Abstract
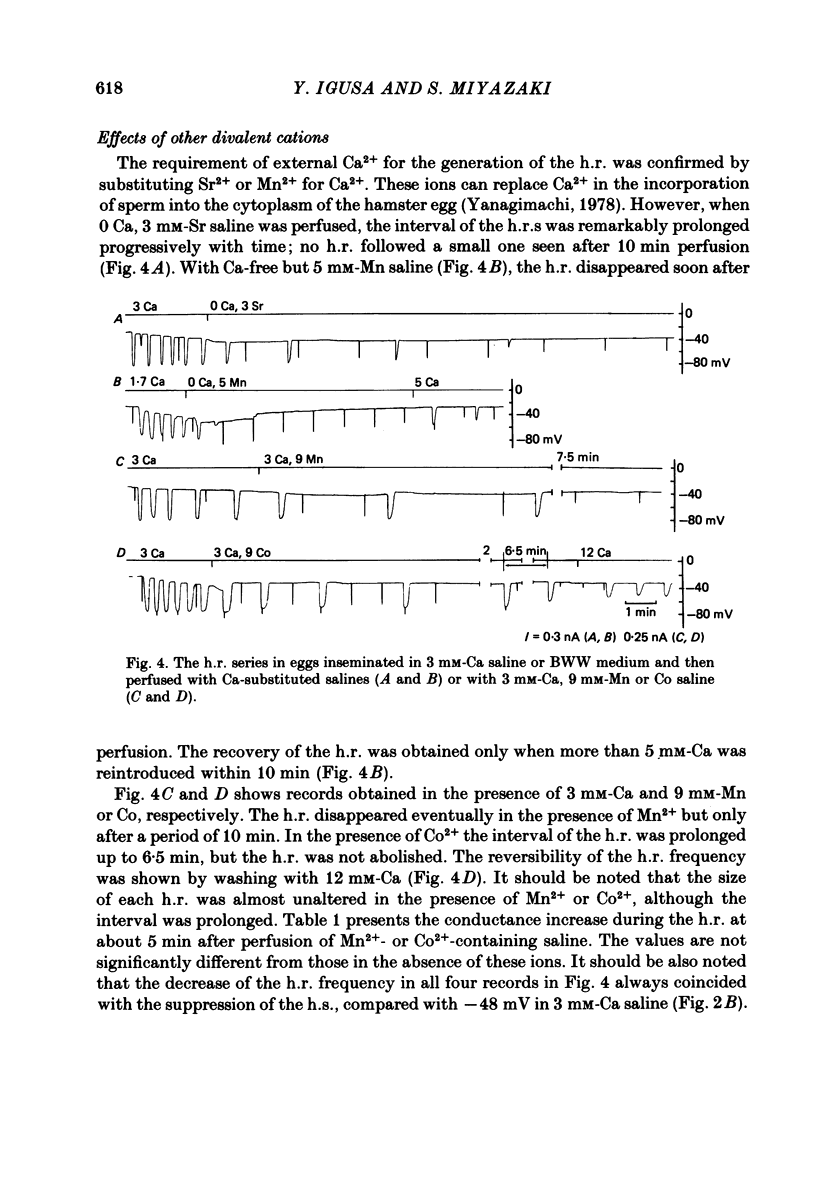
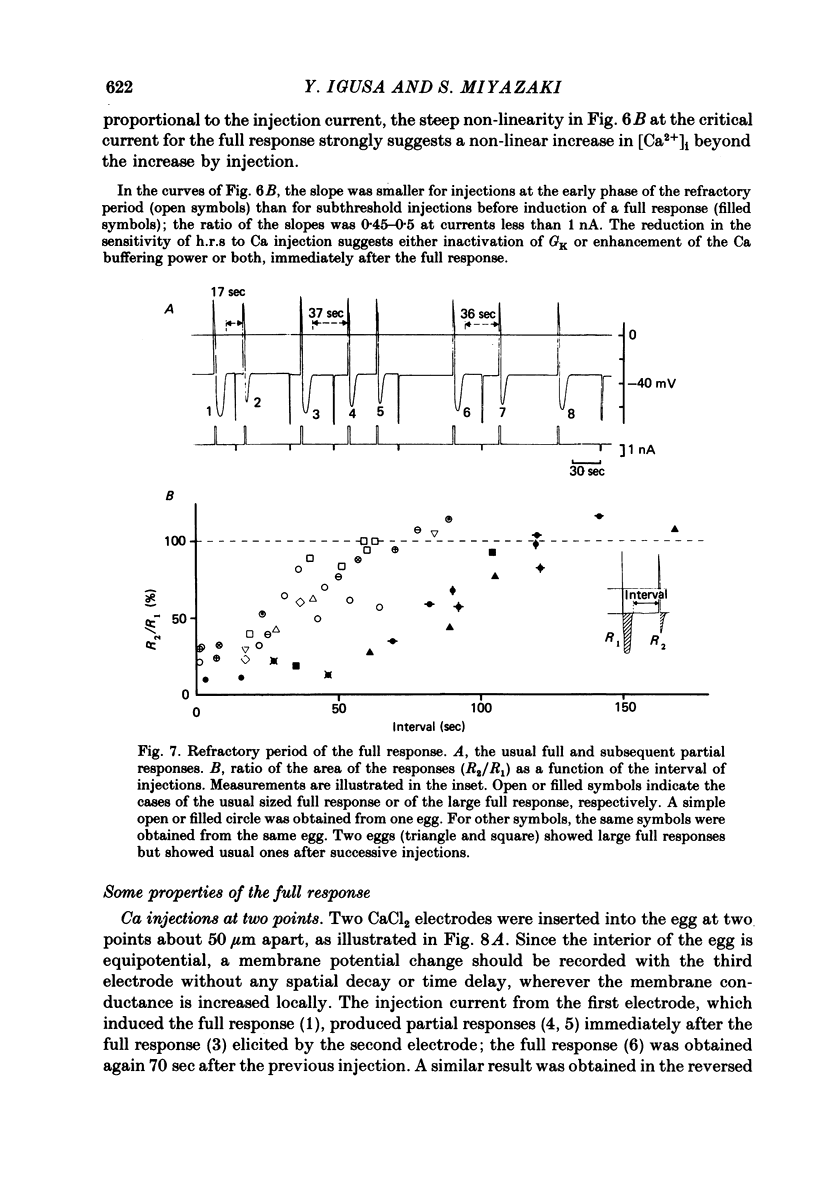
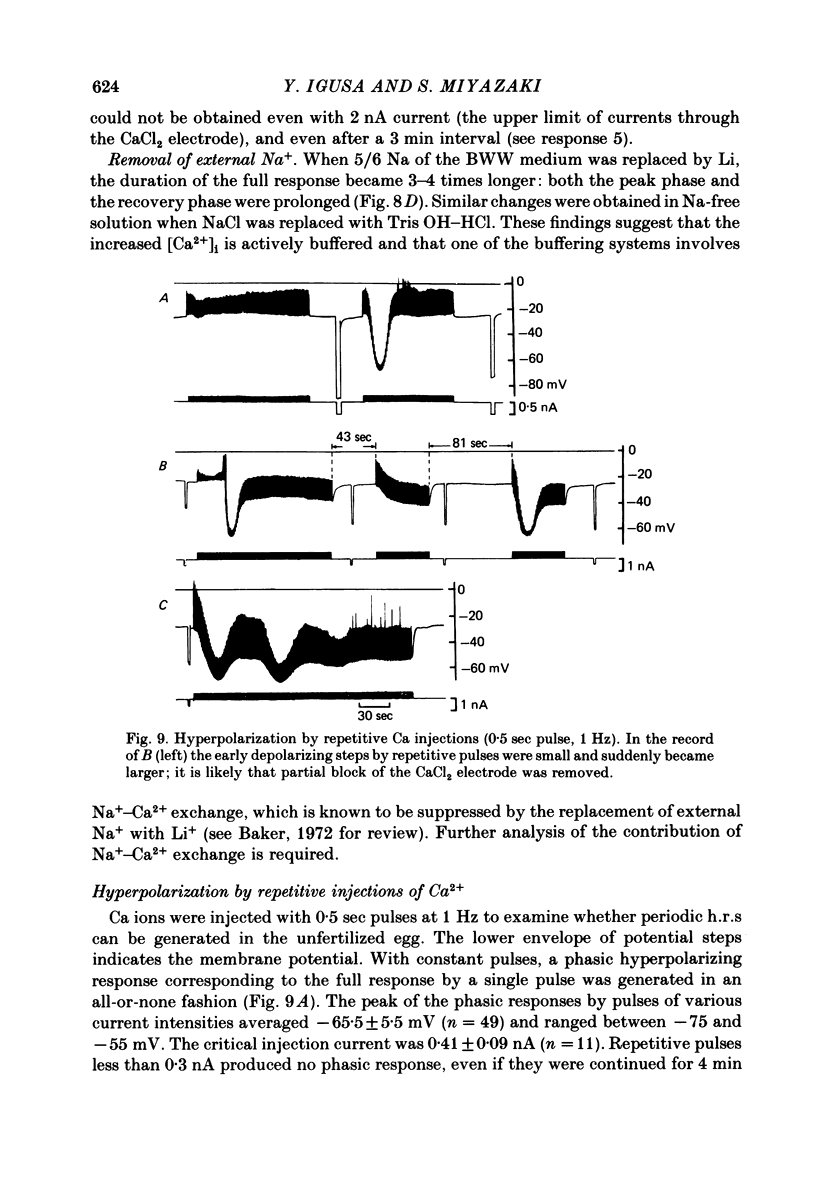
Upon fertilization the hamster egg shows transient, periodic hyperpolarizing responses (h.r.s) due to a Ca-activated K conductance; these are superimposed on a gradual, hyperpolarizing shift of the resting potential (h.s.) (Miyazaki & Igusa, 1981a, 1982a). The h.r.s and h.s. were further analysed by changing external divalent cations or by injection of Ca2+ into the egg, to study the mechanisms of the increase in the intracellular Ca2+ concentration ([Ca2+]i). The series of h.r.s was abolished by the removal of external Ca2+. The frequency of the h.r. was decreased by lowering the [Ca2+]o or by adding Mn2+ or Co2+, and it was increased by raising the [Ca2+]o in a time- and concentration-dependent manner. The h.r. frequency was decreased on sustained depolarization with steady current, and increased on hyperpolarization. In contrast to the h.r. frequency, the amplitude, conductance increase and reversal potential of each h.r. were little affected by [Ca2+]o, Mn2+ or Co2+. The h.s. was decreased by lowering the [Ca2+]o, by adding Mn2+ or Co2+, or by injection of EGTA. The h.s. may reflect continuous Ca influx stimulating a Ca-activated K conductance (GK). In unfertilized eggs a regenerative h.r. was induced by Ca injection with an apparent threshold. The relationship between GK and the injected Ca2+ showed a steep jump at the critical current, associated with a four-fold increase in GK. The regenerative h.r. was followed by a refractory period of 1-2 min. In inseminated eggs the periodic sperm-mediated h.r.s. (s.