Abstract
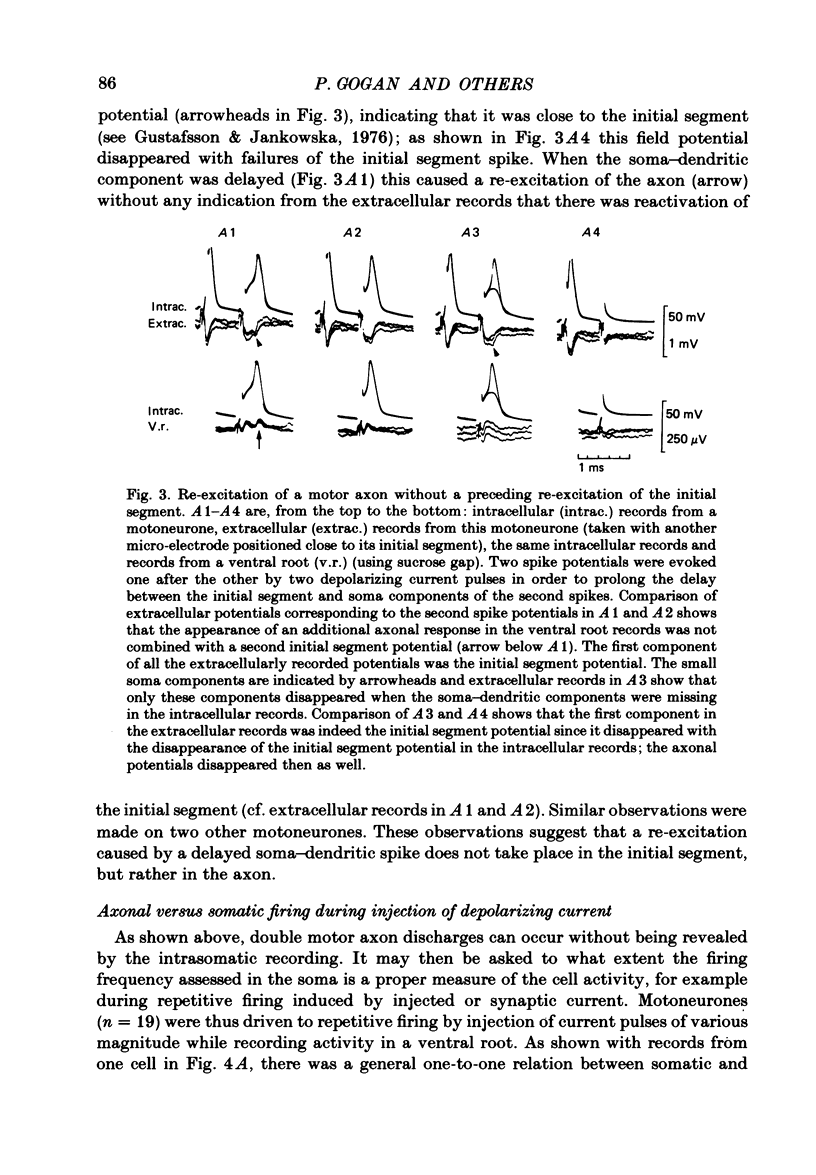
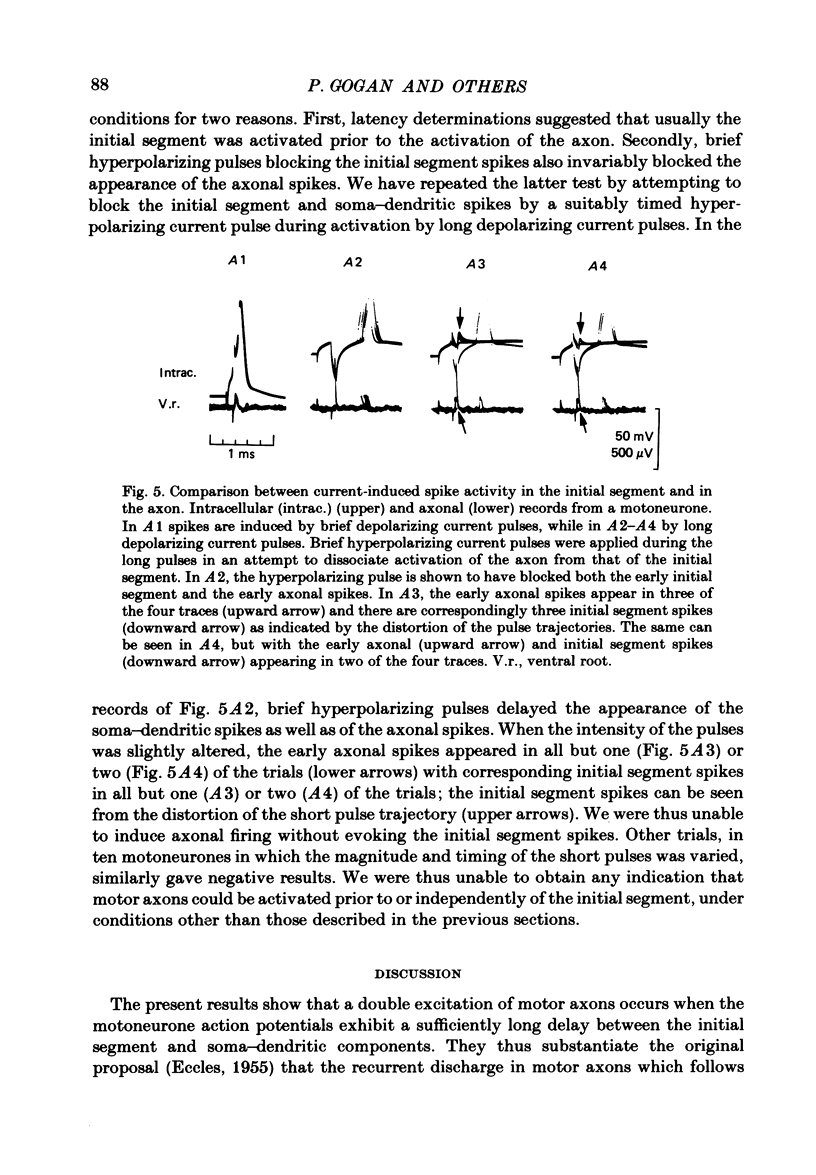
Conditions required for re-excitation of lumbosacral motoneurones, i.e. for double impulses in the motor axons associated with a single soma-dendritic action potential, were examined in cats anaesthetized with pentobarbitone and paralysed with gallamine triethiodide. Simultaneous recording from a motoneurone (intracellular, and in some experiments also extracellular), and from its axon in a ventral root, was used to assess the relations between the soma and the double axonal action potentials. Action potentials (greater than 70 mV) evoked by brief depolarizing current pulses applied intracellularly were never observed to cause re-excitation. Re-excitation could, however, be regularly induced by procedures which increased the delay between the initial segment and soma-dendritic components of these potentials. Re-excitation could be evoked (i) when brief hyperpolarizing pulses were applied before the onset of the soma-dendritic spikes, (ii) when the depolarizing pulses were applied on a background of long hyperpolarizing pulses or (iii) when two action potentials were evoked in a quick succession (by two brief depolarizing pulses). No relationship was found between the presence of re-excitation of motor axons and the presence of the delayed depolarization which follows the soma-dendritic spikes. Neither re-excitation nor delayed depolarization were found to be dependent upon re-excitation of the initial segment. These observations are thus at variance with previous suggestions that the initial segment spikes induce the re-excitation of motor axons and that the initial segment spikes cause the delayed depolarization following soma-dendritic spikes. Since re-excitation of a motor axon occurred without any signs of a second initial segment spike, it is concluded that it is initiated at the level of the axon, most likely at the first node of Ranvier. Re-excitation of motor axons was also observed during repetitive firing induced by intracellular current injection. However, it occurred then only occasionally, and only under strong depolarizing drive. It is thus not expected to be a common phenomenon under natural conditions of repetitive firing.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKS C. M., DOWNMAN C. B. B., ECCLES J. C. After-potentials and excitability of spinal motoneurones following antidromic activation. J Neurophysiol. 1950 Jan;13(1):9–38. doi: 10.1152/jn.1950.13.1.9. [DOI] [PubMed] [Google Scholar]
