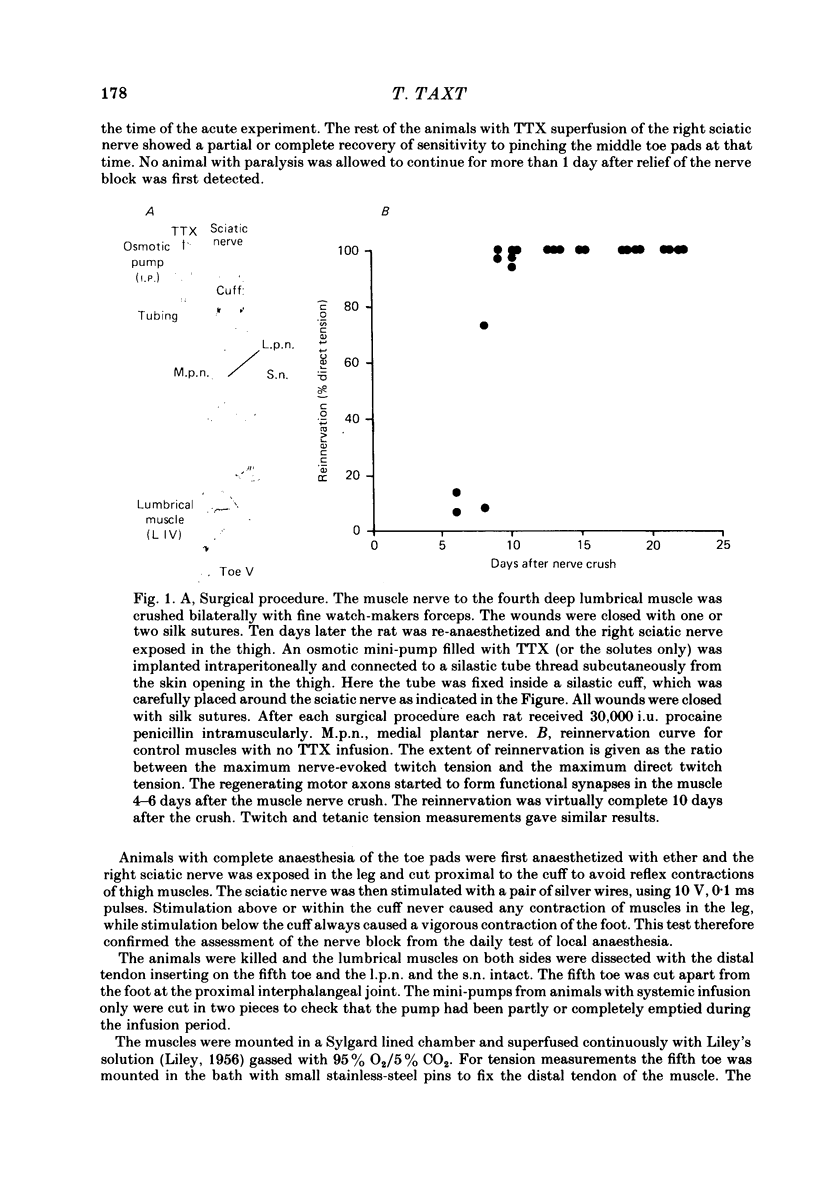
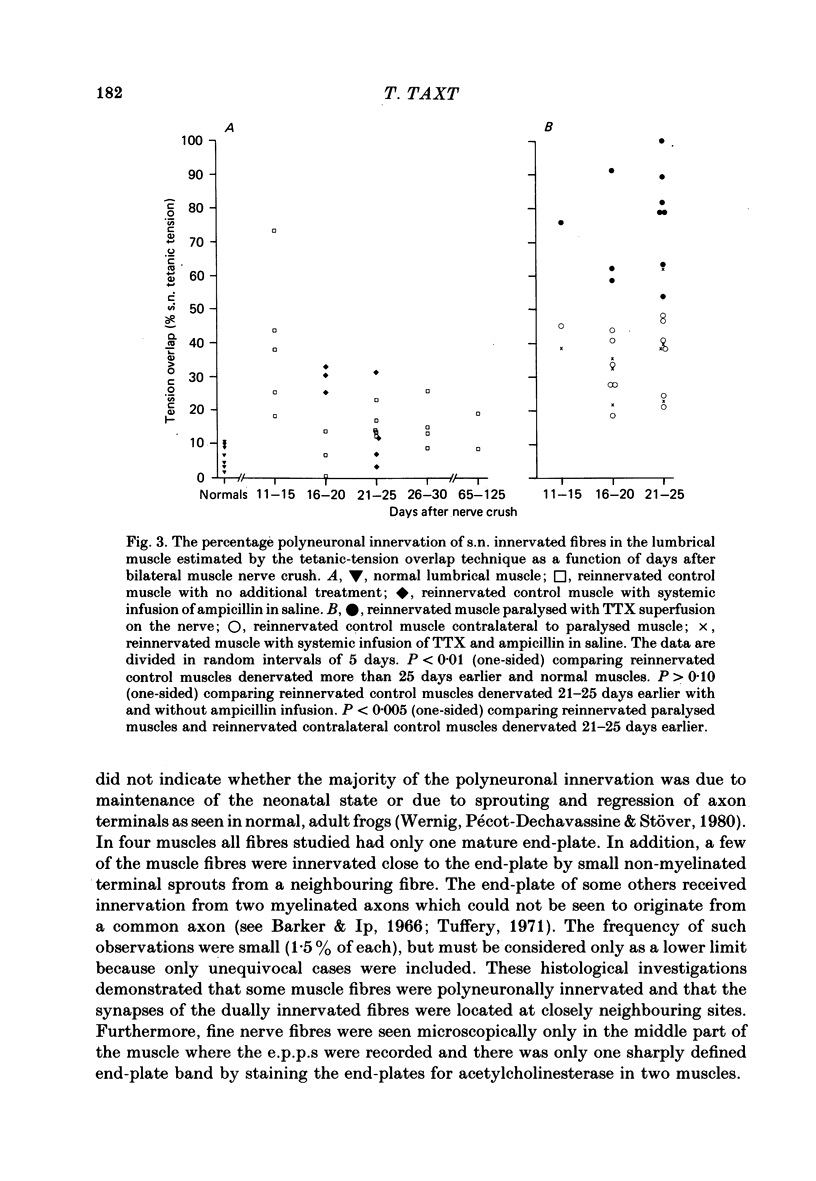
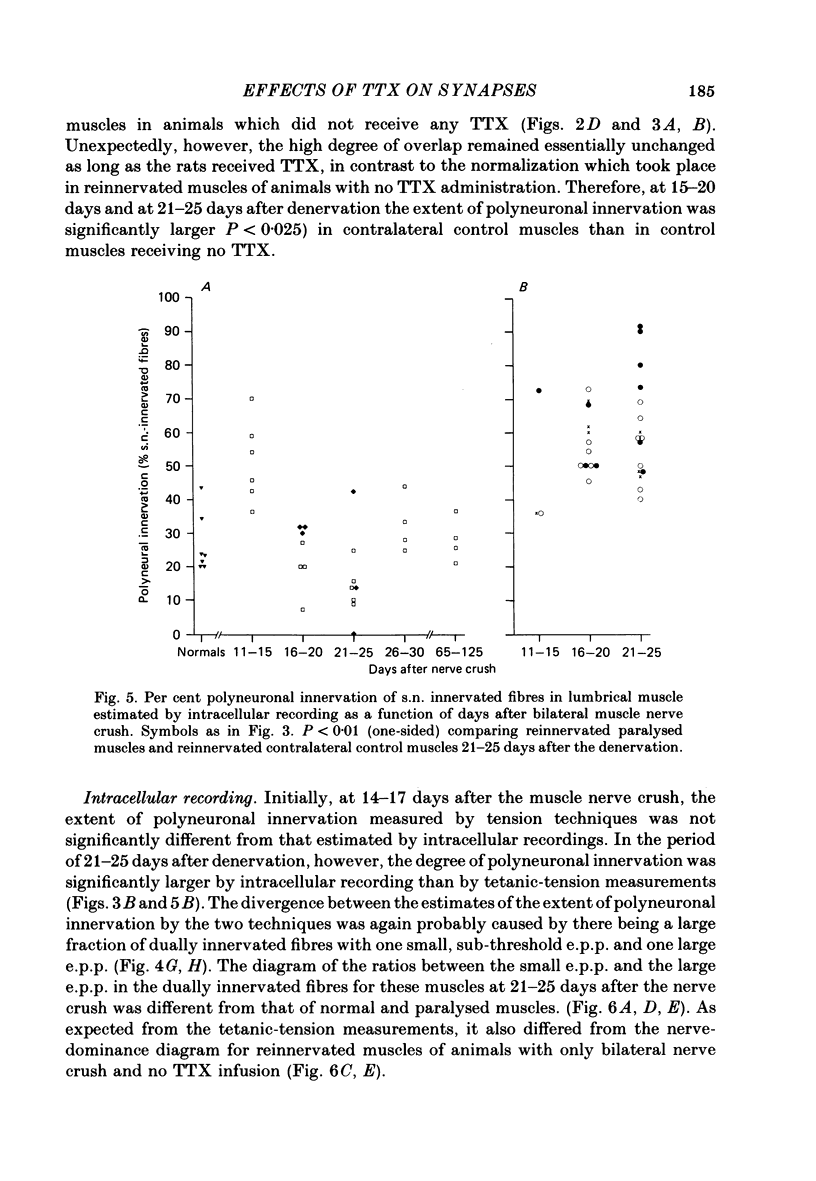
3. Intracellular recordings during muscle reinnervation showed that the extent of polyneuronal innervation reached a maximum of 50% 10-15 days after denervation and that by 16-20 days this had decreased to a level similar to that found in normal muscle.
