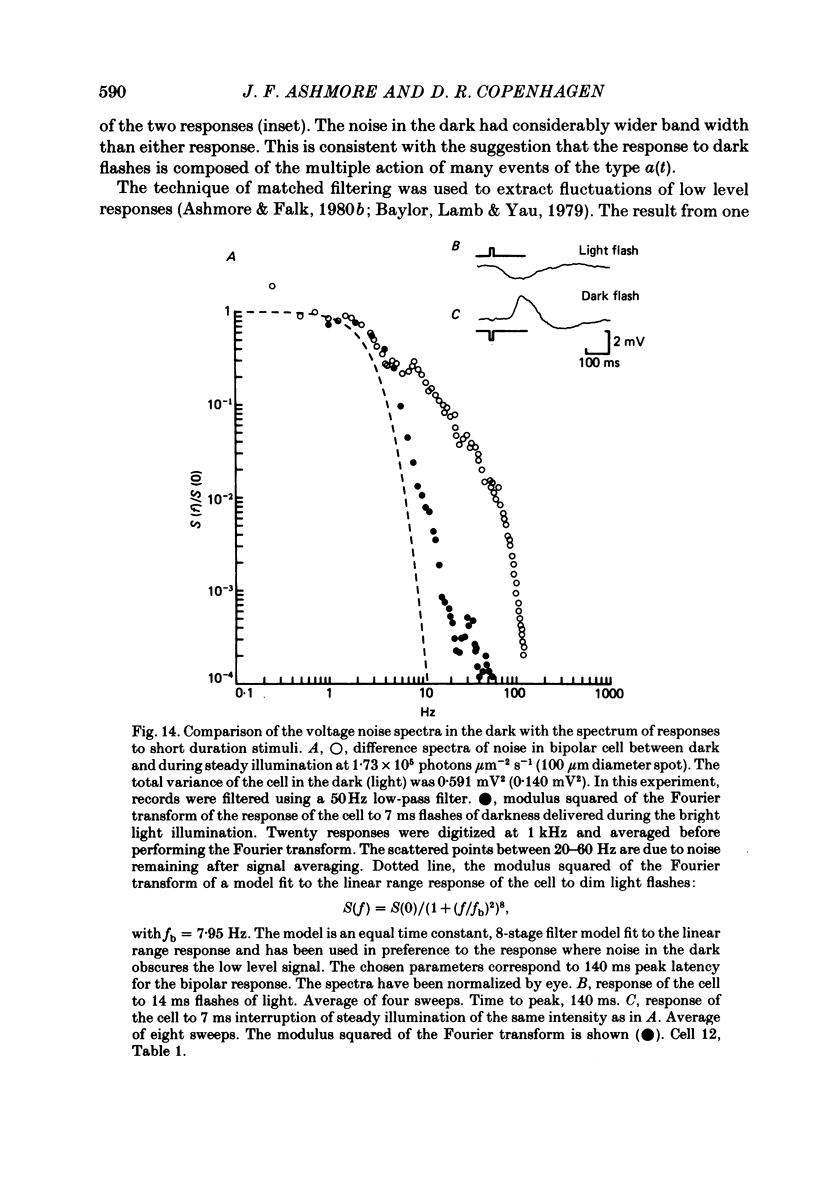
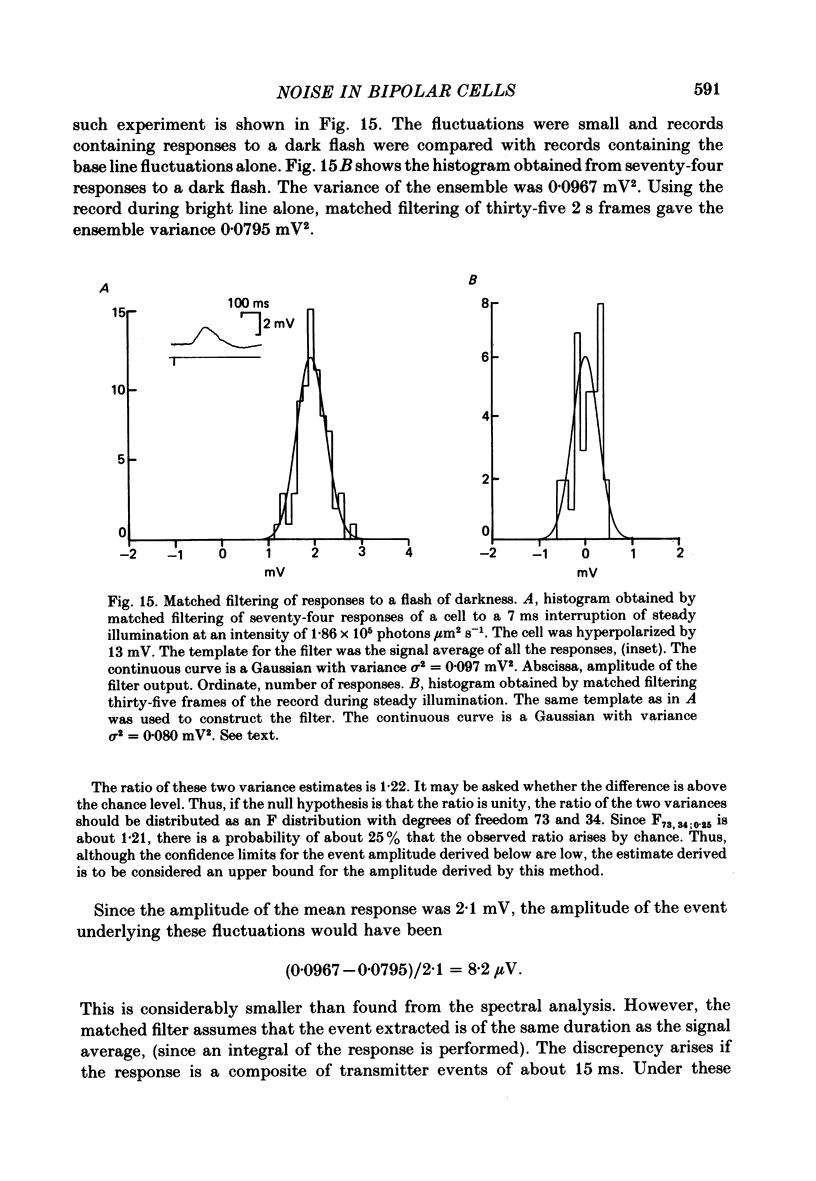
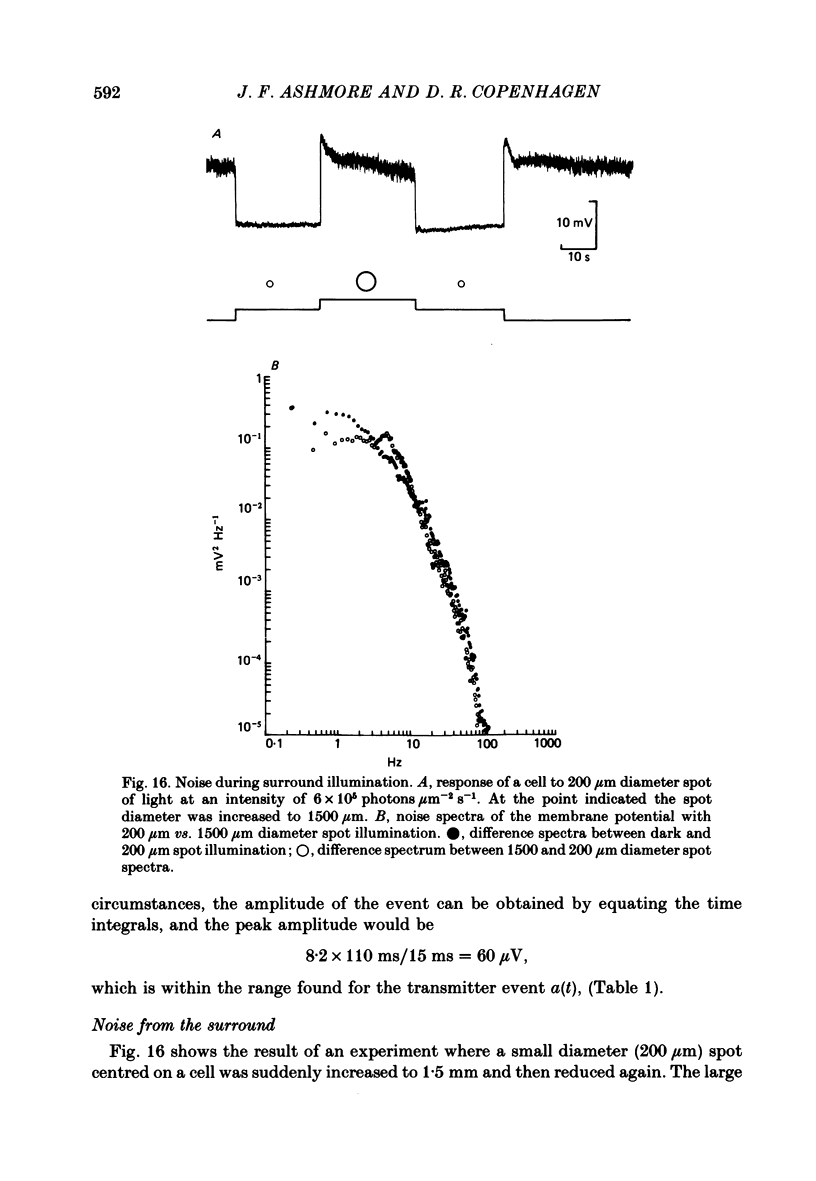
Abstract
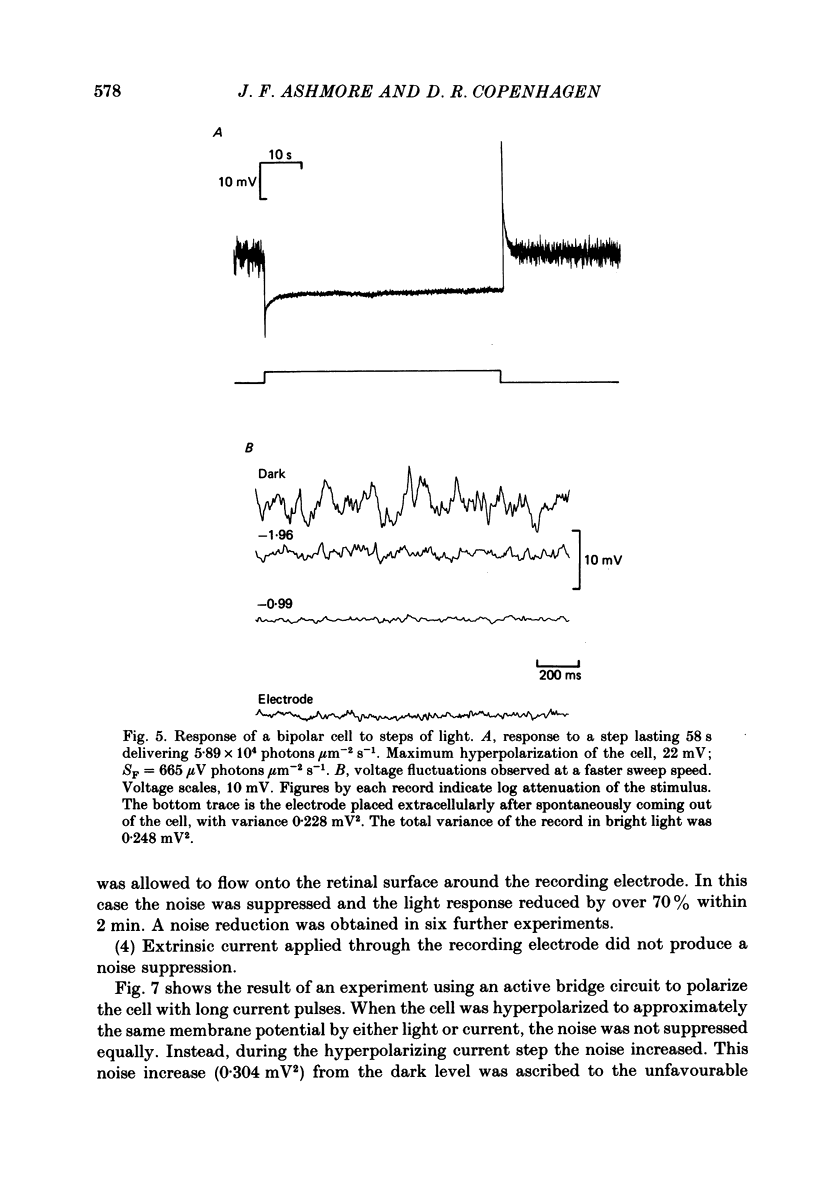
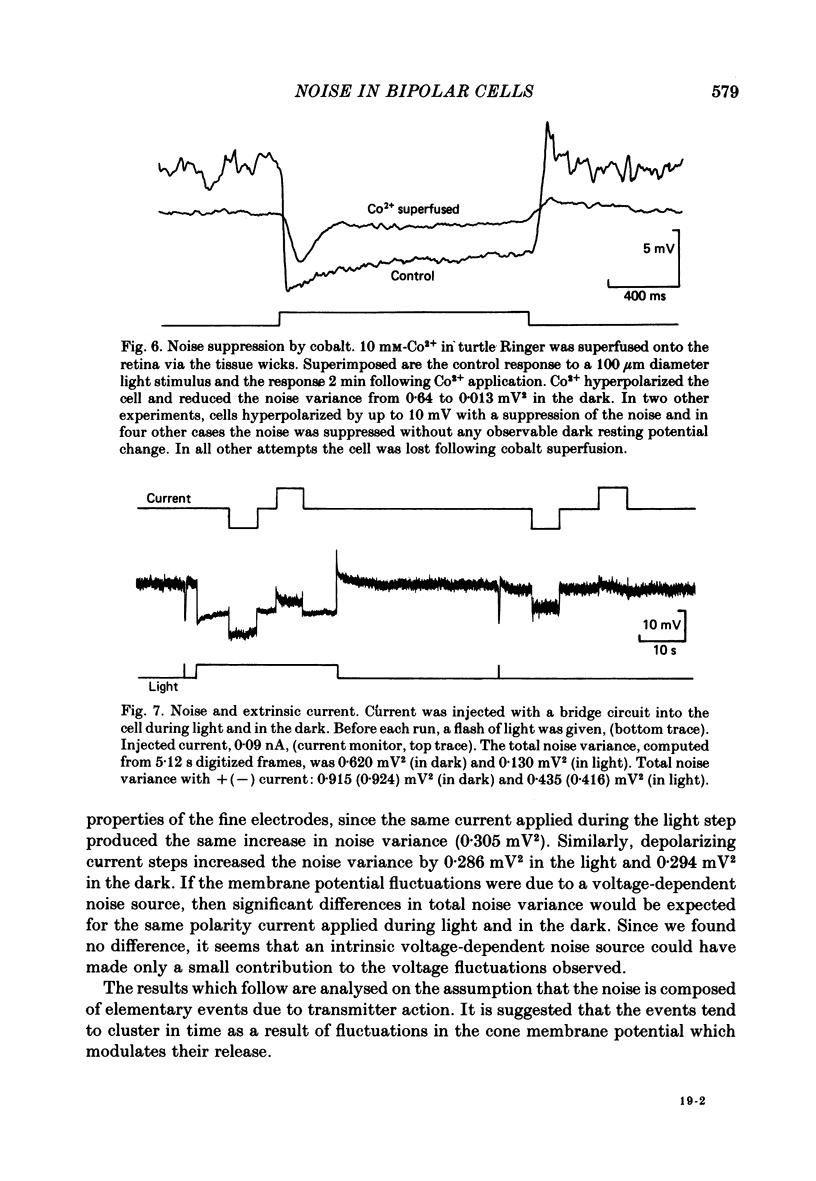
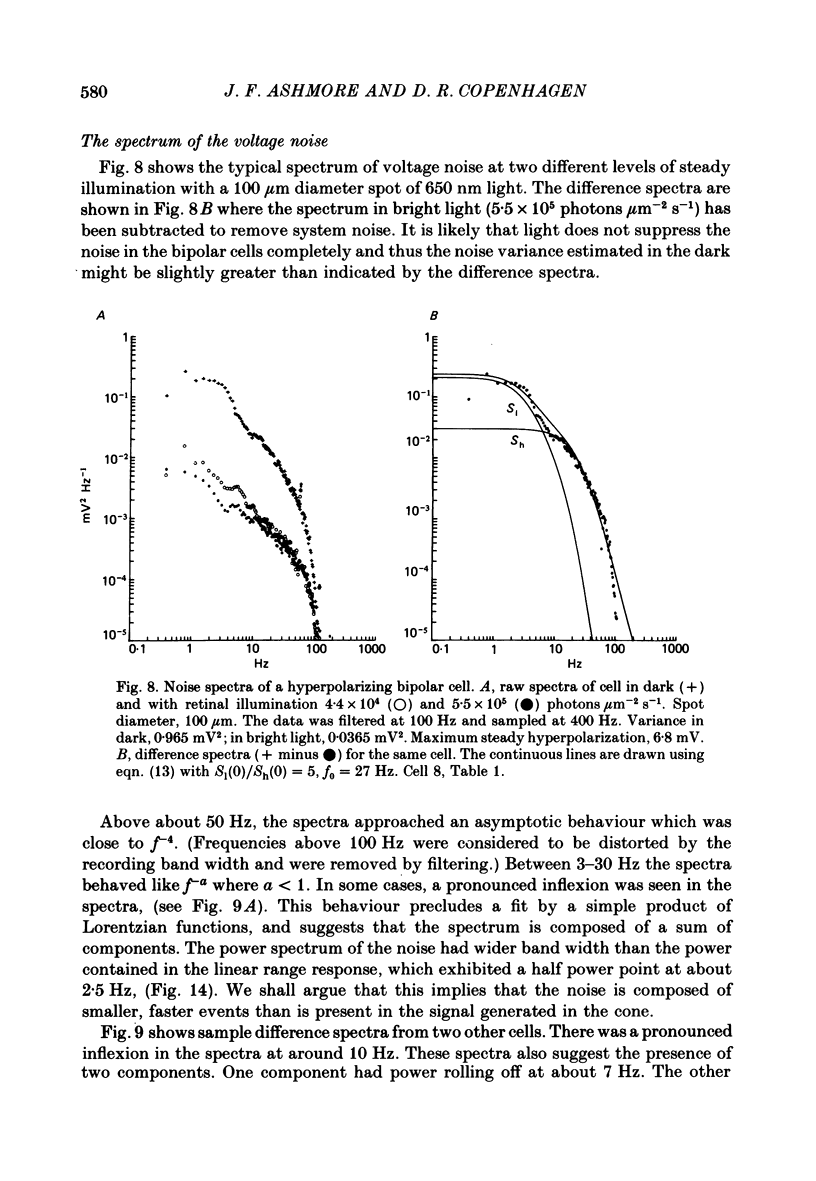
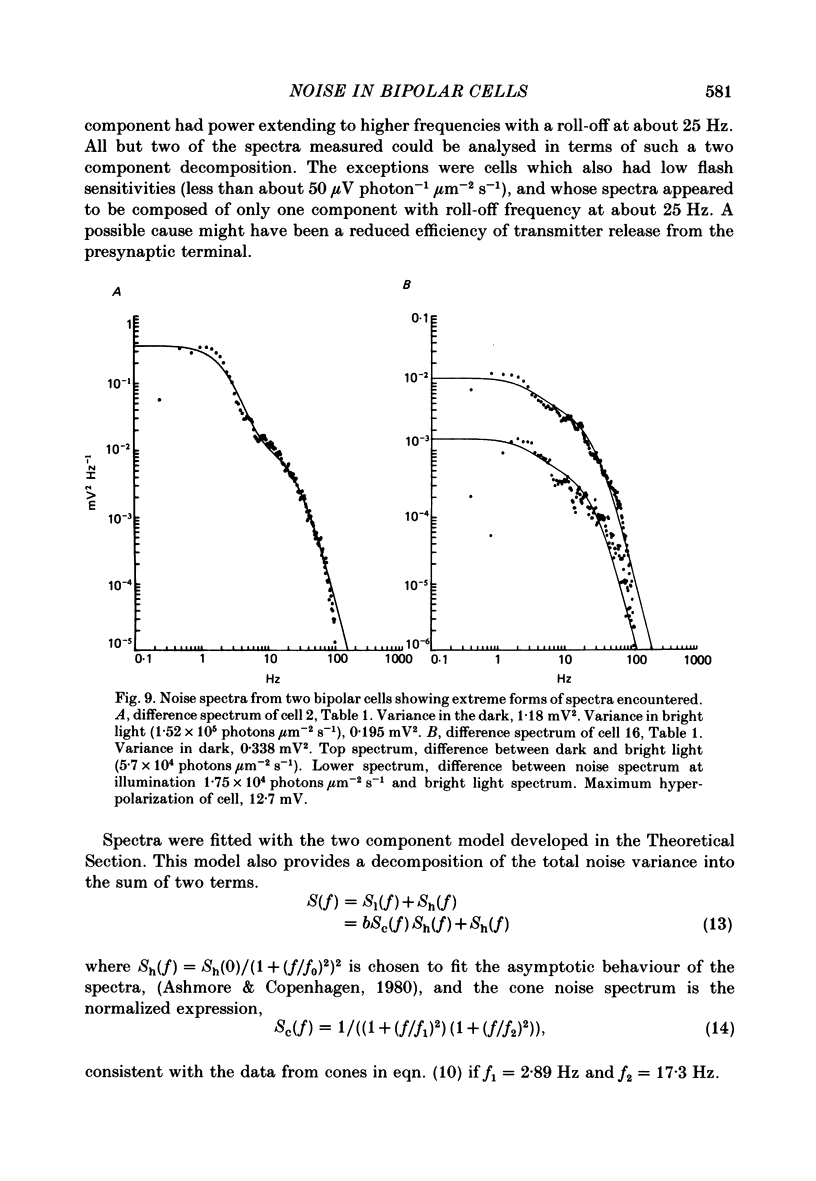
Voltage noise was recorded from centre-hyperpolarizing bipolar cells in the retina of the snapping turtle. The identity of the cells was confirmed by intracellular staining. The variance of the voltage fluctuations of the membrane potential present in the dark was suppressed by up to 30-fold by 100 microns diameter light spot stimuli centred on the cell's receptive field. Such noise reduction is expected when light hyperpolarizes the photoreceptors and reduces the rate of release of transmitter from the terminals. The spectra of the fluctuations were analysed as the sum of two components: (a), a component with power band width limited to below approximately 10 Hz, and (b), a component Sh(f) of the form Sh(f) = Sh(0)/(1 + (f/f0)2)2, with