Abstract
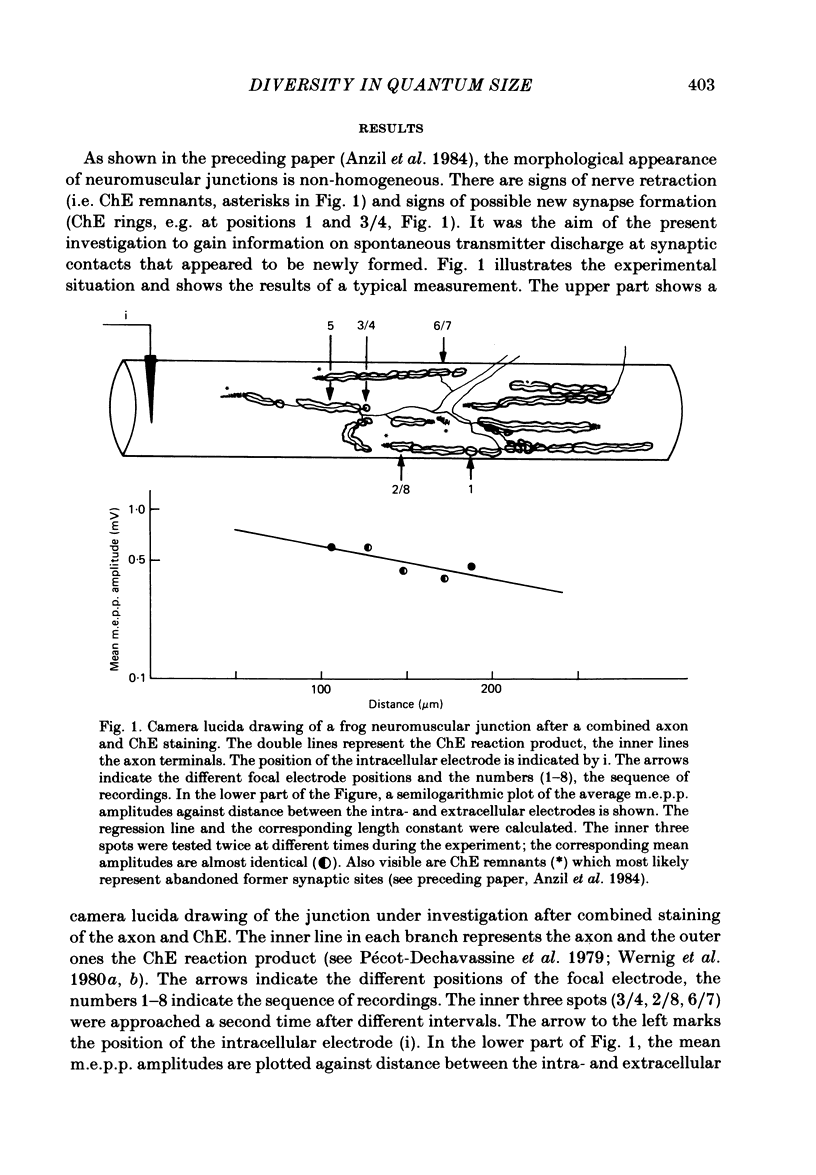
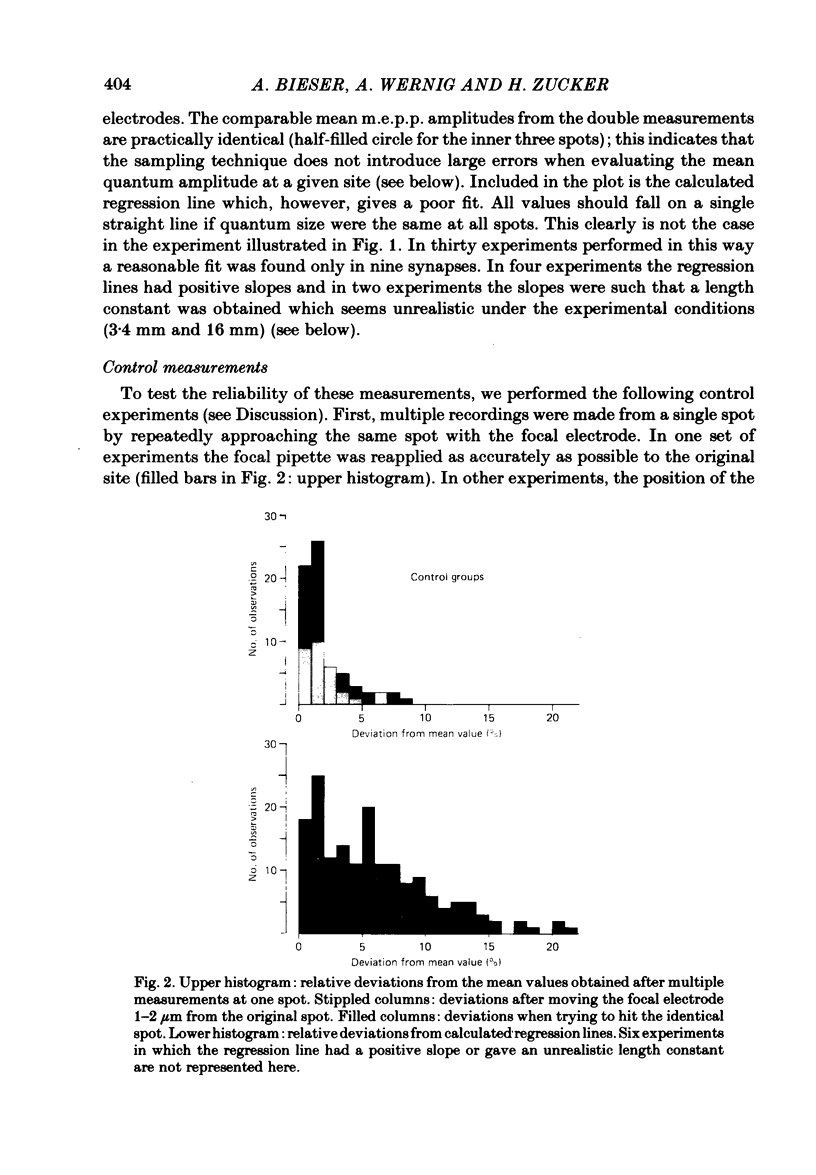
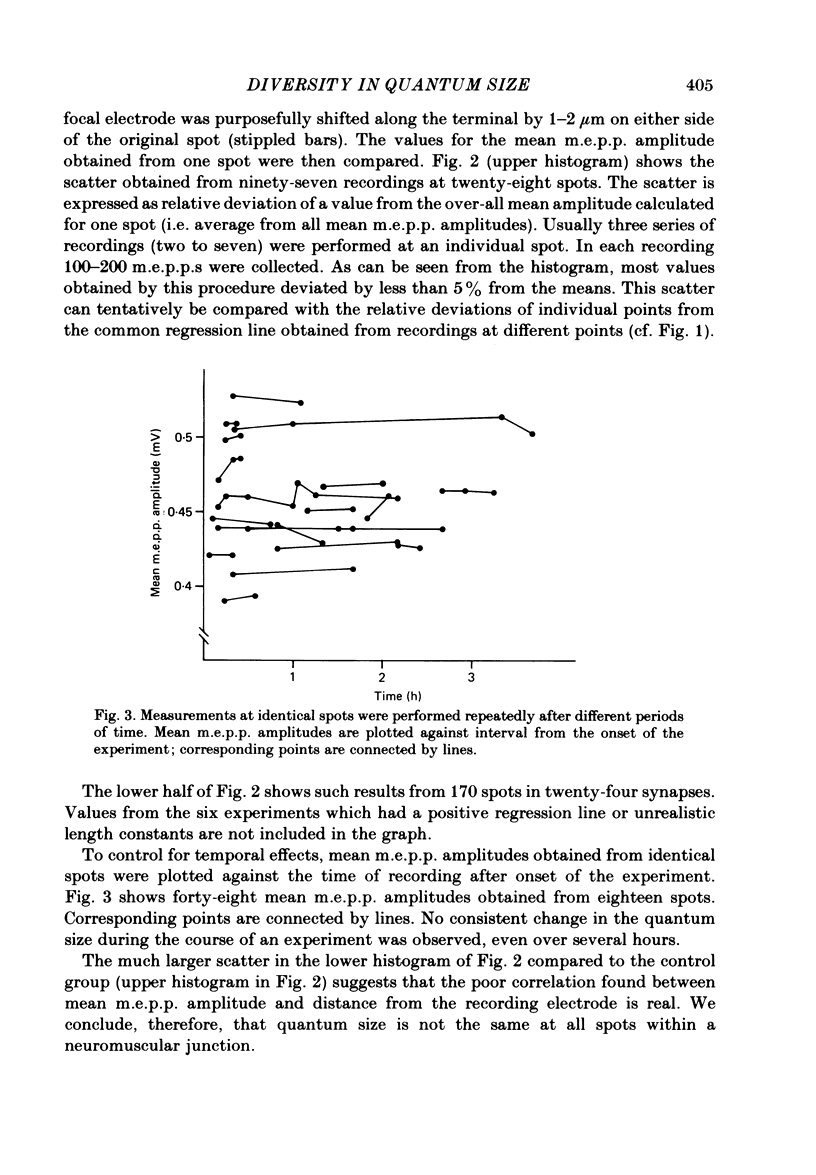
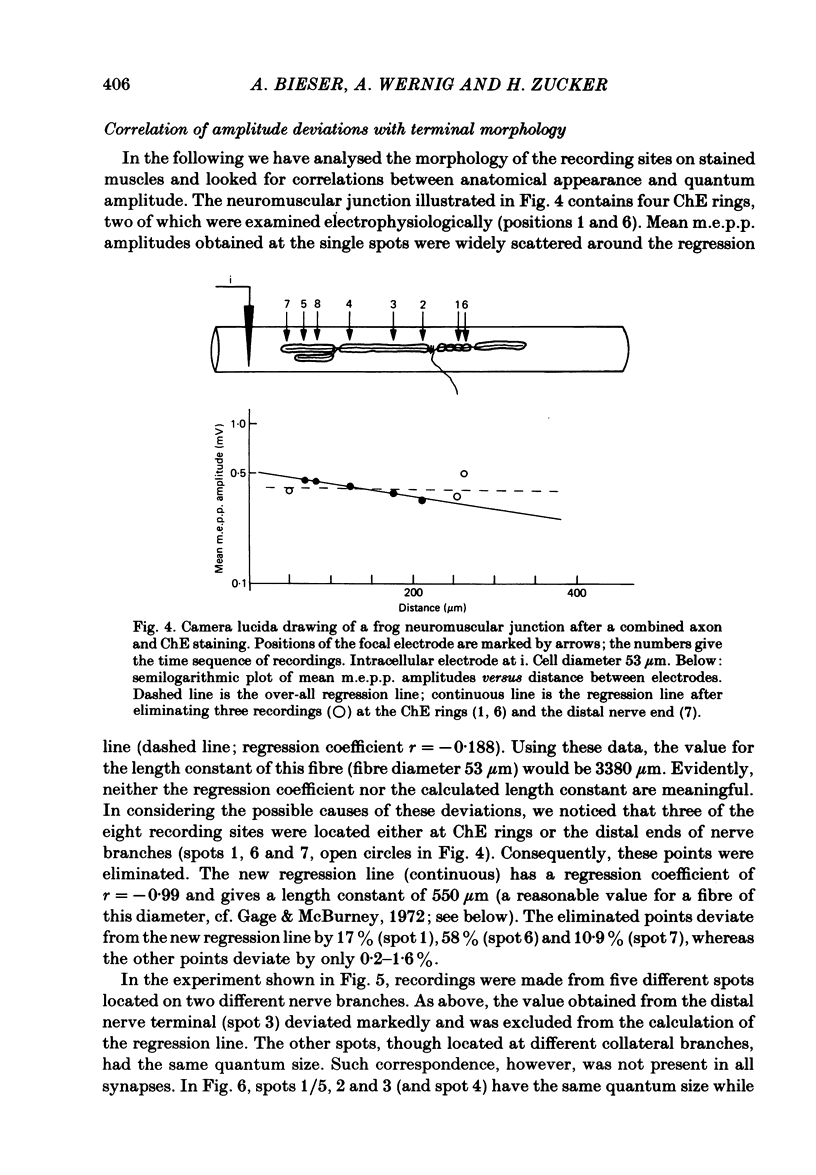
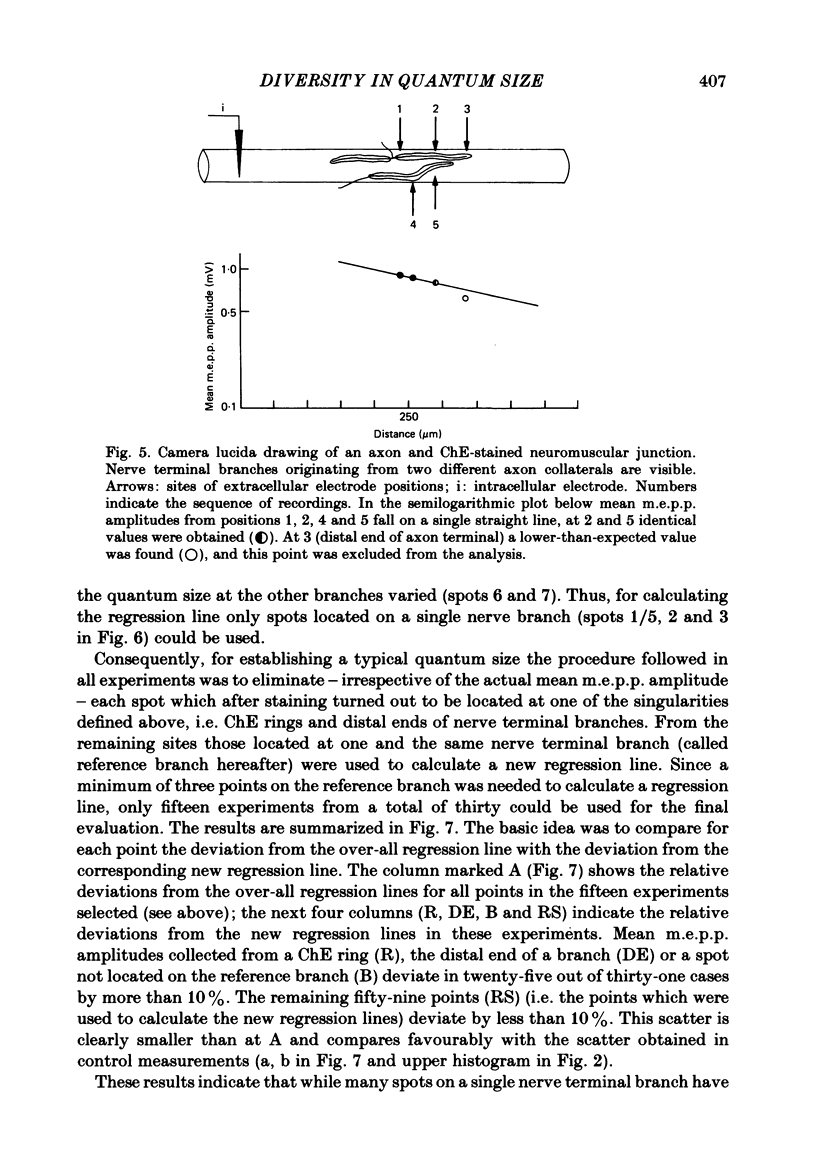
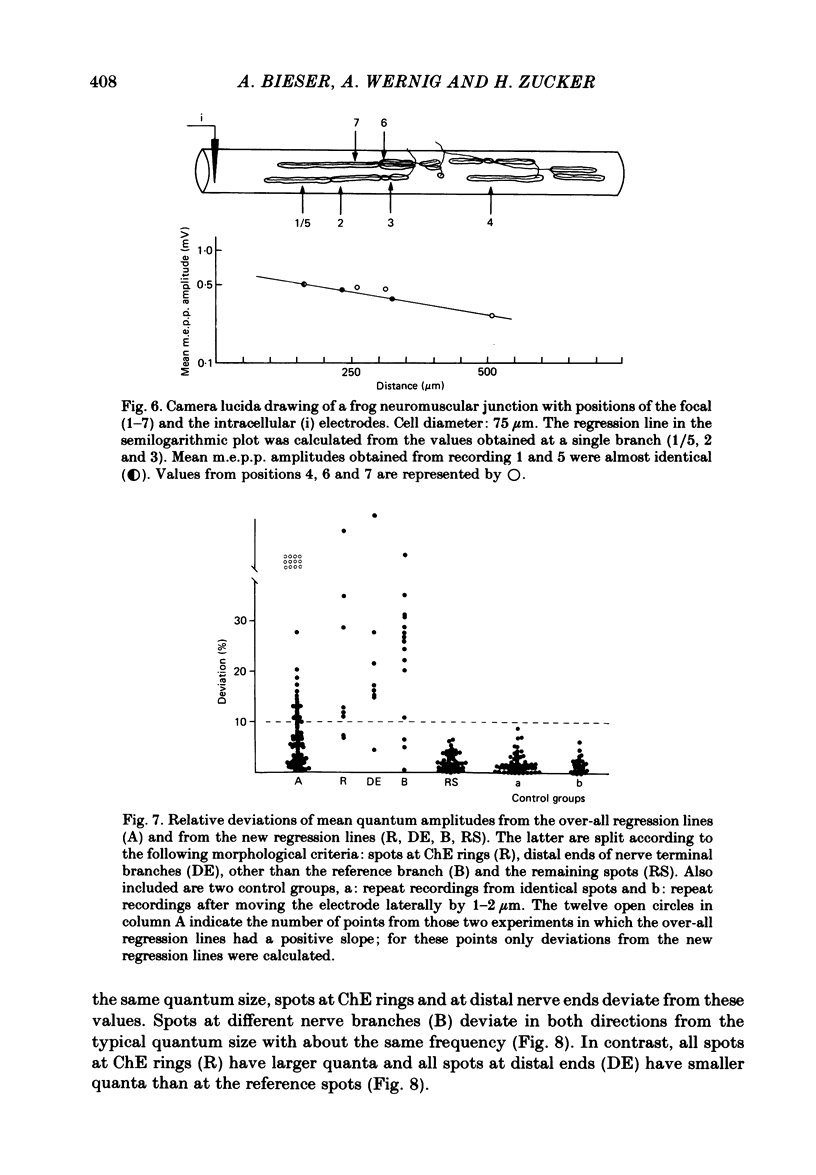
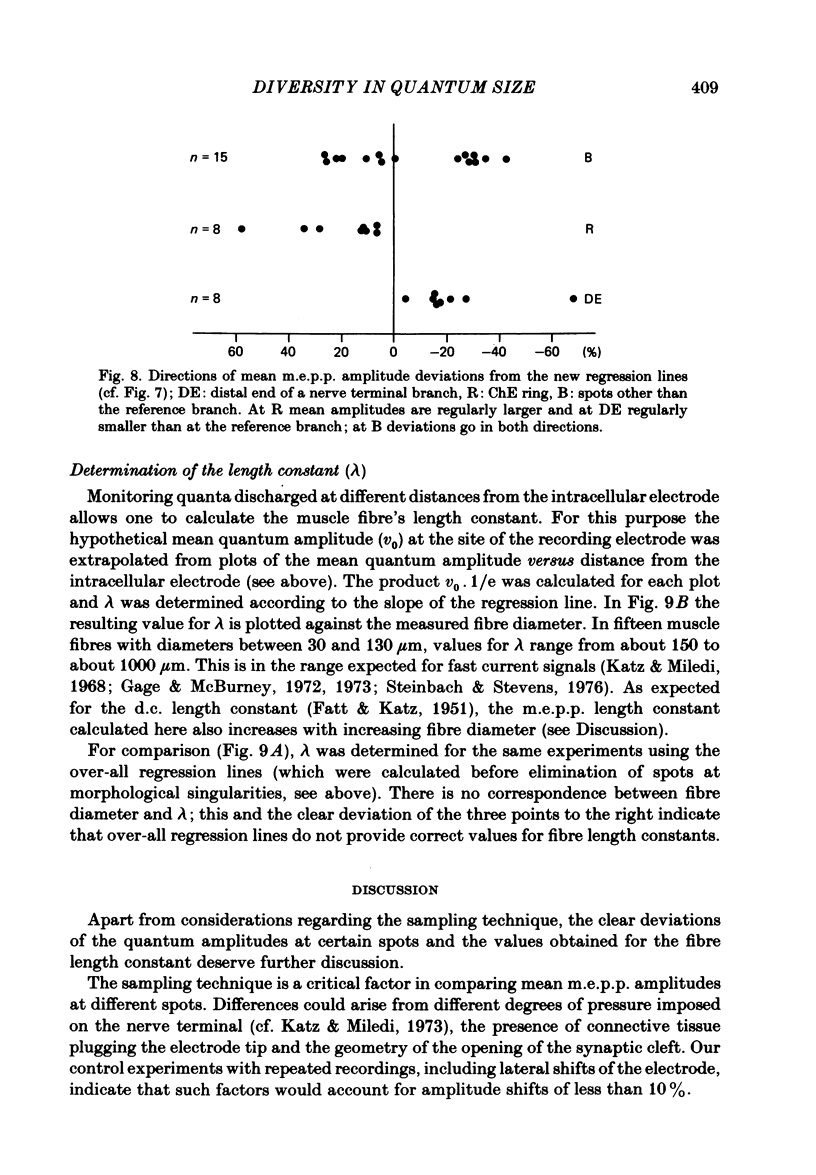
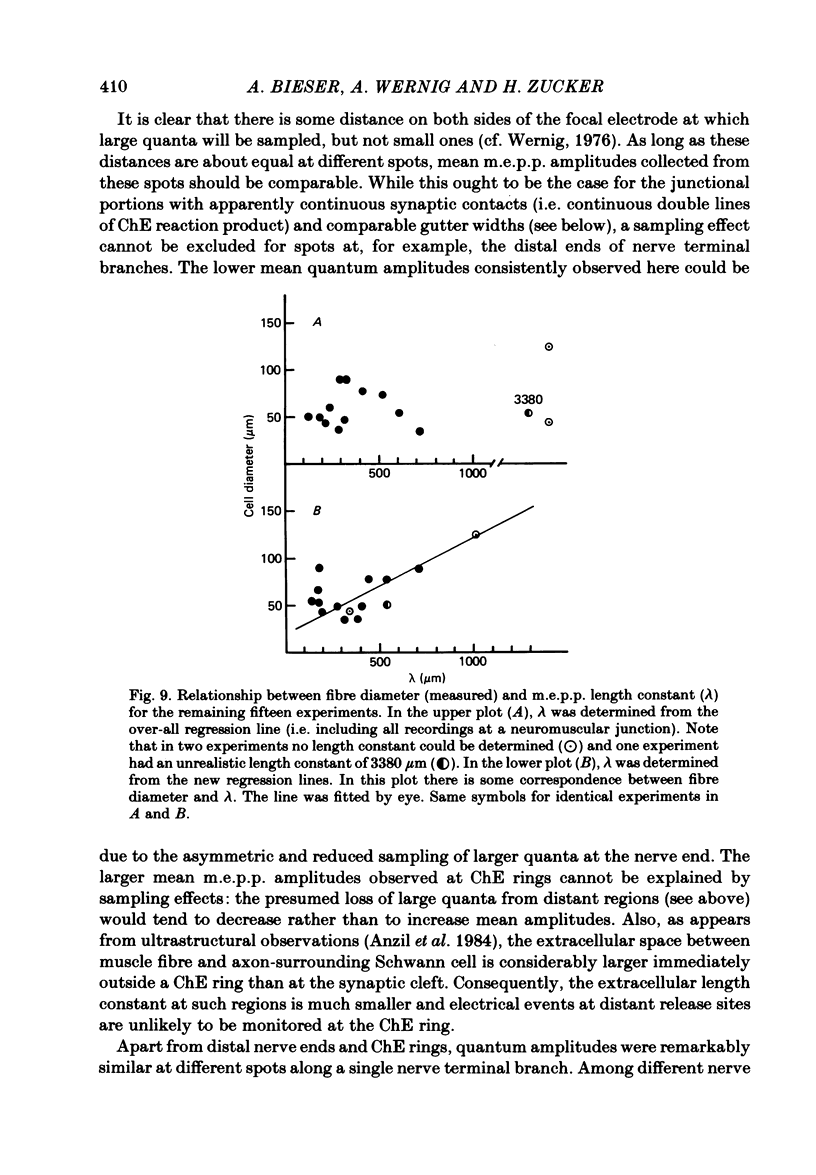
At frog neuromuscular junctions spontaneous miniature end-plate potentials (m.e.p.p.s) were recorded from several isolated spots within single synapses. This was done by consecutively placing an extracellular glass micro-electrode (focal electrode) at