- BROWN M. C., MATTHEWS P. B. The effect on a muscle twitch of the back-response of its motor nerve fibres. J Physiol. 1960 Feb;150:332–346. doi: 10.1113/jphysiol.1960.sp006391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F. Relationships between the spike components and the delayed depolarization in cat spinal neurones. J Physiol. 1976 Jul;259(2):325–338. doi: 10.1113/jphysiol.1976.sp011468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bawa P., Calancie B. Repetitive doublets in human flexor carpi radialis muscle. J Physiol. 1983 Jun;339:123–132. doi: 10.1113/jphysiol.1983.sp014707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E., Harrison P. J., Jankowska E., McCrea D. A., Skoog B. Post-synaptic potentials in a population of motoneurones following activity of single interneurones in the cat. J Physiol. 1983 Oct;343:341–359. doi: 10.1113/jphysiol.1983.sp014896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller A. J., Proske U. Further observations on back-firing in the motor nerve fibres of a muscle during twitch contractions. J Physiol. 1978 Dec;285:59–69. doi: 10.1113/jphysiol.1978.sp012557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., ECCLES J. C. The generation of impulses in motoneurones. J Physiol. 1957 Dec 3;139(2):232–249. doi: 10.1113/jphysiol.1957.sp005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBS J. S., CURTIS D. R., ECCLES J. C. The interpretation of spike potentials of motoneurones. J Physiol. 1957 Dec 3;139(2):198–231. doi: 10.1113/jphysiol.1957.sp005887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin W. H., Hartline D. K. Retrograde invasion of lobster stretch receptor somata in control of firing rate and extra spike patterning. J Neurophysiol. 1977 Jan;40(1):106–118. doi: 10.1152/jn.1977.40.1.106. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O., Conradi S. Evidence for direct synaptic interconnections between cat spinal alpha-motoneurons via the recurrent axon collaterals: a morphological study using intracellular injection of horseradish peroxidase. Brain Res. 1977 Aug 19;132(1):1–10. doi: 10.1016/0006-8993(77)90702-8. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C. The central action of antidromic impulses in motor nerve fibres. Pflugers Arch. 1955;260(5):385–415. doi: 10.1007/BF00363548. [DOI] [PubMed] [Google Scholar]
