Abstract
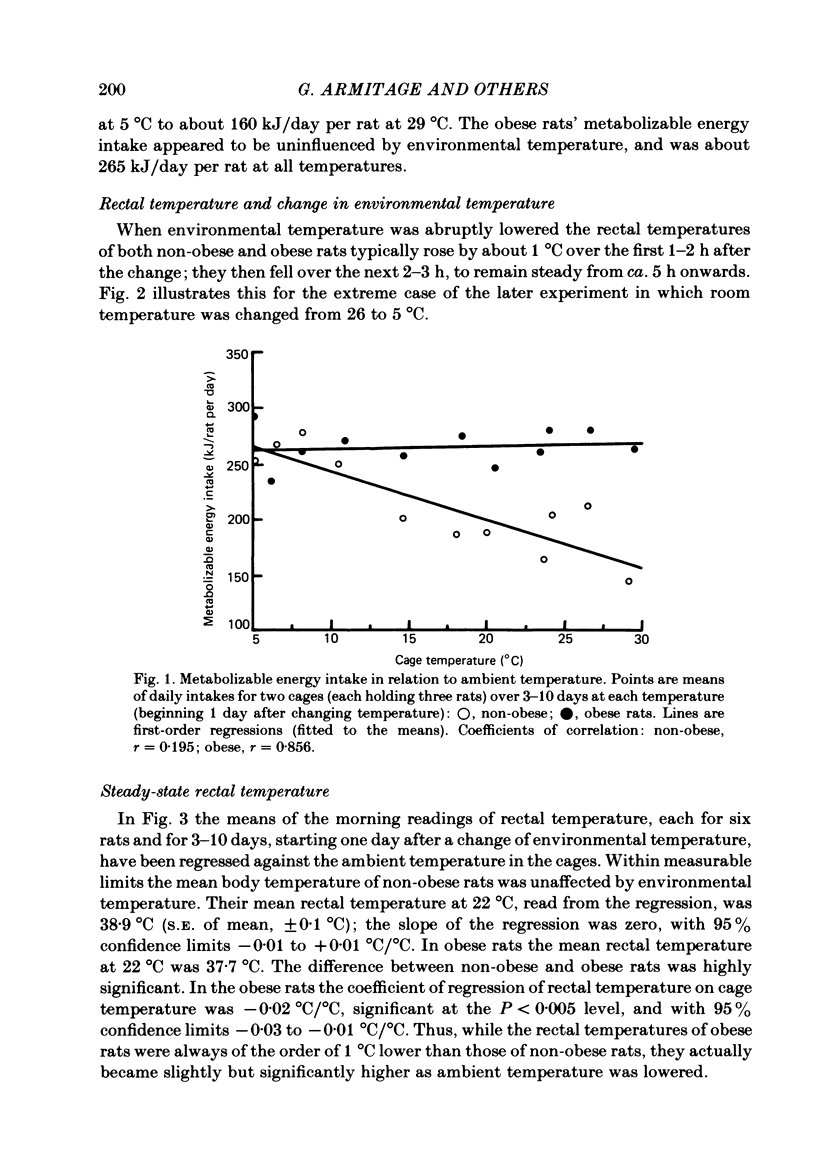
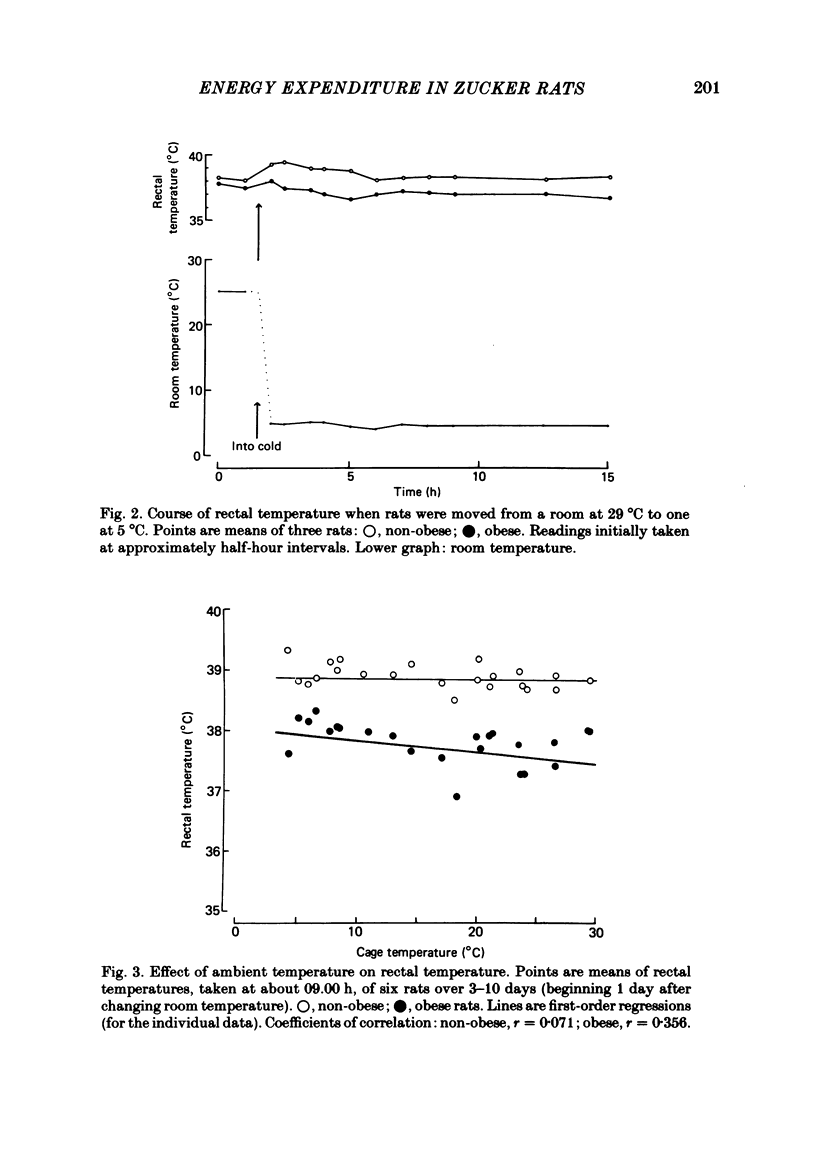
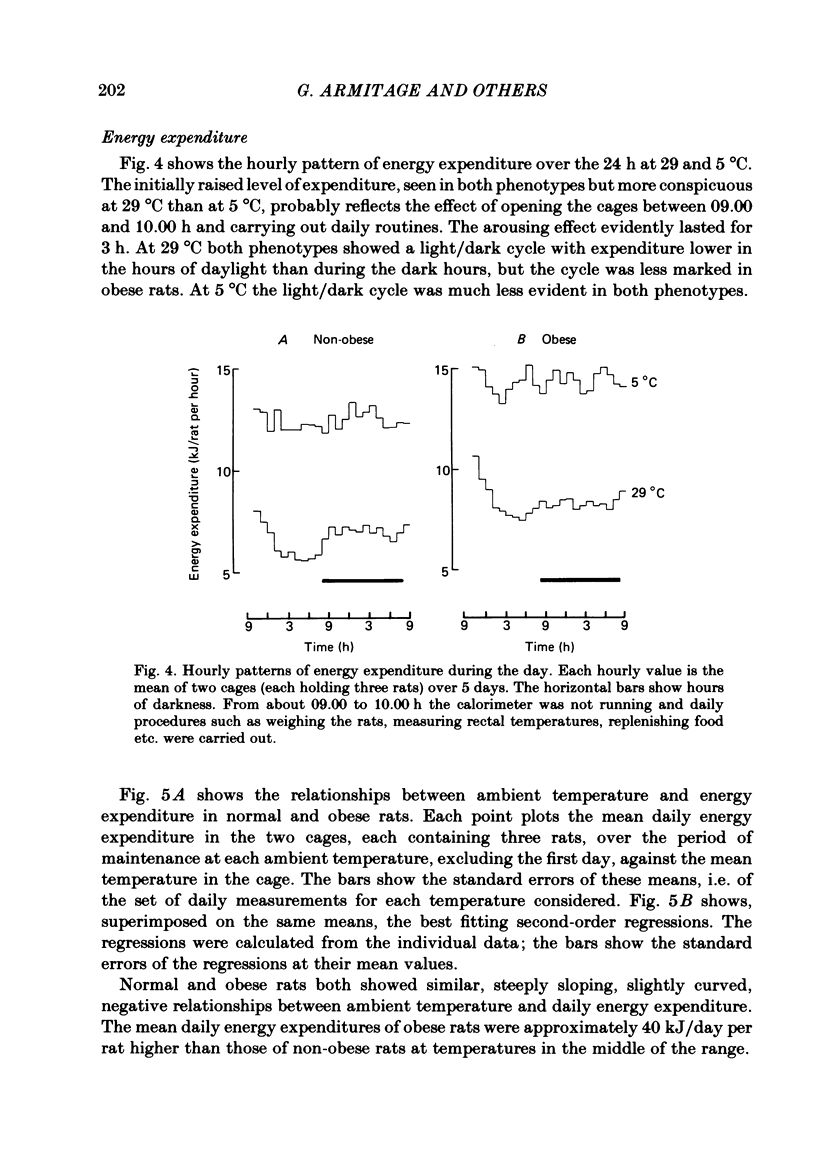
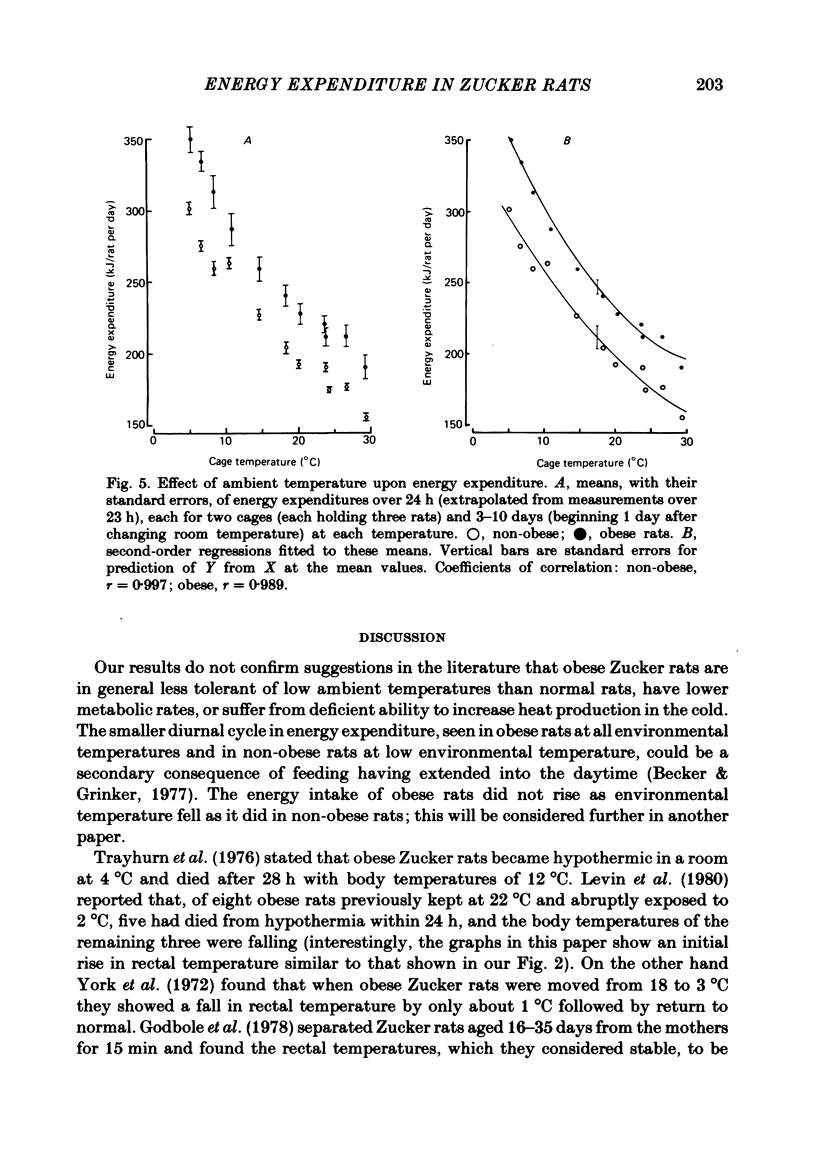
The energy expenditure of normal and congenitally obese adult female Zucker rats has been measured by continuous indirect calorimetry for periods of 3-10 days at ambient temperatures varied from 30 to 5 degrees C. Rectal temperatures were also recorded. Exposure to cold caused no ill-effects in normal or obese rats. The rectal temperatures of obese rats were about 1 degree C lower than those of normal rats. The rectal temperatures of normal rats did not change measurably with ambient temperature; in obese rats rectal temperature rose slightly as ambient temperature fell. In normal and obese rats, energy expenditure showed a smooth, steeply sloping, negative relationship to ambient temperature. Energy expenditure per rat was higher in obese than in normal rats at all temperatures. The two slightly curvilinear regressions were nearly 'parallel', with a separation of about 40 kJ/day per rat at the mid-point. This study therefore does not confirm suggestions that obese Zucker rats suffer from a defect in the level of energy expenditure, or in their capacity to increase it when exposed to cold. It is suggested that in both normal and obese rats the level of energy expenditure was determined by thermoregulatory control. The greater heat