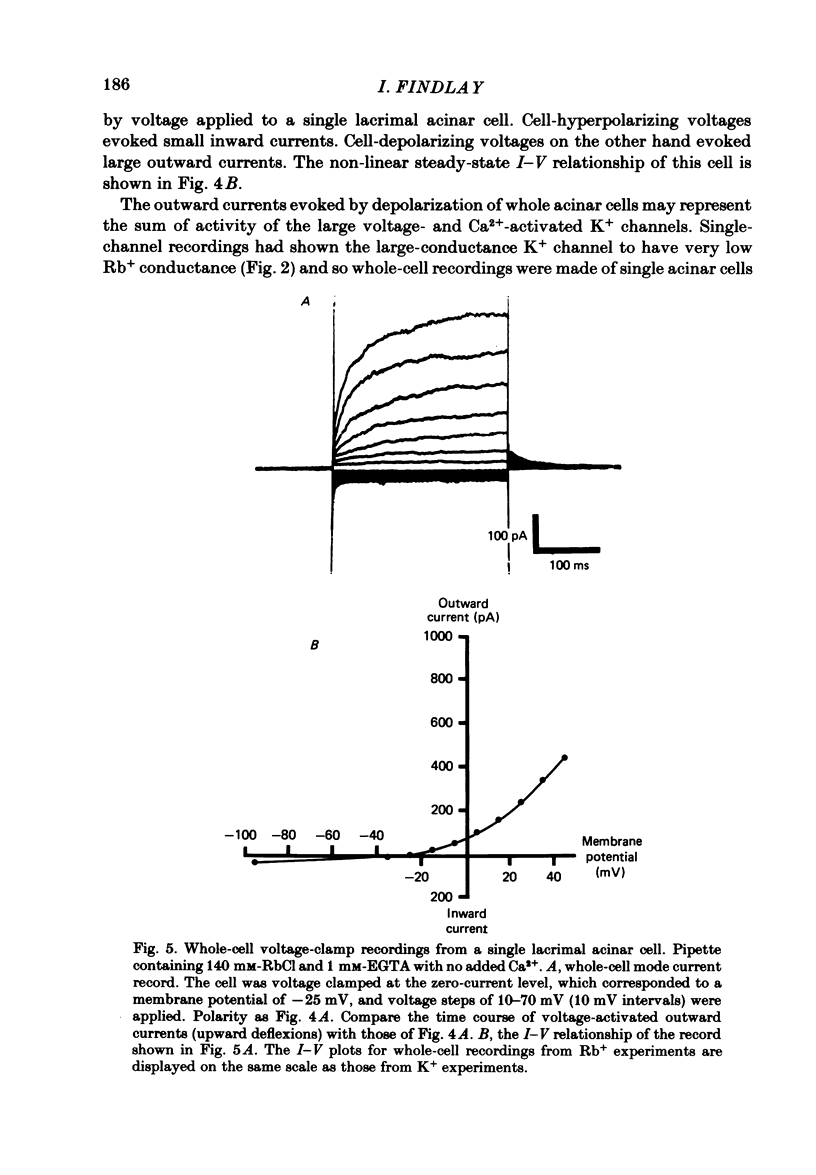
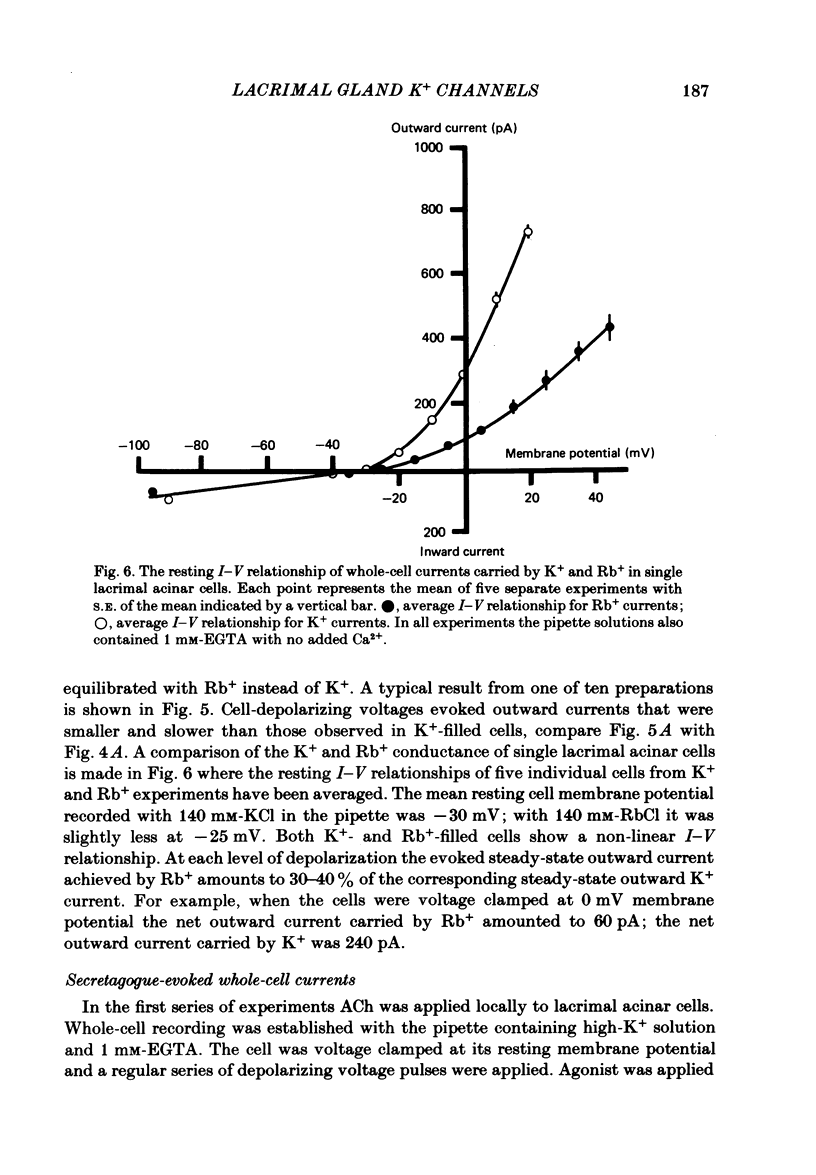
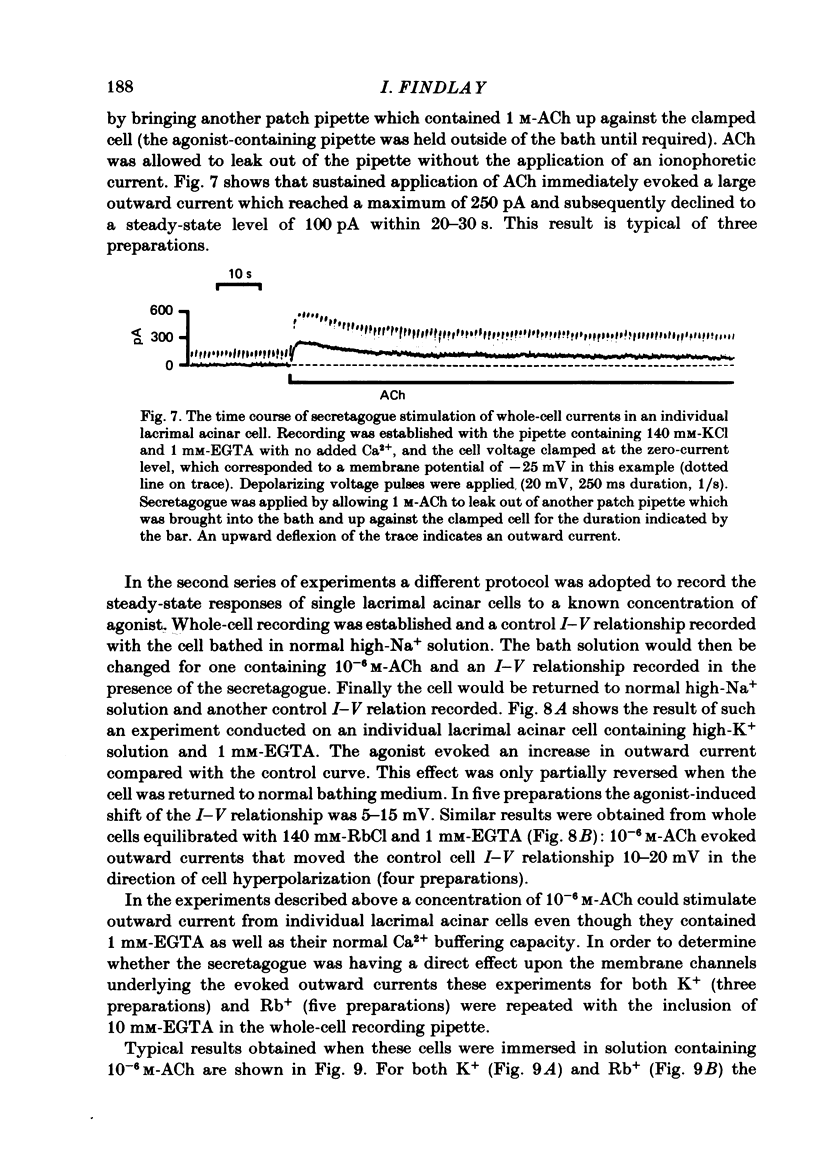
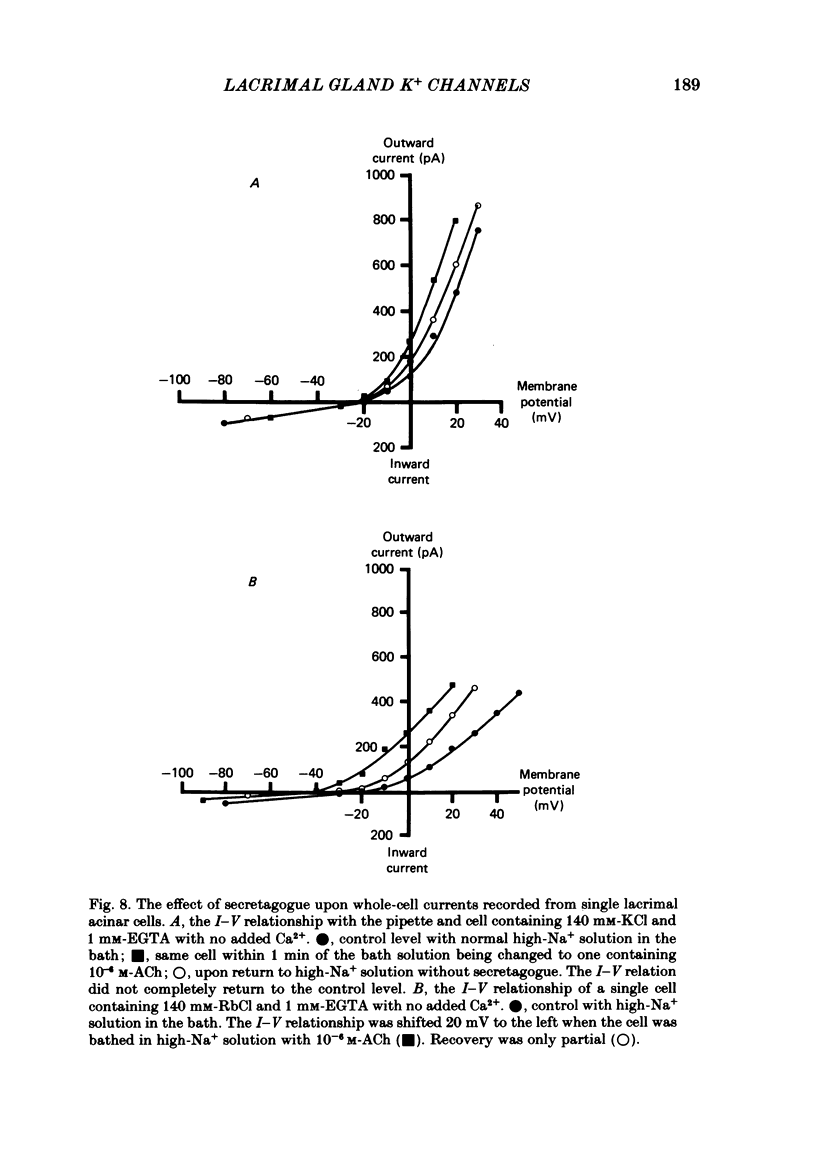
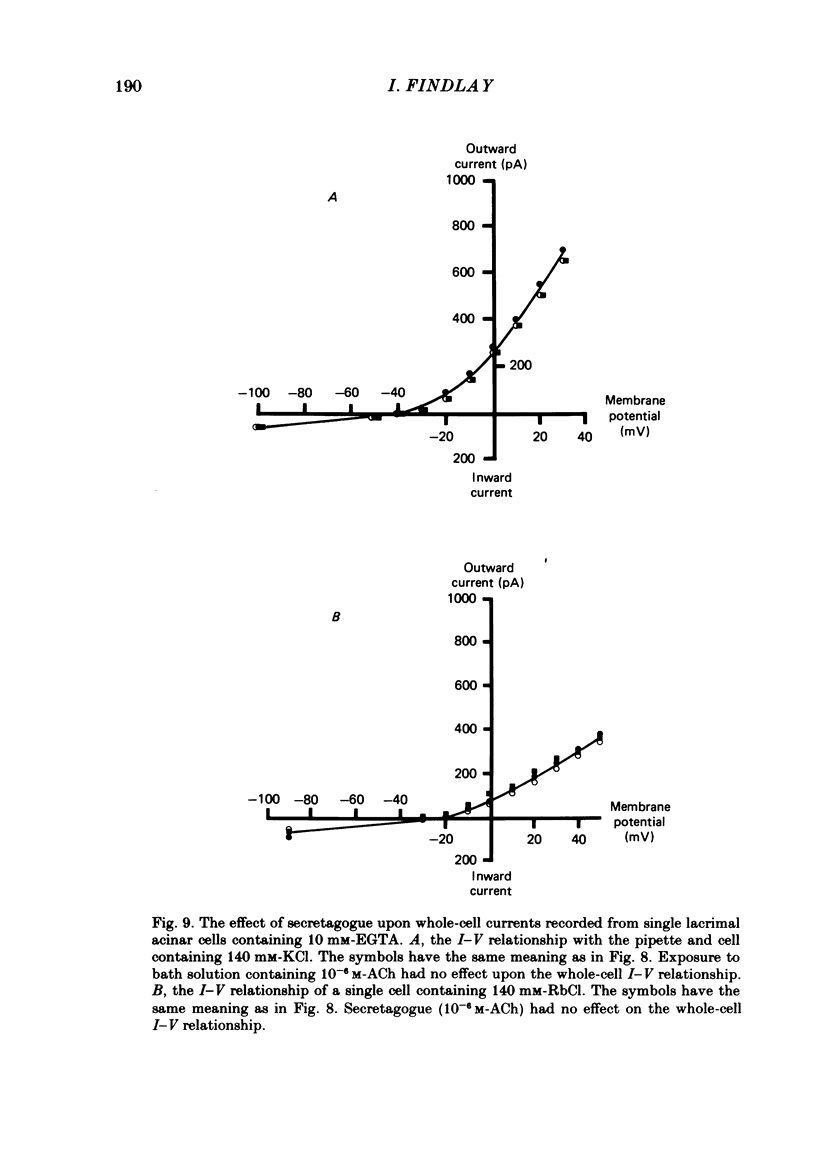
Abstract
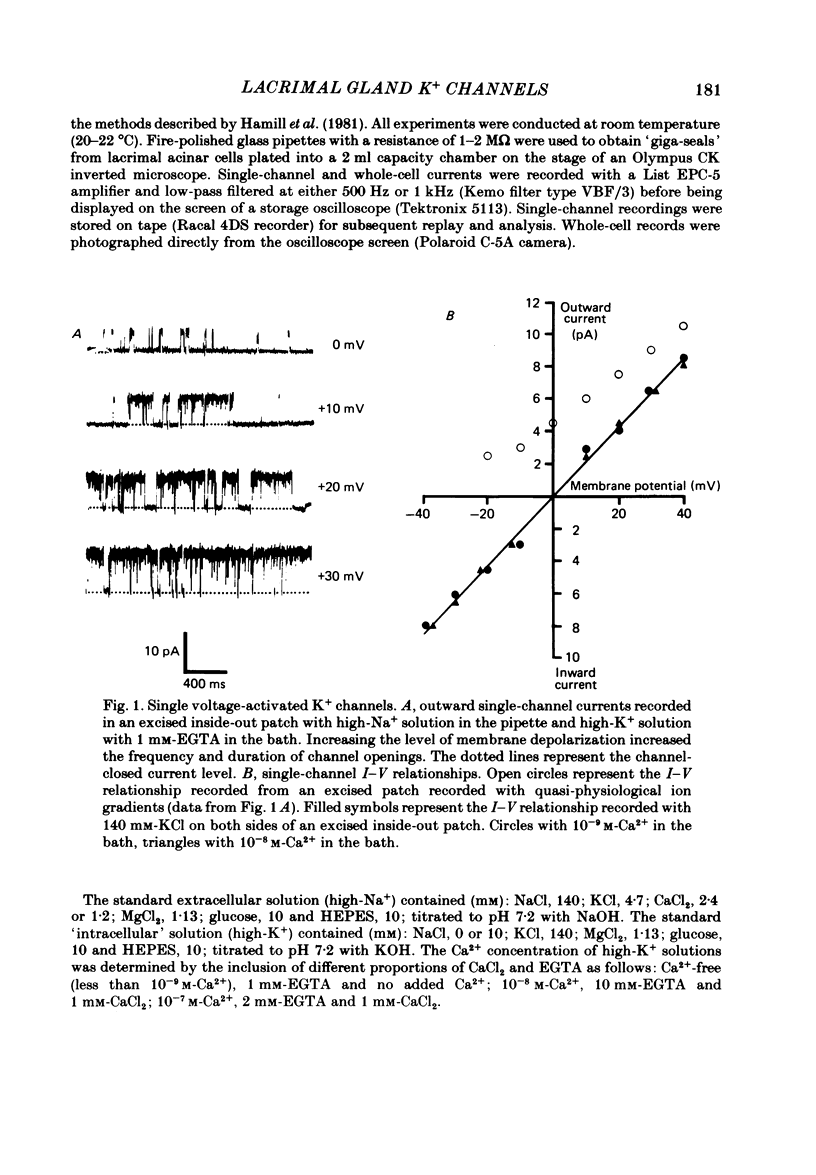
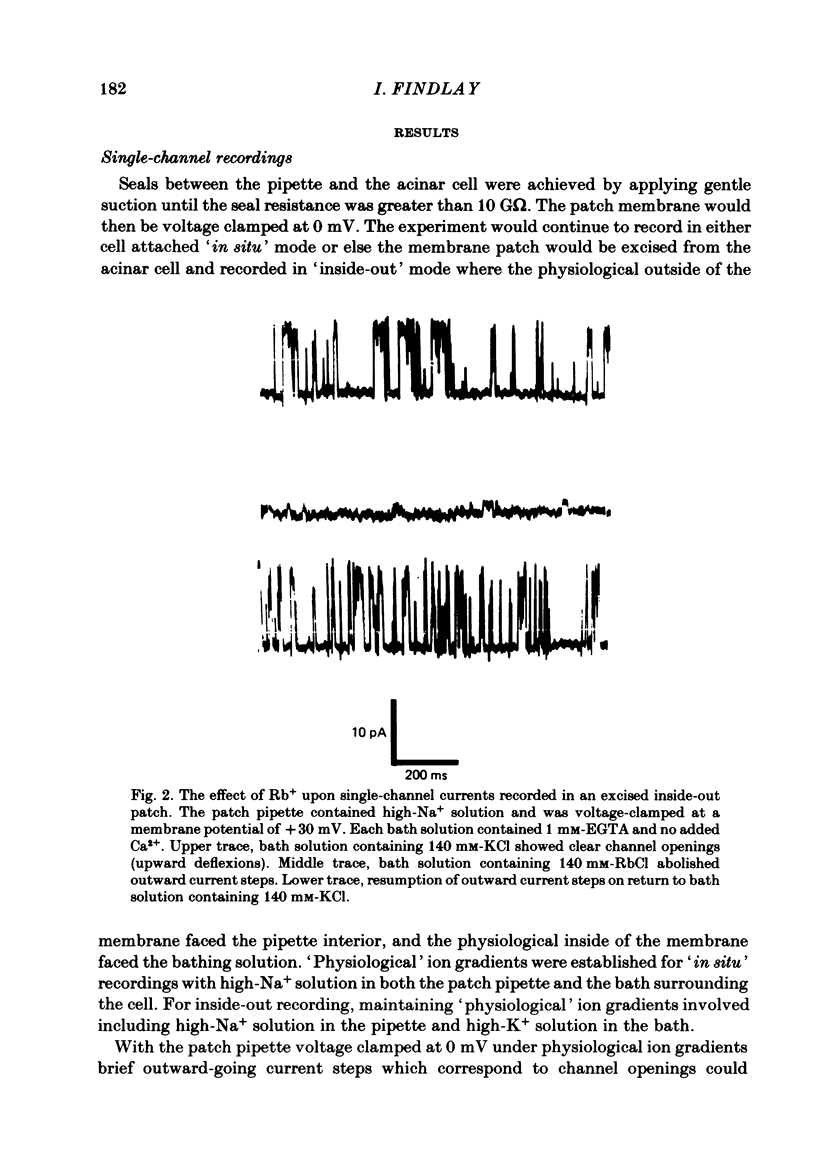
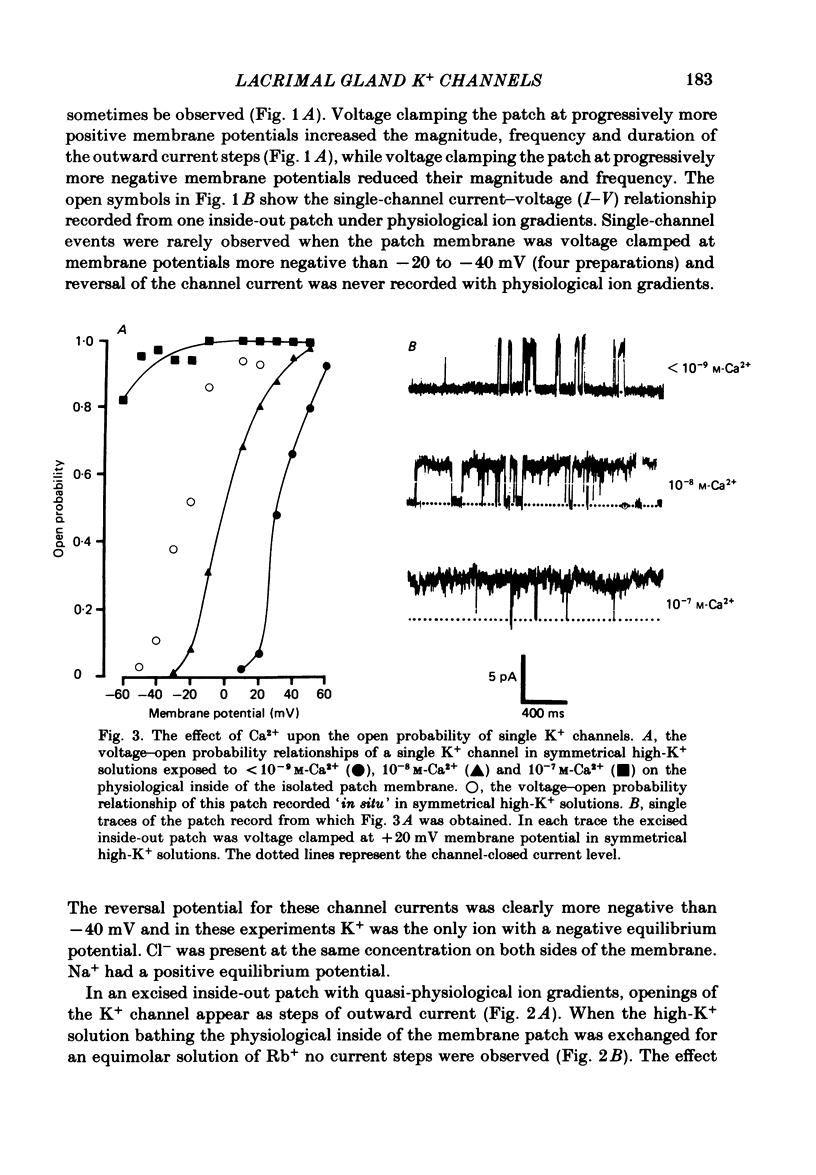
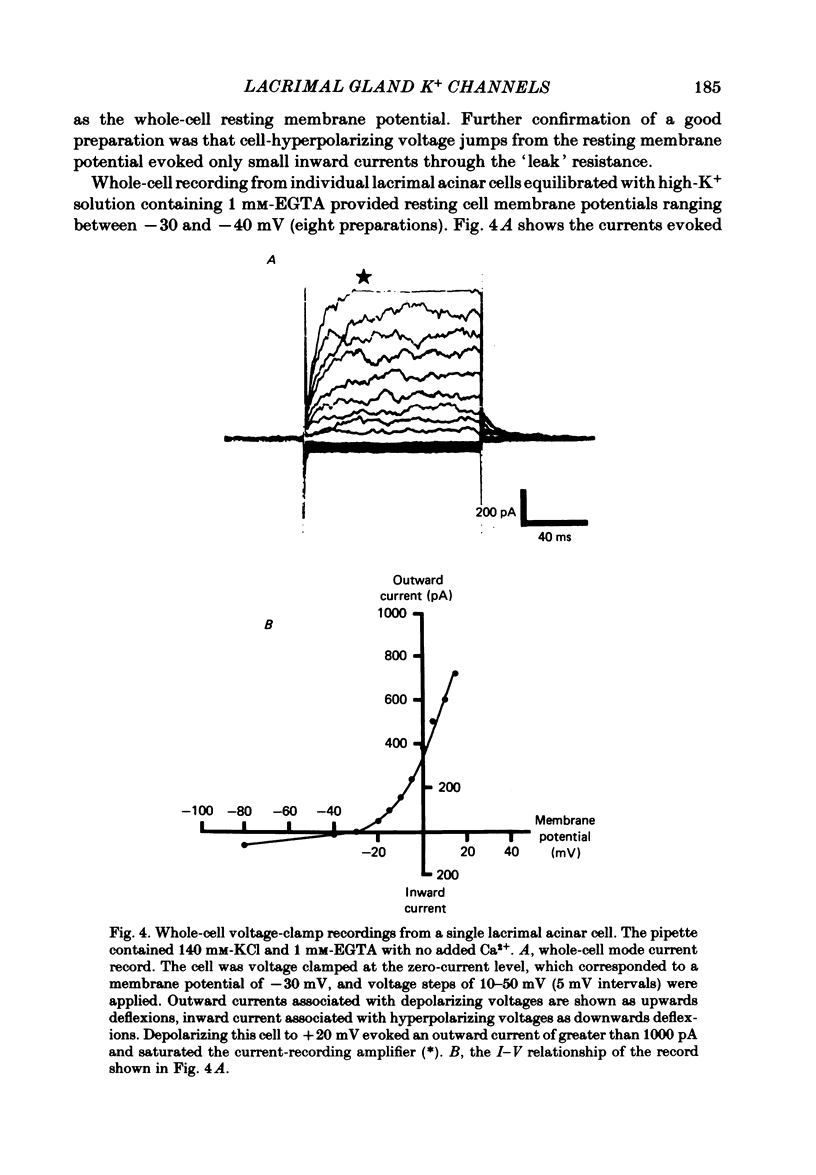
Individual acinar cells were isolated enzymatically from the mouse exorbital lacrimal gland. Their electrical characteristics were studied by the patch-clamp methods of single-channel and whole-cell recording as described by Hamill, Marty, Neher, Sakmann & Sigworth (1981). Recording from cell-attached and excised inside-out patches of acinar membrane with quasi-physiological ion gradients demonstrated large outward current events that correspond to single-channel openings. The amplitude, frequency and duration of channel events increased as the