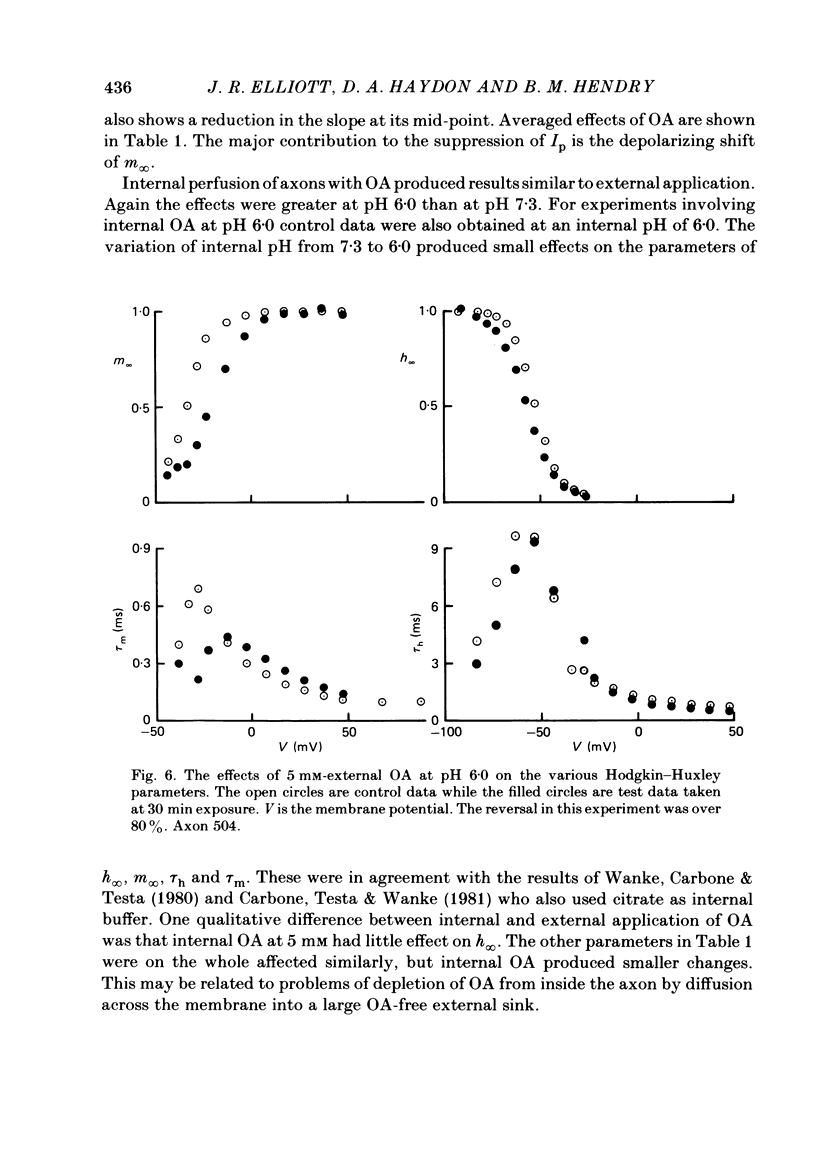
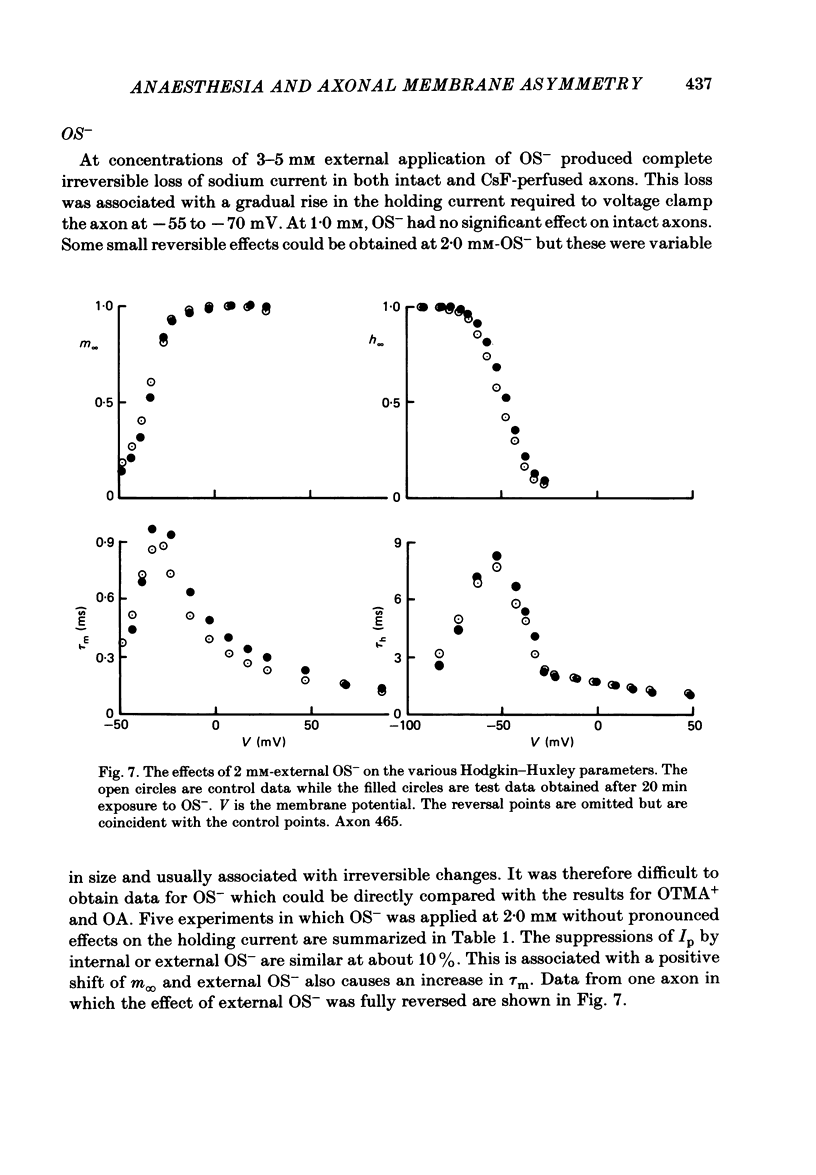
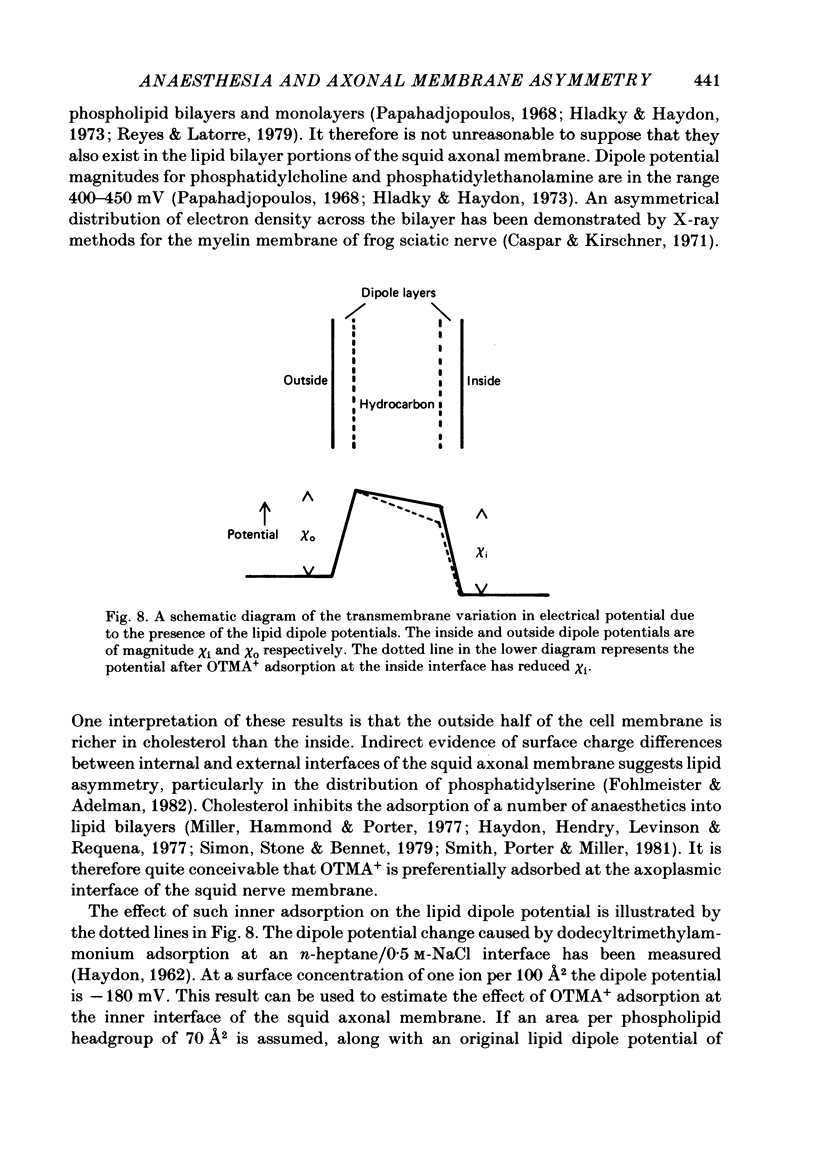
Abstract