f0 = 27 Hz. The two components were attributed (a) to the noise generated in the cones and transmitted through the synapse to the bipolar cells and (b) to the action of transmitter on the bipolar cell membrane. The component Sh(f) attributed to the action of transmitter on the bipolar cells corresponded to an event approximately 14 ms in duration. The event had a peak amplitude in the range 17.6-223 microV with a mean of 69.5 microV. It is estimated that, in the dark, the number of such events contributing to the noise is about 9200 s-1. It is estimated that each elementary noise event in the cones controls approximately thirty of the transmitter-related events at the synapse. Responses to flashes of darkness applied on steady illumination were analysed by a method of matched filtering. The responses fluctuated in amplitude, and the analysis of this fluctuation suggested an elementary event of approximately the same amplitude as found from the noise analysis. Enlarging the diameter of the stimulus spot to 1500 microns repolarized the bipolar cells with an associated increase in voltage noise. Implications for the synaptic mechanisms of the centre-surround organization are discussed.
Full text
PDF

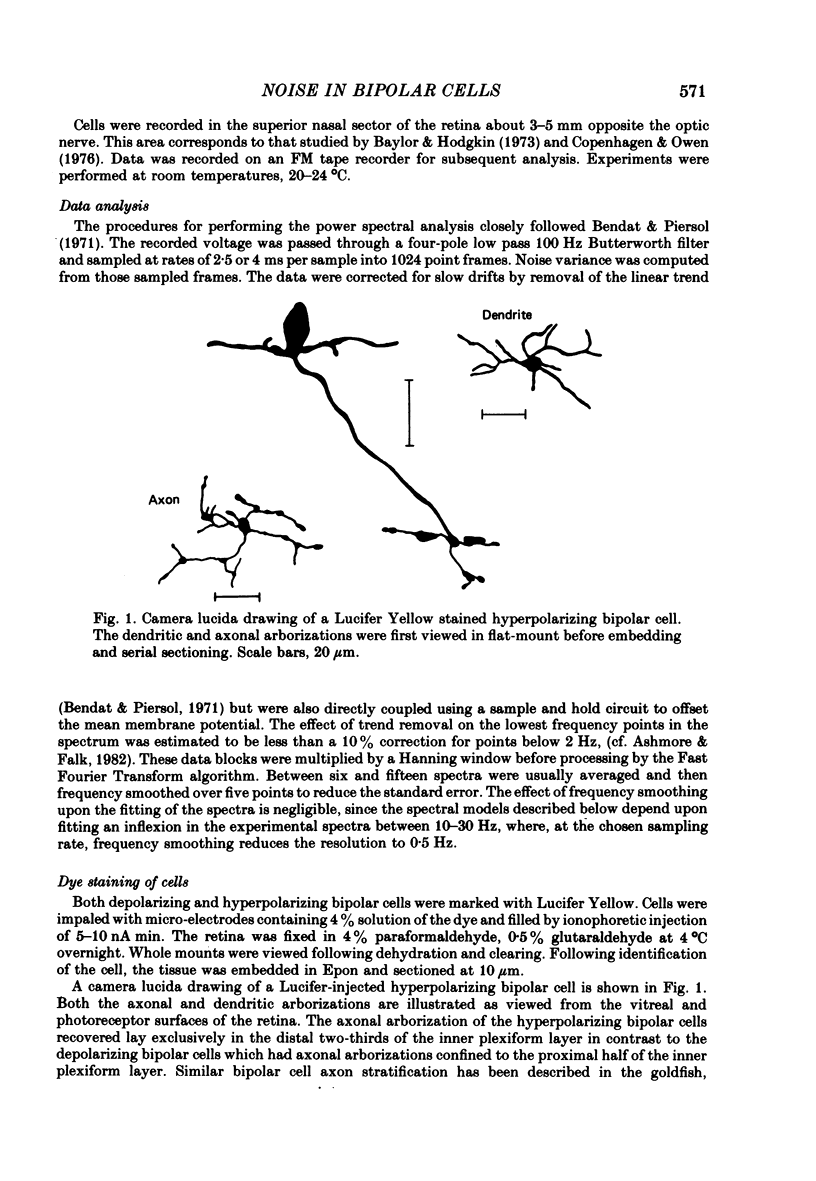
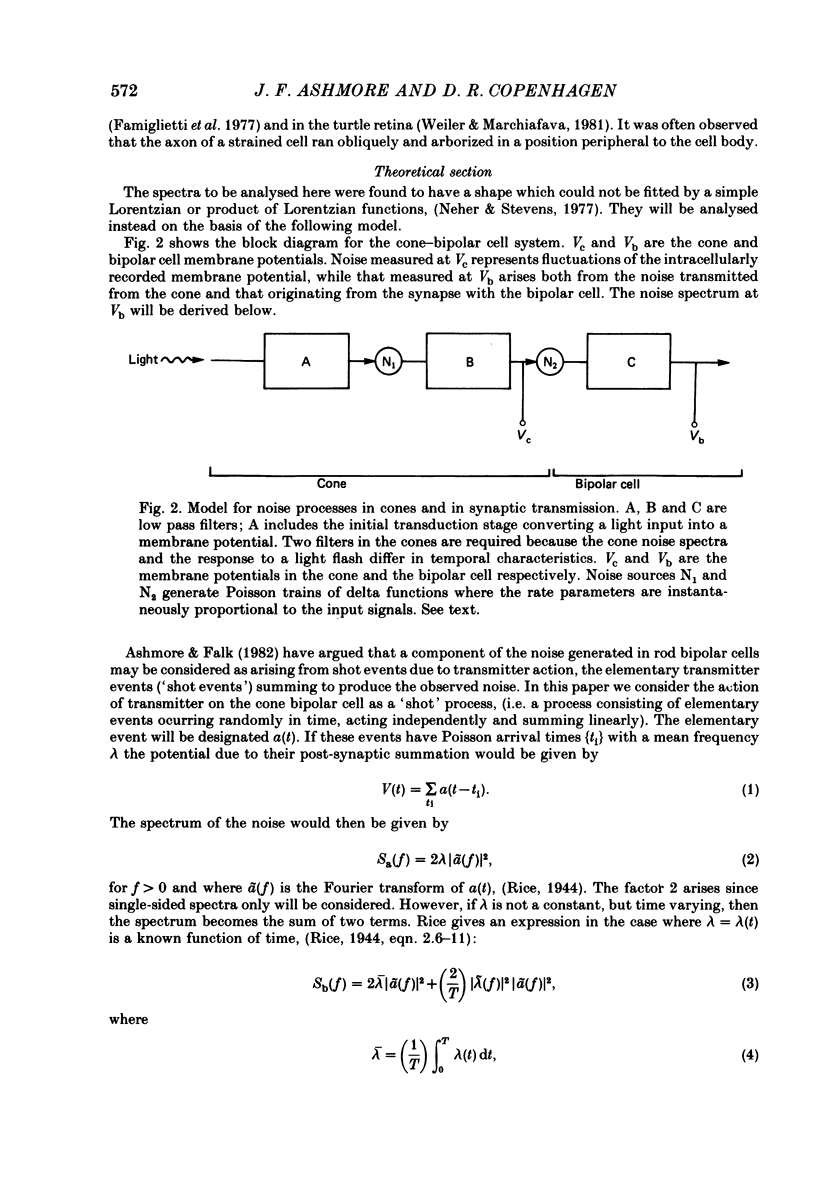


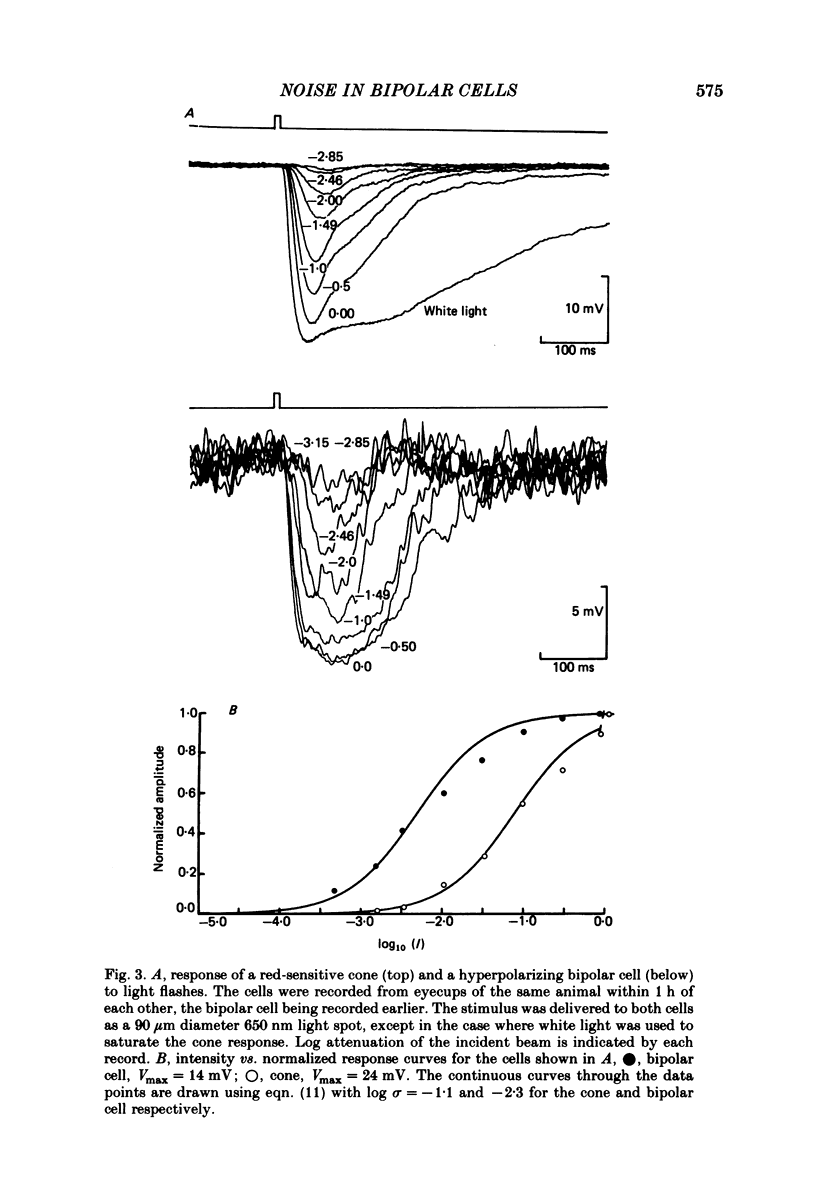

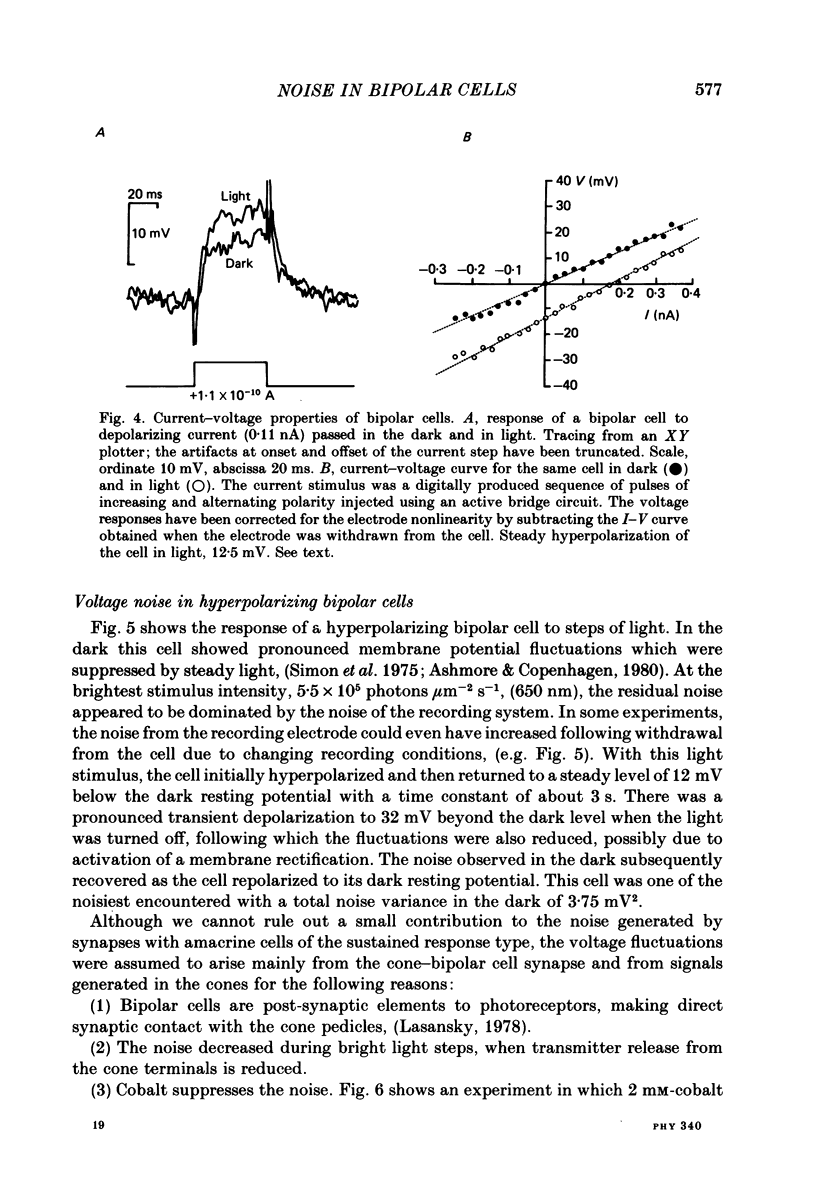









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Copenhagen D. R. Different postsynaptic events in two types of retinal bipolar cell. Nature. 1980 Nov 6;288(5786):84–86. doi: 10.1038/288084a0. [DOI] [PubMed] [Google Scholar]
- Ashmore J. F., Falk G. An analysis of voltage noise in rod bipolar cells of the dogfish retina. J Physiol. 1982 Nov;332:273–297. doi: 10.1113/jphysiol.1982.sp014413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F., Falk G. Responses of rod bipolar cells in the dark-adapted retina of the dogfish, Scyliorhinus canicula. J Physiol. 1980 Mar;300:115–150. doi: 10.1113/jphysiol.1980.sp013155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore J. F., Falk G. The single-photon signal in rod bipolar cells of the dogfish retina. J Physiol. 