membrane patch was depolarized and were reduced by hyperpolarization of the patch membrane. The reversal potential for these channel events is more negative than -40 mV. In excised inside-out patches exposed to quasi-physiological ion gradients single-channel events were abolished when K+ was replaced by Rb+. Since there was no Cl- gradient the channel is clearly highly selective for K+. In excised inside-out patches, when the free Ca2+ concentration bathing the physiological inside of the membrane was raised from less than 10(-9) M to 10(-8) M the frequency and duration of opening of the K+ channel was increased. The channel was almost continuously open when the membrane was exposed to 10(-7) M-free Ca2+. 'Whole cell' recording of lacrimal acinar cells containing 140 mM-KCl and 1 mM-EGTA (with no added Ca2+) provided cell resting membrane potentials of -30 to -40 mV. Depolarizing voltage jumps from the resting membrane potential evoked large outward currents. Hyperpolarizing voltage jumps only evoked small inward currents. Whole-cell recording where RbCl replaced KCl in the pipette provided resting membrane potentials of -20 to -30 mV, reduced the amplitude of outward currents evoked by cell-depolarizing voltage steps by 60% and slowed the time course of the currents. Isolated cells containing 140 mM-KCl and 1 mM-EGTA were voltage clamped at their resting membrane potentials. Acetylcholine (ACh) was applied locally and immediately evoked a strong outward current which rapidly declined to a steady-state level. Sustained agonist responses were obtained by exposing the isolated cell to a solution containing 10(-6) M-ACh. In both K+- and Rb+-filled cells, where the intracellular Ca2+ concentration was buffered by the inclusion of 1 mM-EGTA, 10(-6) M-ACh evoked sustained outward currents that corresponded to cell hyperpolarizations