-h.r.s) were interrupted by interposed h.r.(s) induced by Ca injection(s): the periodicity of s.-h.r.s was reset by Ca-induced h.r. In inseminated eggs the regenerative h.r. was induced by Ca injection with a much smaller pulse than necessary in unfertilized eggs. The refractory period was shortened to 40-50 sec, comparable to the period of s.-h.r.s. In inseminated eggs periodic h.r.s similar to s.-h.r.s were produced by continuous, repetitive injections of Ca2+ with constant pulses. The frequency of these h.r.s was dependent on the injection current. It is concluded that each h.r. indicates an enhancement of the increase in [Ca2+]i, probably the result of Ca-induced Ca release from intracellular stores. A possible mechanism for periodic increase in [Ca2+]i reflected in s.-h.r.s is proposed, based on a linkage of the continuous Ca influx to Ca release.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTIN C. R. The capacitation of the mammalian sperm. Nature. 1952 Aug 23;170(4321):326–326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- Gilkey J. C., Jaffe L. F., Ridgway E. B., Reynolds G. T. A free calcium wave traverses the activating egg of the medaka, Oryzias latipes. J Cell Biol. 1978 Feb;76(2):448–466. doi: 10.1083/jcb.76.2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Jaffe L. A. Electrical properties of egg cell membranes. Annu Rev Biophys Bioeng. 1979;8:385–416. doi: 10.1146/annurev.bb.08.060179.002125. [DOI] [PubMed] [Google Scholar]
