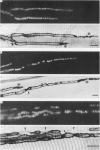
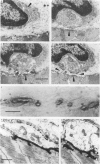
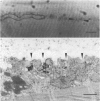
Abstract
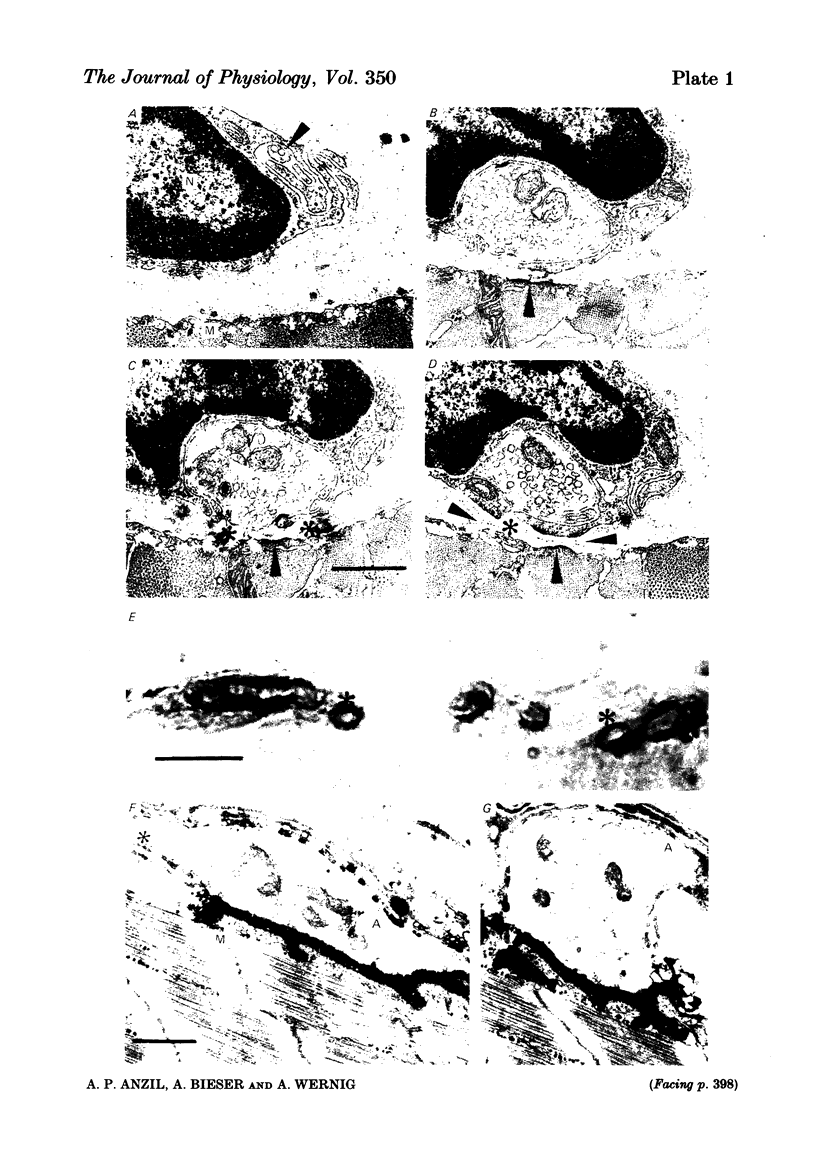
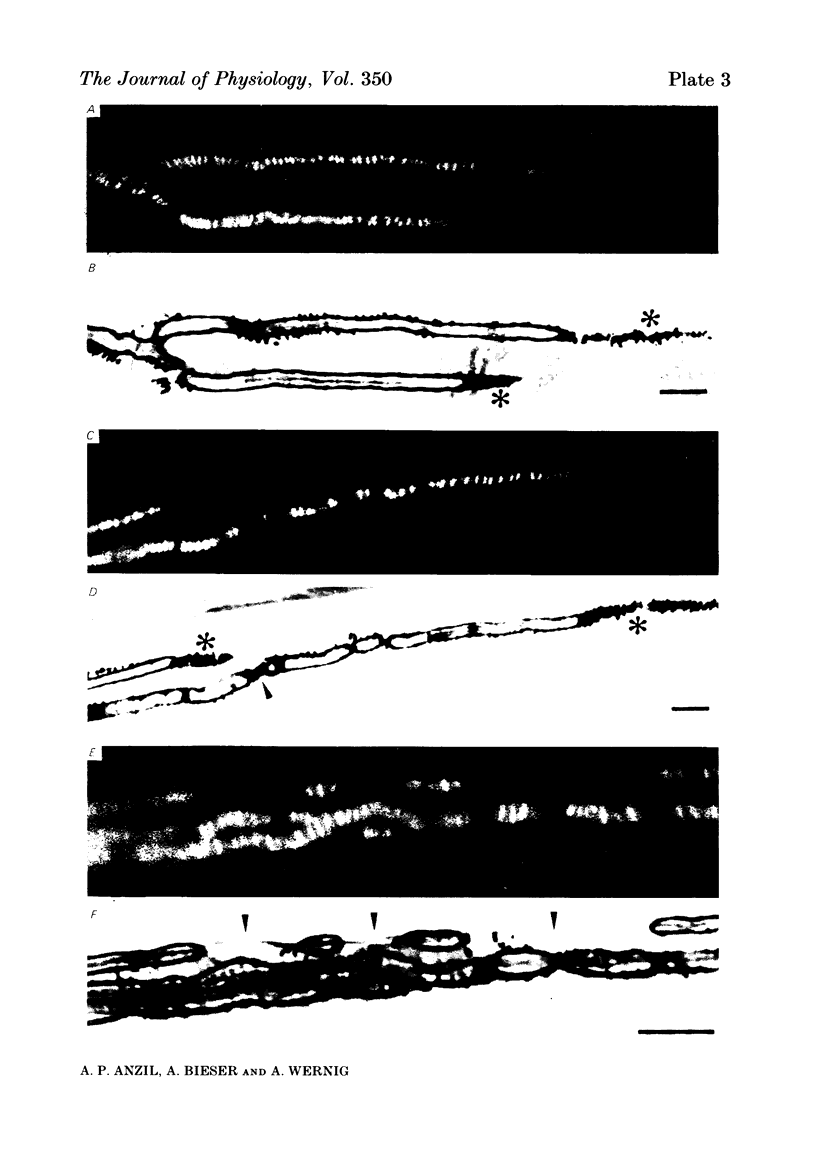
A combined light and electron microscopic study was performed on neuromuscular junctions of normal adult frogs. In a previous investigation signs of new synapse formation, as well as abandoned former synaptic sites, have been observed in normal muscles (Wernig, Pécot-Dechavassine & Stöver, 1980a, b). Here we performed a detailed light and electron microscopic correlation to investigate those parts of junctions which, after staining for cholinesterase (ChE) and presynaptic axon terminals, were suspected either to be newly formed or sites abandoned by the presynaptic nerve and the Schwann cell. Thin presynaptic nerve branches, enclosed by Schwann cell sheaths along most of their length, formed synaptic contacts with the muscle fibre only at small circumscribed areas. In these regions post-synaptic secondary folds (invariably present at mature synapses) were either missing or were less well developed. At these small contacts, binding sites for fluorescein-labelled alpha-bungarotoxin were usually present. At other sites the ChE reaction product was present but an axon could not be detected in silver-stained preparations. Electron microscopic observation revealed post-synaptic secondary folds filled with ChE reaction product while the presynaptic axon and Schwann cell were missing. The sites with ChE remnants can thus be regarded as abandoned former synaptic contacts. No binding of fluorescein-labelled alpha-bungarotoxin could be detected at such sites. These findings confirm earlier suggestions that synaptic contacts in frog muscle are normally undergoing continual remodelling. The lack of binding sites for fluorescein-labelled alpha-bungarotoxin at abandoned synaptic sites suggests that a neural or Schwann cell factor is important for the maintainance of synaptic acetylcholine receptors.