4. After a week of total muscle paralysis the extent of polyneuronal innervation had increased to about 80%, estimated by both tension measurements and intracellular recordings. Subsequently, there was no sign of any net elimination of the polyneuronal innervation, even in muscles paralysed for up to two weeks. Many of the polyneuronally innervated fibres were innervated by at least two motor axons. each producing suprathreshold e.p.p.s.
5. In muscles contralateral to the paralysed muscles, the extent of polyneuronal innervation reached a maximum of 50% 10-15 days after denervation as in reinnervated muscles not exposed to TTX. But in contrast to the subsequent decrease in the extent of polyneuronal innervation in animals which received no TTX, this level of polyneuronal innervation persisted in muscles contralateral to the paralysed muscles. The same was true for reinnervated muscles in animals which only received TTX systemically.
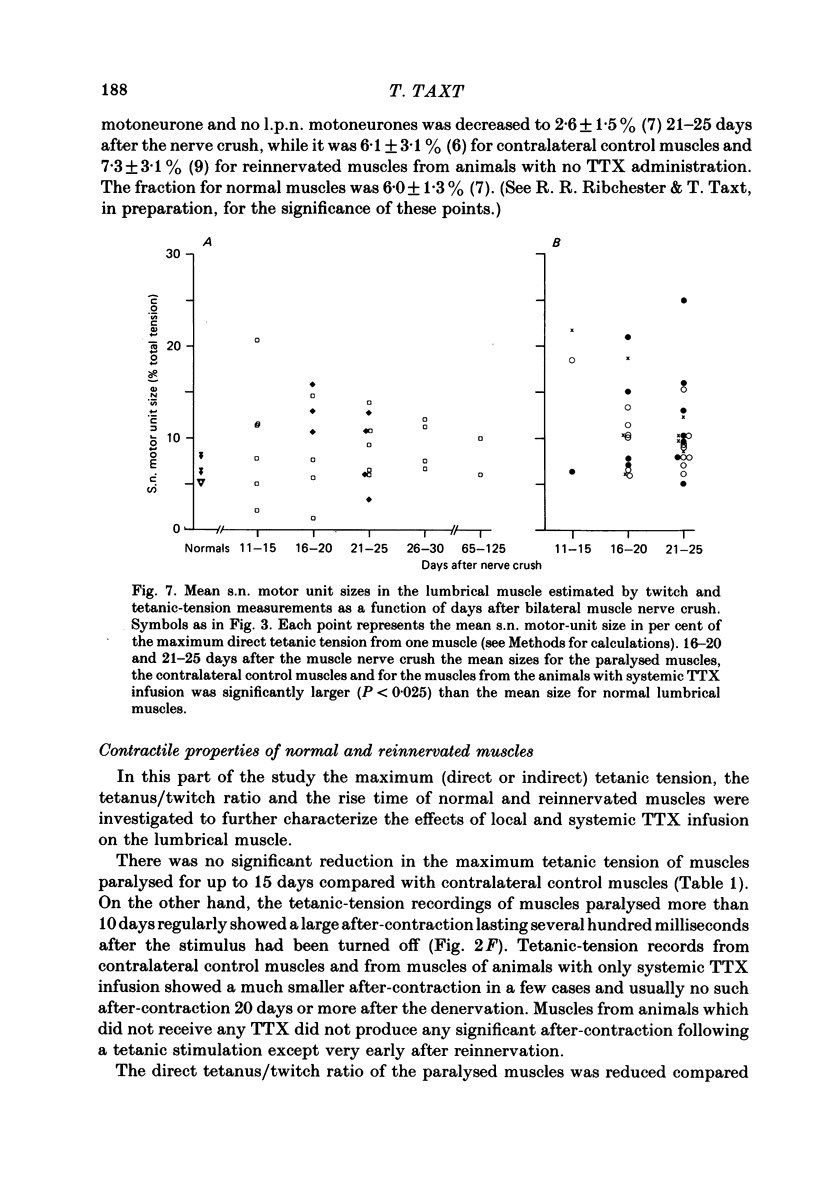
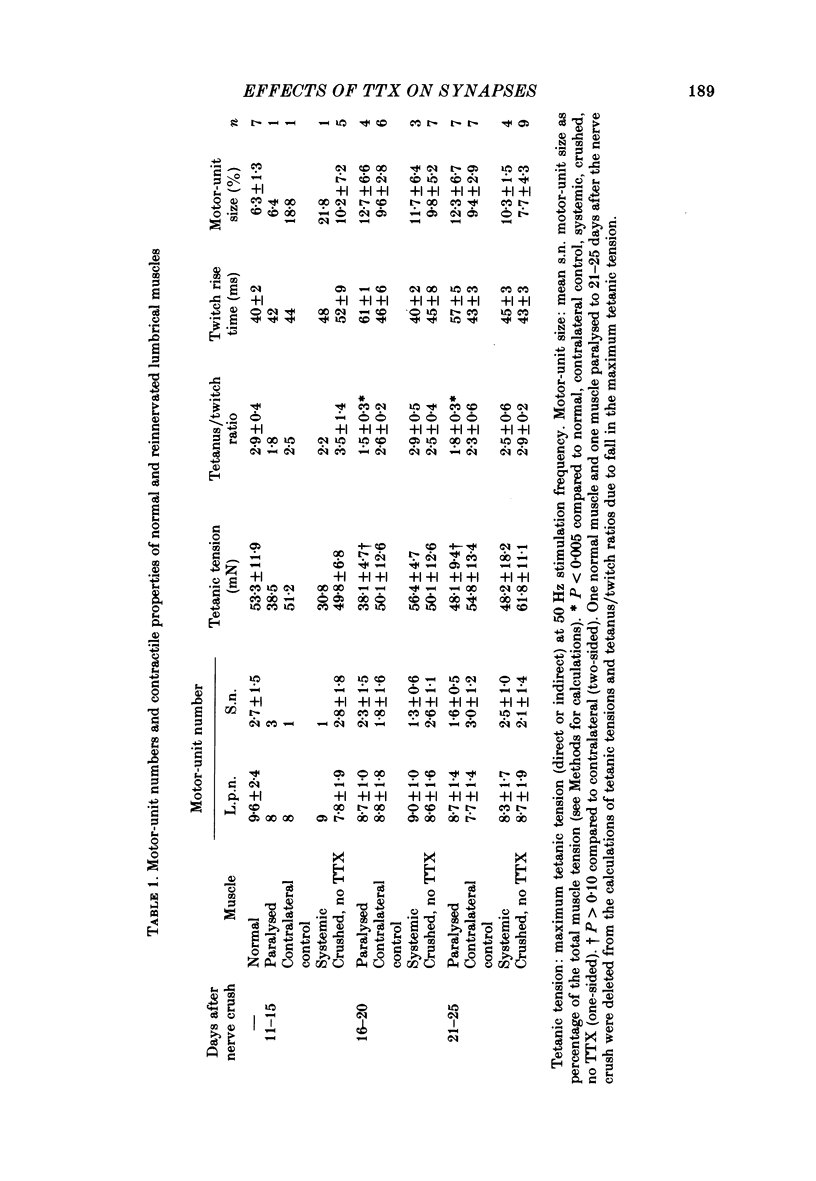
6. The increased level of polyneuronal innervation after TTX application was not caused by differences in the number of motor units or in number of muscle fibres.
7. Paralysed muscles relaxed much more slowly than non-paralysed muscles at the end of a fused tetanic contraction. The tetanus/twitch ratio of these muscles was also smaller than in contralateral control muscles and the rise time of the twitch was greater.