- Henkart M. P., Nelson P. G. Evidence for an intracellular calcium store releasable by surface stimuli ifibroblasts (L cells). J Gen Physiol. 1979 May;73(5):655–673. doi: 10.1085/jgp.73.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock S. E. Regulation of motility in nonmuscle cells. J Cell Biol. 1977 Jul;74(1):1–15. doi: 10.1083/jcb.74.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K. Release of calcium ions linked to the activation of potassium conductance in a caffeine-treated sympathetic neurone. J Physiol. 1980 Jan;298:251–269. doi: 10.1113/jphysiol.1980.sp013079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba K., Takeshita S. Simulation of intracellular Ca2+ oscillation in a sympathetic neurone. J Theor Biol. 1981 Dec 21;93(4):1009–1031. doi: 10.1016/0022-5193(81)90352-0. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. Effect of measured calcium chloride injections on the membrane potential and internal pH of snail neurones. J Physiol. 1980 Jan;298:111–129. doi: 10.1113/jphysiol.1980.sp013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Igusa Y. Ca-mediated activation of a K current at fertilization of golden hamster eggs. Proc Natl Acad Sci U S A. 1982 Feb;79(3):931–935. doi: 10.1073/pnas.79.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Igusa Y. Fertilization potential in golden hamster eggs consists of recurring hyperpolarizations. Nature. 1981 Apr 23;290(5808):702–704. doi: 10.1038/290702a0. [DOI] [PubMed] [Google Scholar]
- Okada Y., Tsuchiya W., Inouye A. Oscillations of membrane potential in L cells. IV. Role of intracellular Ca2+ in hyperpolarizing excitability. J Membr Biol. 1979 Jun 7;47(4):357–376. doi: 10.1007/BF01869744. [DOI] [PubMed] [Google Scholar]