production of obese rats may have been a response to their lower core temperature. A steady state in which greater heat production is associated with lower core temperature implies lower insulation between body core and surface. This could be due to greater blood flow.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armitage G., Hervey G. R., Rolls B. J., Rowe E. A., Tobin G. The effects of supplementation of the diet with highly palatable foods upon energy balance in the rat. J Physiol. 1983 Sep;342:229–251. doi: 10.1113/jphysiol.1983.sp014848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. E., Grinker J. A. Meal patterns in the genetically obese Zucker rat. Physiol Behav. 1977 Apr;18(4):685–692. doi: 10.1016/0031-9384(77)90067-1. [DOI] [PubMed] [Google Scholar]
- Benzinger T. H. Heat regulation: homeostasis of central temperature in man. Physiol Rev. 1969 Oct;49(4):671–759. doi: 10.1152/physrev.1969.49.4.671. [DOI] [PubMed] [Google Scholar]
- Bertin R., Razanamaniraka I., De Marco F., Portet R. Effects of cold acclimation on the feeding pattern and energetic metabolism of genetically obese Zucker rats. Comp Biochem Physiol A Comp Physiol. 1983;74(4):855–860. doi: 10.1016/0300-9629(83)90358-4. [DOI] [PubMed] [Google Scholar]
- Bray G. A. Oxygen consumption of genetically obese rats. Experientia. 1969 Oct 15;25(10):1100–1101. doi: 10.1007/BF01901456. [DOI] [PubMed] [Google Scholar]