different recording sites, while the intracellular electrode remained in one place. After each set of recordings, muscles were stained to reveal both axon terminals and cholinesterase (ChE) such that the exact position of each recording site could be determined. In many nerve terminal branches a similar quantum size was found at several different spots. In other instances, however, mean quantum amplitudes varied by 10-60% at different spots along the same terminal branches. As a control, individual spots were recorded from repeatedly after repositioning the focal electrode. In these recordings mean m.e.p.p. amplitude varied by only 5-10%. It is concluded that quantum size within a single junction is similar at many spots, but deviates markedly at others. Correlation of this variation with the stained preparations suggested that spots where quanta significantly larger or smaller than normal were recorded were either at ChE rings or at the distal ends of nerve branches, respectively; at different nerve terminal branches within the same junction, quantum amplitudes were similar in many cases but deviated in others. The results are consistent with ultrastructural evidence that frog neuromuscular junctions are non-homogeneous structures which undergo continual remodelling.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anzil A. P., Bieser A., Wernig A. Light and electron microscopic identification of nerve terminal sprouting and retraction in normal adult frog muscle. J Physiol. 1984 May;350:393–399. doi: 10.1113/jphysiol.1984.sp015207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. An analysis of the relationship between the current and potential generated by a quantum of acetylcholine in muscle fibers