- Okada Y., Tsuchiya W., Yada T., Yano J., Yawo H. Phagocytic activity and hyperpolarizing responses in L-strain mouse fibroblasts. J Physiol. 1981;313:101–119. doi: 10.1113/jphysiol.1981.sp013653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- Ridgway E. B., Gilkey J. C., Jaffe L. F. Free calcium increases explosively in activating medaka eggs. Proc Natl Acad Sci U S A. 1977 Feb;74(2):623–627. doi: 10.1073/pnas.74.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Kurihara S., Yoshioka T. Action of manganese ions on excitation-contractions coupling of frog skeletal muscle fibres. Jpn J Physiol. 1974 Oct;24(5):513–530. doi: 10.2170/jjphysiol.24.513. [DOI] [PubMed] [Google Scholar]
- Steinhardt R., Zucker R., Schatten G. Intracellular calcium release at fertilization in the sea urchin egg. Dev Biol. 1977 Jul 1;58(1):185–196. doi: 10.1016/0012-1606(77)90084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagimachi R. Calcium requirement for sperm-egg fusion in mammals. Biol Reprod. 1978 Dec;19(5):949–958. doi: 10.1095/biolreprod19.5.949. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. In vitro capacitation of golden hamster spermatozoa by homologous and heterologous blood sera. Biol Reprod. 1970 Oct;3(2):147–153. doi: 10.1093/biolreprod/3.2.147. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R. In vitro capacitation of hamster spermatozoa by follicular fluid. J Reprod Fertil. 1969 Mar;18(2):275–286. doi: 10.1530/jrf.0.0180275. [DOI] [PubMed] [Google Scholar]
- Yavin E., Yavin Z. Attachment and culture of dissociated cells from rat embryo cerebral hemispheres on polylysine-coated surface. J Cell Biol. 1974 Aug;62(2):540–546. doi: 10.1083/jcb.62.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]