- Bray G. A., York D. A. Studies on food intake of genetically obese rats. Am J Physiol. 1972 Jul;223(1):176–179. doi: 10.1152/ajplegacy.1972.223.1.176. [DOI] [PubMed] [Google Scholar]
- Bray G. A., York D. A., Swerloff R. S. Genetic obesity in rats. I. The effects of food restriction on body composition and hypothalamic function. Metabolism. 1973 Mar;22(3):435–442. doi: 10.1016/0026-0495(73)90035-8. [DOI] [PubMed] [Google Scholar]
- DAVIS T. R., MAYER J. Imperfect homeothermia in the hereditary obese-hyperglycemic syndrome of mice. Am J Physiol. 1954 May;177(2):222–226. doi: 10.1152/ajplegacy.1954.177.2.222. [DOI] [PubMed] [Google Scholar]
- Godbole V., York D. A., Bloxham D. P. Developmental changes in the fatty (fafa) rat: evidence for defective thermogenesis preceding the hyperlipogenesis and hyperinsulinaemia. Diabetologia. 1978 Jul;15(1):41–44. doi: 10.1007/BF01219327. [DOI] [PubMed] [Google Scholar]
- James W. P., Trayhurn P. An integrated view of the metabolic and genetic basis for obesity. Lancet. 1976 Oct 9;2(7989):770–773. doi: 10.1016/s0140-6736(76)90602-4. [DOI] [PubMed] [Google Scholar]
- James W. P., Trayhurn P. Thermogenesis and obesity. Br Med Bull. 1981 Jan;37(1):43–48. doi: 10.1093/oxfordjournals.bmb.a071674. [DOI] [PubMed] [Google Scholar]