8. It is concluded that a substantial fraction of the fibres in the normal lumbrical muscle of young rats is polyneuronally innervated. After reinnervation, the normal innervation pattern is re-established, but no net elimination of the polyneuronal innervation occurs unless either nerve or muscle or both are active. A net elimination of synapses is also prevented when TTX is present systemically in low concentrations.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akert K., Sandri C. An electron-microscopic study of zinc iodide-osmium impregnation of neurons. I. Staining of synaptic vesicles at cholinergic junctions. Brain Res. 1968 Feb;7(2):286–295. doi: 10.1016/0006-8993(68)90104-2. [DOI] [PubMed] [Google Scholar]
- BARSTAD J. A. Presynaptic effect of the neuro-muscular transmitter. Experientia. 1962 Dec 15;18:579–580. doi: 10.1007/BF02172193. [DOI] [PubMed] [Google Scholar]
- BROWN M. C., MATTHEWS P. B. An investigation into the possible existence of polyneuronal innervation of individual skeletal muscle fibres in certain hind-limb muscles of the cat. J Physiol. 1960 Jun;151:436–457. doi: 10.1113/jphysiol.1960.sp006450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN M. C., MATTHEWS P. B. The effect on a muscle twitch of the back-response of its motor nerve fibres. J Physiol. 1960 Feb;150:332–346. doi: 10.1113/jphysiol.1960.sp006391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D., Ip M. C. Sprouting and degeneration of mammalian motor axons in normal and de-afferentated skeletal muscle. Proc R Soc Lond B Biol Sci. 1966 Jan 18;163(993):538–554. doi: 10.1098/rspb.1966.0008. [DOI] [PubMed] [Google Scholar]
- Benoit P., Changeux J. P. Consequences of blocking the nerve with a local anaesthetic on the evolution of multiinnervation at the regenerating neuromuscular junction of the rat. Brain Res. 1978 Jun 23;149(1):89–96. doi: 10.1016/0006-8993(78)90589-9. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. Sprouting of active nerve terminals in partially inactive muscles of the rat. J Physiol. 1980 Jun;303:281–297. doi: 10.1113/jphysiol.1980.sp013285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The size of motor units during post-natal development of rat lumbrical muscle. J Physiol. 1979 Dec;297(0):463–478. doi: 10.1113/jphysiol.1979.sp013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby J. L., van Essen D. C. Regional differences in the timing of synapse elimination in skeletal muscles of the neonatal rabbit. Brain Res. 1979 Jun 22;169(2):275–286. doi: 10.1016/0006-8993(79)91030-8. [DOI] [PubMed] [Google Scholar]
- Braithwaite A. W., Harris A. J. Neural influence on acetylcholine receptor clusters in embryonic development of skeletal muscles. Nature. 1979 Jun 7;279(5713):549–551. doi: 10.1038/279549a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Hopkins W. G. Restoration of focal multiple innervation in rat muscles by transmission block during a critical stage of development. J Physiol. 1981 Sep;318:355–364. doi: 10.1113/jphysiol.1981.sp013869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Ironton R. Nodal and terminal sprouting from motor nerves in fast and slow muscles of the mouse. J Physiol. 1980 Sep;306:493–510. doi: 10.1113/jphysiol.1980.sp013410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Motor neurone sprouting induced by prolonged tetrodotoxin block of nerve action potentials. Nature. 1977 Feb 3;265(5593):459–461. doi: 10.1038/265459a0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Ironton R. Sprouting and regression of neuromuscular synapses in partially denervated mammalian muscles. J Physiol. 1978 May;278:325–348. doi: 10.1113/jphysiol.1978.sp012307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley G. A., Heaton J. A quantitative study of cholinesterase in myoneural junctions from rat and guinea-pig extraocular muscles. J Physiol. 1968 Dec;199(3):743–749. doi: 10.1113/jphysiol.1968.sp008676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangiano A., Lømo T., Lutzemberger L., Sveen O. Effects of chronic nerve conduction block on formation of neuromuscular junctions and junctional AChE in the rat. Acta Physiol Scand. 1980 Jul;109(3):283–296. doi: 10.1111/j.1748-1716.1980.tb06599.x. [DOI] [PubMed] [Google Scholar]