1980 Mar;300:151–166. doi: 10.1113/jphysiol.1980.sp013156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fettiplace R. Kinetics of synaptic transfer from receptors to ganglion cells in turtle retina. J Physiol. 1977 Oct;271(2):425–448. doi: 10.1113/jphysiol.1977.sp012007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fettiplace R. Light path and photon capture in turtle photoreceptors. J Physiol. 1975 Jun;248(2):433–464. doi: 10.1113/jphysiol.1975.sp010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. E., Wagner R. F., Jennings R. J., Barlow H. B. Efficiency of human visual signal discrimination. Science. 1981 Oct 2;214(4516):93–94. doi: 10.1126/science.7280685. [DOI] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Functional characteristics of lateral interactions between rods in the retina of the snapping turtle. J Physiol. 1976 Jul;259(2):251–282. doi: 10.1113/jphysiol.1976.sp011465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacheux R. F. Connections of the small bipolar cells with the photoreceptors in the turtle. An electron microscope study of Golgi-impregnated, gold-toned retinas. J Comp Neurol. 1982 Feb 10;205(1):55–62. doi: 10.1002/cne.902050106. [DOI] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Kaneko A., Tachibana M. Neuronal architecture of on and off pathways to ganglion cells in carp retina. Science. 1977 Dec 23;198(4323):1267–1269. doi: 10.1126/science.73223. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Hayashida Y., Matsuura S. Quantal analysis of the size of excitatory post-synaptic potentials at synapses between hair cells and afferent nerve fibres in goldfish. J Physiol. 1978 Mar;276:211–226. doi: 10.1113/jphysiol.1978.sp012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A. Electrical signs of information flow in photoreceptors. Cold Spring Harb Symp Quant Biol. 1965;30:403–418. doi: 10.1101/sqb.1965.030.01.040. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Simon E. J. Analysis of electrical noise in turtle cones. J Physiol. 