- KEATINGE W. R. The effects of subcutaneous fat and of previous exposure to cold on the body temperature, peripheral blood flow and metabolic rate of men in cold water. J Physiol. 1960 Aug;153:166–178. doi: 10.1113/jphysiol.1960.sp006526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. L. Oxygen consumption by Zucker obese rats, obese yellow mice, and obese hyperglycemic mice with body protein used for metabolic mass. Int J Obes. 1981;5(1):51–56. [PubMed] [Google Scholar]
- Levin B. E., Triscari J., Sullivan A. C. Abnormal sympatho- adrenal function and plasma catecholamines in obese Zucker rats. Pharmacol Biochem Behav. 1980 Jul;13(1):107–113. doi: 10.1016/0091-3057(80)90128-8. [DOI] [PubMed] [Google Scholar]
- PUGH L. G., EDHOLM O. G. The physiology of channel swimmers. Lancet. 1955 Oct 8;269(6893):761–768. doi: 10.1016/s0140-6736(55)92454-5. [DOI] [PubMed] [Google Scholar]
- Pullar J. D., Webster A. J. Heat loss and energy retention during growth in congenitally obese and lean rats. Br J Nutr. 1974 May;31(3):377–392. doi: 10.1079/bjn19740046. [DOI] [PubMed] [Google Scholar]
- QUAADE F. INSULATION IN LEANNESS AND OBESITY. Lancet. 1963 Aug 31;2(7305):429–432. doi: 10.1016/s0140-6736(63)92172-x. [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Stock M. J. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979 Sep 6;281(5726):31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- Thurlby P. L., Trayhurn P. Regional blood flow in genetically obese (ob/ob) mice. The importance of brown adipose tissue to the reduced energy expenditure on non-shivering thermogenesis. Pflugers Arch. 1980 Jun;385(3):193–201. doi: 10.1007/BF00647457. [DOI] [PubMed] [Google Scholar]