of 5-15 and 10-20 mV, respectively. Increasing intracellular Ca2+ buffering by including 10 mM-EGTA abolished secretagogue-induced outward current in both K+- and Rb+-filled cells. It is concluded that the lacrimal acinar cell membrane contains voltage- and Ca2+-activated K+ channels.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. H., van Lennep E. W., Young J. A. Water and electrolyte secretion by the exorbital lacrimal gland of the rat studied by micropuncture and catheterization techniques. Pflugers Arch. 1972;337(4):299–309. doi: 10.1007/BF00586647. [DOI] [PubMed] [Google Scholar]
- Barrett J. N., Magleby K. L., Pallotta B. S. Properties of single calcium-activated potassium channels in cultured rat muscle. J Physiol. 1982 Oct;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho S. Y., Dartt D. A. Effect of calcium antagonism or chelation on rabbit lacrimal gland secretion and membrane potentials. J Physiol. 1980 Jul;304:397–403. doi: 10.1113/jphysiol.1980.sp013331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V., Petersen O. H. Stimulus-secretion coupling in mammalian salivary glands. Int Rev Physiol. 1983;28:1–52. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hisada M., Botelho S. Y. Membrane potentials of in situ lacrimal gland in the cat. Am J Physiol. 1968 Jun;214(6):1262–1267. doi: 10.1152/ajplegacy.1968.214.6.1262. [DOI] [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Intracellular Ca2+ injection causes membrane hyperpolarization and conductance increase in lacrimal acinar cells. Pflugers Arch. 1978 Nov 14;377(2):185–187. [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Membrane potential, resistance, and intercellular communication in the lacrimal gland: effects of acetylcholine and adrenaline. J Physiol. 