- Gogan P., Gueritaud J. P., Horcholle-Bossavit G., Tyc-Dumont S. Direct excitatory interactions between spinal motoneurones of the cat. J Physiol. 1977 Nov;272(3):755–767. doi: 10.1113/jphysiol.1977.sp012071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogan P., Gueritaud J. P., Tyc-Dumont S. Comparison of antidromic and orthodromic action potentials of identified motor axons in the cat's brain stem. J Physiol. 1983 Feb;335:205–220. doi: 10.1113/jphysiol.1983.sp014529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B. Changes in motoneurone electrical properties following axotomy. J Physiol. 1979 Aug;293:197–215. doi: 10.1113/jphysiol.1979.sp012885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol. 1976 Jun;258(1):33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Lipski J. Effect of membrane polarization and synaptic activity on the timing of antidromic invasion. Brain Res. 1980 Jan 6;181(1):61–74. doi: 10.1016/0006-8993(80)91259-7. [DOI] [PubMed] [Google Scholar]
- Hoffer J. A., O'Donovan M. J., Pratt C. A., Loeb G. E. Discharge patterns of hindlimb motoneurons during normal cat locomotion. Science. 1981 Jul 24;213(4506):466–467. doi: 10.1126/science.7244644. [DOI] [PubMed] [Google Scholar]
- Lagerbäck P. A., Ronnevi L. O., Cullheim S., Kellerth J. O. An ultrastructural study of the synaptic contacts of alpha-motoneurone axon collaterals. I. Contacts in lamina IX and with identified alpha-motoneurone dendrites in lamina VII. Brain Res. 1981 Mar 2;207(2):247–266. doi: 10.1016/0006-8993(81)90363-2. [DOI] [PubMed] [Google Scholar]
- Nelson P. G., Burke R. E. Delayed depolarization in cat spinal motoneurons. Exp Neurol. 1967 Jan;17(1):16–26. doi: 10.1016/0014-4886(67)90118-5. [DOI] [PubMed] [Google Scholar]
- Zajac F. E., Young J. L. Discharge properties of hindlimb motoneurons in decerebrate cats during locomotion induced by mesencephalic stimulation. J Neurophysiol. 1980 May;43(5):1221–1235. doi: 10.1152/jn.1980.43.5.1221. [DOI] [PubMed] [Google Scholar]