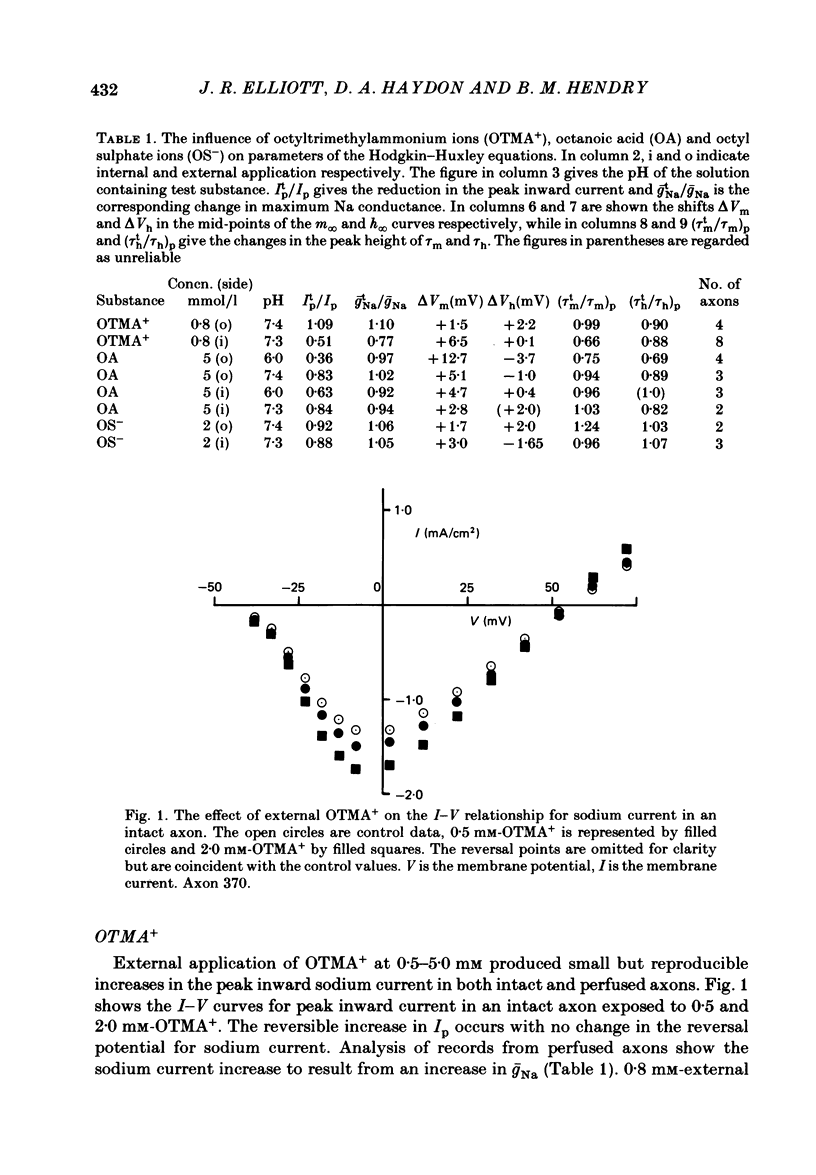
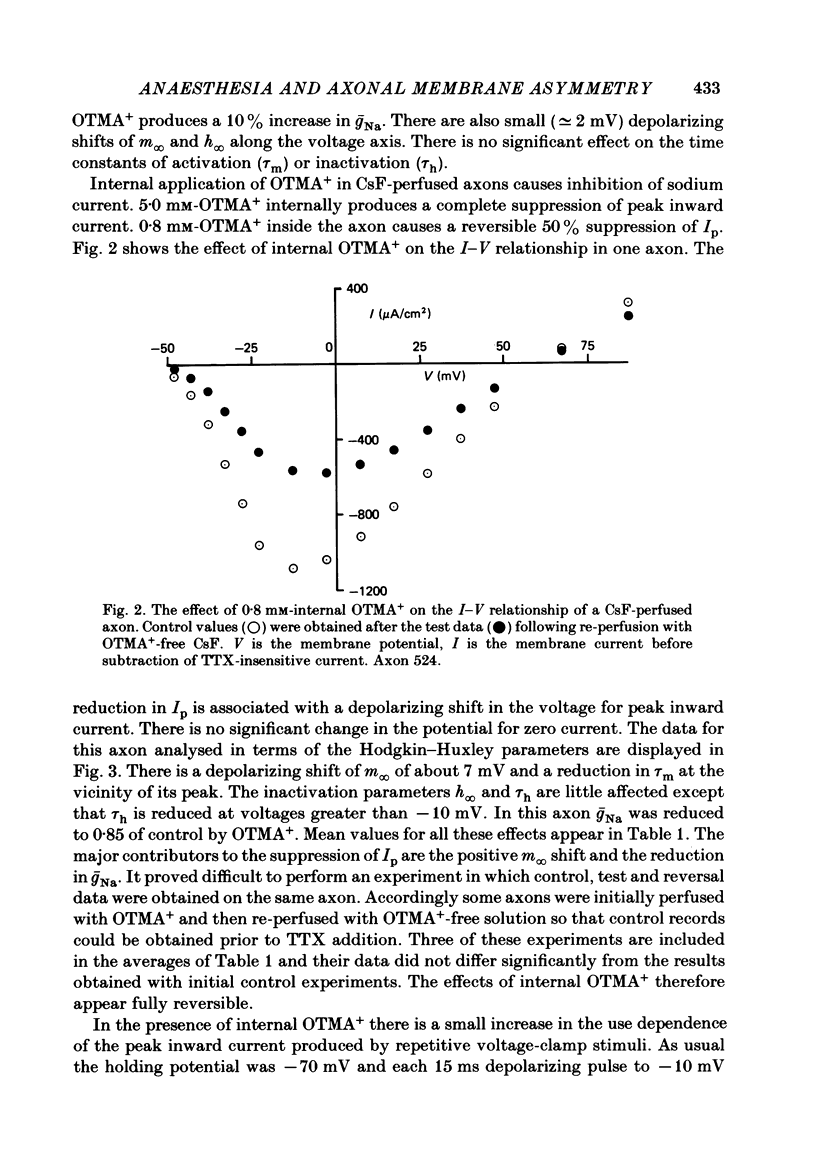
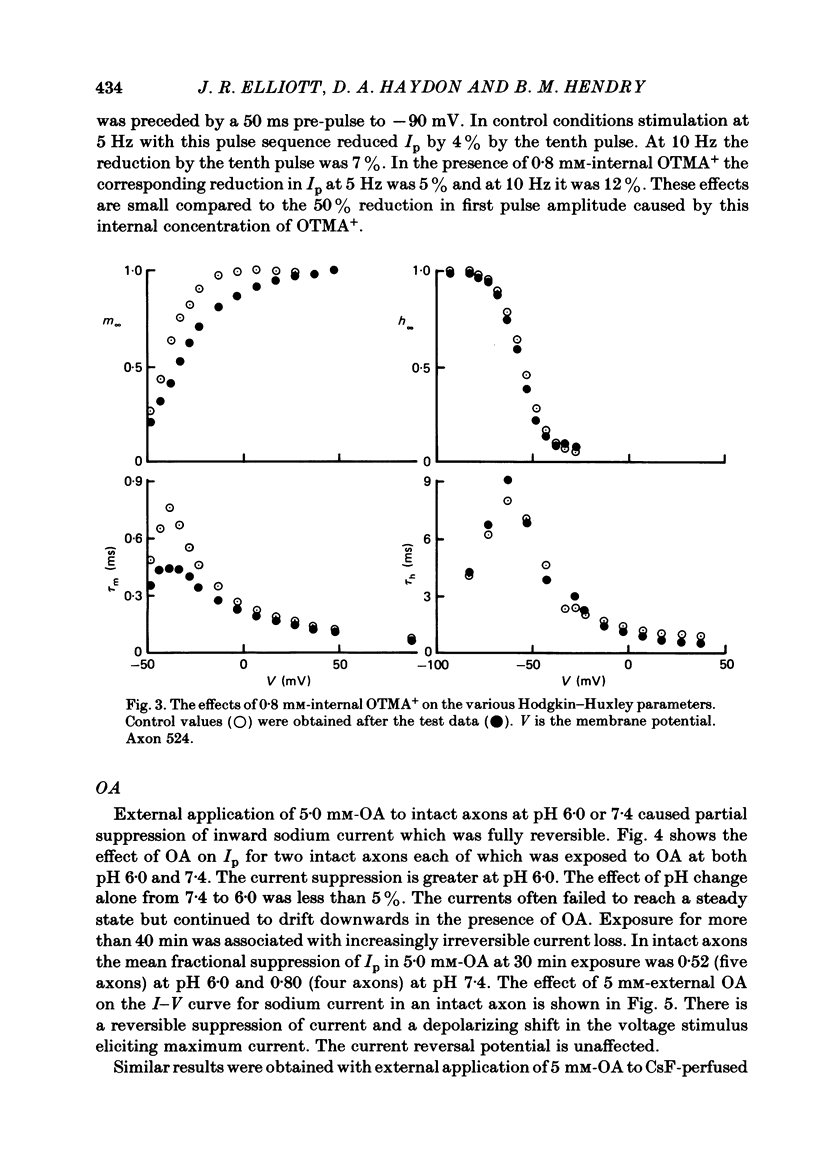