without transverse tubules. J Membr Biol. 1973;12(3):247–272. doi: 10.1007/BF01870004. [DOI] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Miniature end-plate currents and potentials generated by quanta of acetylcholine in glycerol-treated toad sartorius fibres. J Physiol. 1972 Oct;226(1):79–94. doi: 10.1113/jphysiol.1972.sp009974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The binding of acetylcholine to receptors and its removal from the synaptic cleft. J Physiol. 1973 Jun;231(3):549–574. doi: 10.1113/jphysiol.1973.sp010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The effect of local blockage of motor nerve terminals. J Physiol. 1968 Dec;199(3):729–741. doi: 10.1113/jphysiol.1968.sp008675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M., Martin A. R. Non-linear summation of end-plate potentials in the frog and mouse. J Physiol. 1981 Feb;311:307–324. doi: 10.1113/jphysiol.1981.sp013586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecot-Dechavassine M., Wernig A., Stöver H. A combined silver and cholinesterase method for studying exact relations between the pre- and the postsynaptic elements at the frog neuromuscular junction. Stain Technol. 1979 Jan;54(1):25–28. doi: 10.3109/10520297909110671. [DOI] [PubMed] [Google Scholar]
- Wernig A. Localization of active sites in the neuromuscular junction of the frog. Brain Res. 1976 Dec 10;118(1):63–72. doi: 10.1016/0006-8993(76)90841-6. [DOI] [PubMed] [Google Scholar]
- Wernig A., Pécot-Dechavassine M., Stover H. Sprouting and regression of the nerve at the frog neuromuscular junction in normal conditions and after prolonged paralysis with curare. J Neurocytol. 1980 Jun;9(3):278–303. doi: 10.1007/BF01181538. [DOI] [PubMed] [Google Scholar]