- Thurlby P. L., Trayhurn P. The role of thermoregulatory thermogenesis in the development of obesity in genetically-obese (ob/ob) mice pair-fed with lean siblings. Br J Nutr. 1979 Nov;42(3):377–385. doi: 10.1079/bjn19790127. [DOI] [PubMed] [Google Scholar]
- Trayhurn P., James W. P. Thermoregulation and non-shivering thermogenesis in the genetically obese (ob/ob) mouse. Pflugers Arch. 1978 Feb 22;373(2):189–193. doi: 10.1007/BF00584859. [DOI] [PubMed] [Google Scholar]
- Trayhurn P., Thurlby P. L., James W. P. A defective response to cold in the obese (obob) mouse and the obese Zucker (fafa) rat [proceedings]. Proc Nutr Soc. 1976 Dec;35(3):133A–133A. [PubMed] [Google Scholar]
- Triandafillou J., Himms-Hagen J. Brown adipose tissue in genetically obese (fa/fa) rats: response to cold and diet. Am J Physiol. 1983 Feb;244(2):E145–E150. doi: 10.1152/ajpendo.1983.244.2.E145. [DOI] [PubMed] [Google Scholar]
- Wickler S. J., Horwitz B. A., Stern J. S. Regional blood flow in genetically--obese rats during nonshivering thermogenesis. Int J Obes. 1982;6(5):481–490. [PubMed] [Google Scholar]
- York D. A., Hershman J. M., Utiger R. D., Bray G. A. Thyrotropin secretion in genetically obese rats. Endocrinology. 1972 Jan;90(1):67–72. doi: 10.1210/endo-90-1-67. [DOI] [PubMed] [Google Scholar]