1977 Nov;272(2):435–468. doi: 10.1113/jphysiol.1977.sp012053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A. Contacts between receptors and electrophysiologically identified neurones in the retina of the larval tiger salamander. J Physiol. 1978 Dec;285:531–542. doi: 10.1113/jphysiol.1978.sp012587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchiafava P. L. Horizontal cells influence membrane potential of bipolar cells in the retina of the turtle. Nature. 1978 Sep 14;275(5676):141–142. doi: 10.1038/275141a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Piccolino M., Gerschenfeld H. M. Characteristics and ionic processes involved in feedback spikes of turtle cones. Proc R Soc Lond B Biol Sci. 1980 Jan 17;206(1165):439–463. doi: 10.1098/rspb.1980.0007. [DOI] [PubMed] [Google Scholar]
- Richter A., Simon E. J. Properties of centre-hyperpolarizing, red-sensitive bipolar cells in the turtle retina. J Physiol. 1975 Jun;248(2):317–334. doi: 10.1113/jphysiol.1975.sp010976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T., Kondo H., Toyoda J. I. Ionic mechanisms of two types of on-center bipolar cells in the carp retina. I. The responses to central illumination. J Gen Physiol. 1979 Jan;73(1):73–90. doi: 10.1085/jgp.73.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer S. F., Raviola E., Heuser J. E. Membrane specializations in the outer plexiform layer of the turtle retina. J Comp Neurol. 1982 Jan 20;204(3):253–267. doi: 10.1002/cne.902040305. [DOI] [PubMed] [Google Scholar]
- Schnapf J. L., Copenhagen D. R. Differences in the kinetics of rod and cone synaptic transmission. Nature. 1982 Apr 29;296(5860):862–864. doi: 10.1038/296862a0. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of bipolar cells in the retina of the turtle. J Physiol. 1974 Jan;236(1):211–224. doi: 10.1113/jphysiol.1974.sp010431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Voltage noise observed in rods of the turtle retina. J Physiol. 1977 Nov;272(2):217–246. doi: 10.1113/jphysiol.1977.sp012042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F. J. Interpreting power spectra from nonstationary membrane current fluctuations. Biophys J. 1981 Aug;35(2):289–300. doi: 10.1016/S0006-3495(81)84790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon E. J., Lamb T. D., Hodgkin A. L. Spontaneous voltage fluctuations in retinal cones and bipolar cells. Nature. 1975 Aug 21;256(5519):661–662. doi: 10.1038/256661a0. [DOI] [PubMed] [Google Scholar]
- Toyoda J. Membrane resistance changes underlying the bipolar cell response in the carp retina. Vision Res. 1973 Feb;13(2):283–294. doi: 10.1016/0042-6989(73)90107-7. [DOI] [PubMed] [Google Scholar]
- Weiler R., Marchiafava P. L. Physiological and morphological study of the inner plexiform layer in the turtle retina. Vision Res. 1981;21(11):1635–1638. doi: 10.1016/0042-6989(81)90047-x. [DOI] [PubMed] [Google Scholar]
- van Meeteren A. On the detective quantum efficiency of the human eye. Vision Res. 1978;18(3):257–267. doi: 10.1016/0042-6989(78)90160-8. [DOI] [PubMed] [Google Scholar]