1978 Feb;275:507–520. doi: 10.1113/jphysiol.1978.sp012204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Lux H. D., Neher E., Marty A. Single channel activity associated with the calcium dependent outward current in Helix pomatia. Pflugers Arch. 1981 Mar;389(3):293–295. doi: 10.1007/BF00584792. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Gallacher D. V., Petersen O. H. Voltage and Ca2+-activated K+ channel in baso-lateral acinar cell membranes of mammalian salivary glands. Nature. 1983 Apr 28;302(5911):827–829. doi: 10.1038/302827a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H. Cholecystokinin activation of single-channel currents is mediated by internal messenger in pancreatic acinar cells. Nature. 1982 Nov 4;300(5887):61–63. doi: 10.1038/300061a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H., Flanagan P., Pearson G. T. Quantification of Ca2+-activated K+ channels under hormonal control in pig pancreas acinar cells. Nature. 1983 Sep 15;305(5931):228–232. doi: 10.1038/305228a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Peterson O. H. Single-channel currents in isolated patches of plasma membrane from basal surface of pancreatic acini. Nature. 1982 Sep 9;299(5879):159–161. doi: 10.1038/299159a0. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S., Magleby K. L., Barrett J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981 Oct 8;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- Parod R. J., Dambach G. E., Putney J. W., Jr Membrane potential changes in lacrimal gland acinar cells elicited by carbachol and epinephrine. J Pharmacol Exp Ther. 1980 Jun;213(3):473–479. [PubMed] [Google Scholar]
- Parod R. J., Leslie B. A., Putney J. W., Jr Muscarinic and alpha-adrenergic stimulation of Na and Ca uptake by dispersed lacrimal cells. Am J Physiol. 1980 Aug;239(2):G99–105. doi: 10.1152/ajpgi.1980.239.2.G99. [DOI] [PubMed] [Google Scholar]
- Parod R. J., Putney J. W., Jr An alpha-adrenergic receptor mechanism controlling potassium permeability in the rat lacrimal gland acinar cell. J Physiol. 1978 Aug;281:359–369. doi: 10.1113/jphysiol.1978.sp012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parod R. J., Putney J. W., Jr Stimulus-permeability coupling in rat lacrimal gland. Am J Physiol. 1980 Aug;239(2):G106–G113. doi: 10.1152/ajpgi.1980.239.2.G106. [DOI] [PubMed] [Google Scholar]
- Parod R. J., Putney J. W., Jr The role of calcium in the receptor mediated control of potassium permeability in the rat lacrimal gland. J Physiol. 1978 Aug;281:371–381. doi: 10.1113/jphysiol.1978.sp012428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B. S., Lecar H., Adler M. Single calcium-dependent potassium channels in clonal anterior pituitary cells. Biophys J. 1982 Sep;39(3):313–317. doi: 10.1016/S0006-3495(82)84522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