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRKS R., KATZ B., MILEDI R. Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol. 1960 Jan;150:145–168. doi: 10.1113/jphysiol.1960.sp006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieser A., Wernig A., Zucker H. Different quantal responses within single frog neuromuscular junctions. J Physiol. 1984 May;350:401–412. doi: 10.1113/jphysiol.1984.sp015208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardasis C. A., Padykula H. A. Ultrastructural evidence indicating reorganization at the neuromuscular junction in the normal rat soleus muscle. Anat Rec. 1981 May;200(1):41–59. doi: 10.1002/ar.1092000105. [DOI] [PubMed] [Google Scholar]
- Dreyer F., Peper K. The spread of acetylcholine sensitivity after denervation of frog skeletal muscle fibers. Pflugers Arch. 1974 May 6;348(4):287–292. doi: 10.1007/BF00589218. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kullberg R. W., Lentz T. L., Cohen M. W. Development of the myotomal neuromuscular junction in Xenopus laevis: an electrophysiological and fine-structural study. Dev Biol. 1977 Oct 1;60(1):101–129. doi: 10.1016/0012-1606(77)90113-0. [DOI] [PubMed] [Google Scholar]
- Letinsky M. S., Decino P. A. Histological staining of pre- and postsynaptic components of amphibian neuromuscular junctions. J Neurocytol. 1980 Jun;9(3):305–320. doi: 10.1007/BF01181539. [DOI] [PubMed] [Google Scholar]
- Letinsky M. S., Fischbeck K. H., McMahan U. J. Precision of reinnervation of original postsynaptic sites in frog muscle after a nerve crush. J Neurocytol. 1976 Dec;5(6):691–718. doi: 10.1007/BF01181582. [DOI] [PubMed] [Google Scholar]