- Dennis M. J., Yip J. W. Formation and elimination of foreign synapses on adult salamander muscle. J Physiol. 1978 Jan;274:299–310. doi: 10.1113/jphysiol.1978.sp012148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Ziskind-Conhaim L., Harris A. J. Development of neuromuscular junctions in rat embryos. Dev Biol. 1981 Jan 30;81(2):266–279. doi: 10.1016/0012-1606(81)90290-6. [DOI] [PubMed] [Google Scholar]
- Hall Z. W. Multiple forms of acetylcholinesterase and their distribution in endplate and non-endplate regions of rat diaphragm muscle. J Neurobiol. 1973;4(4):343–361. doi: 10.1002/neu.480040404. [DOI] [PubMed] [Google Scholar]
- Harris W. A. Neural activity and development. Annu Rev Physiol. 1981;43:689–710. doi: 10.1146/annurev.ph.43.030181.003353. [DOI] [PubMed] [Google Scholar]
- Harris W. A. The effects of eliminating impulse activity on the development of the retinotectal projection in salamanders. J Comp Neurol. 1980 Nov 15;194(2):303–317. doi: 10.1002/cne.901940203. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Histochemical composition, contraction speed and fatiguability of rat soleus motor units. J Neurol Sci. 1973 Oct;20(2):177–198. doi: 10.1016/0022-510x(73)90029-4. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. Spontaneous release of transmitter substance in multiquantal units. J Physiol. 1957 May 23;136(3):595–605. doi: 10.1113/jphysiol.1957.sp005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J. J. Complex end-plate potentials at the regenerating neuromuscular junction of the rat. Exp Neurol. 1975 Dec;49(3):629–638. doi: 10.1016/0014-4886(75)90048-5. [DOI] [PubMed] [Google Scholar]
- Moore J. W., Blaustein M. P., Anderson N. C., Narahashi T. Basis of tetrodotoxin's selectivity in blockage of squid axons. J Gen Physiol. 1967 May;50(5):1401–1411. doi: 10.1085/jgp.50.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDIC M., STRAUGHAN D. W. ANTIDROMIC ACTIVITY IN THE RAT PHRENIC NERVE-DIAPHRAGM PREPARATION. J Physiol. 1964 Sep;173:130–148. doi: 10.1113/jphysiol.1964.sp007447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogart R. Sodium channels in nerve and muscle membrane. Annu Rev Physiol. 1981;43:711–725. doi: 10.1146/annurev.ph.43.030181.003431. [DOI] [PubMed] [Google Scholar]
- Roper S., Ko C. P. Impulse blockade in frog cardiac ganglion does not resemble partial denervation in changing synaptic organization. Science. 1978 Oct 6;202(4363):66–68. doi: 10.1126/science.308697. [DOI] [PubMed] [Google Scholar]
- Rotshenker S. Synapse formation in intact innervated cutaneous-pectoris muscles of the frog following denervation of the opposite muscle. J Physiol. 1979 Jul;292:535–547. doi: 10.1113/jphysiol.1979.sp012870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalbruch H. Skeletal muscle fibers of newborn rats are coupled by gap junctions. Dev Biol. 1982 Jun;91(2):485–490. doi: 10.1016/0012-1606(82)90056-2. [DOI] [PubMed] [Google Scholar]
- Thompson W., Kuffler D. P., Jansen J. K. The effect of prolonged, reversible block of nerve impulses on the elimination of polyneuronal innervation of new-born rat skeletal muscle fibers. Neuroscience. 1979;4(2):271–281. doi: 10.1016/0306-4522(79)90088-5. [DOI] [PubMed] [Google Scholar]
- Thompson W. Reinnervation of partially denervated rat soleus muscle. Acta Physiol Scand. 1978 May;103(1):81–91. doi: 10.1111/j.1748-1716.1978.tb06193.x. [DOI] [PubMed] [Google Scholar]
- Tuffery A. R. Growth and degeneration of motor end-plates in normal cat hind limb muscles. J Anat. 1971 Nov;110(Pt 2):221–247. [PMC free article] [PubMed] [Google Scholar]
- Weinberg C. B., Hall Z. W. Junctional form of acetylcholinesterase restored at nerve-free endplates. Dev Biol. 1979 Feb;68(2):631–635. doi: 10.1016/0012-1606(79)90233-1. [DOI] [PubMed] [Google Scholar]
- Wernig A., Pécot-Dechavassine M., Stover H. Sprouting and regression of the nerve at the frog neuromuscular junction in normal conditions and after prolonged paralysis with curare. J Neurocytol. 1980 Jun;9(3):278–303. doi: 10.1007/BF01181538. [DOI] [PubMed] [Google Scholar]