- Matthews-Bellinger J., Salpeter M. M. Distribution of acetylcholine receptors at frog neuromuscular junctions with a discussion of some physiological implications. J Physiol. 1978 Jun;279:197–213. doi: 10.1113/jphysiol.1978.sp012340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudell B. M., Grinnell A. D. Inverse relationship between transmitter release and terminal length in synapses on frog muscle fibers of uniform input resistance. J Neurosci. 1982 Feb;2(2):216–224. doi: 10.1523/JNEUROSCI.02-02-00216.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suszkiw J. B., Ichiki M. Fluorescein conjugated alpha-bungarotoxin: its properties and interaction with acetylcholine receptors. Anal Biochem. 1976 May 21;73(1):109–114. doi: 10.1016/0003-2697(76)90146-9. [DOI] [PubMed] [Google Scholar]
- Verma V. Degenerative changes in the frog neuromuscular junction after long durations of denervation. Cytologia (Tokyo) 1980 Dec;45(4):605–614. doi: 10.1508/cytologia.45.605. [DOI] [PubMed] [Google Scholar]
- Wernig A., Anzil A. P., Bieser A. Light and electron microscopic identification of a nerve sprout in muscle of normal adult frog. Neurosci Lett. 1981 Feb 6;21(3):261–266. doi: 10.1016/0304-3940(81)90214-7. [DOI] [PubMed] [Google Scholar]
- Wernig A., Anzil A. P., Bieser A., Schwarz U. Abandoned synaptic sites in muscles of normal adult frog. Neurosci Lett. 1981 May 6;23(2):105–110. doi: 10.1016/0304-3940(81)90025-2. [DOI] [PubMed] [Google Scholar]
- Wernig A., Pécot-Dechavassine M., Stover H. Sprouting and regression of the nerve at the frog neuromuscular junction in normal conditions and after prolonged paralysis with curare. J Neurocytol. 1980 Jun;9(3):278–303. doi: 10.1007/BF01181538. [DOI] [PubMed] [Google Scholar]