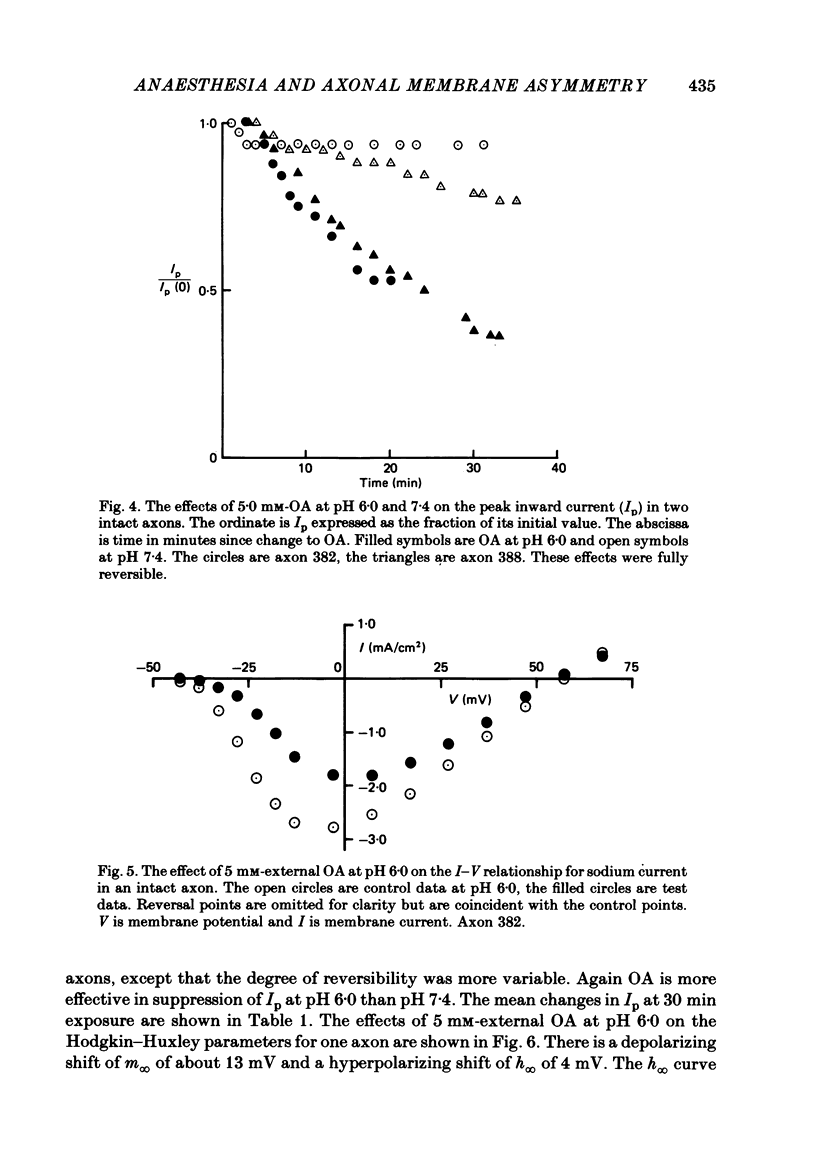
The effects of octyltrimethylammonium ions (OTMA+), octyl sulphate ions (OS-) and octanoic acid (OA) on the sodium current of the voltage-clamped squid giant axon have been investigated using intracellular and extracellular application of the test substances. OTMA+ applied externally at concentrations of 0.8-5.0 mM produces a small reversible increase in the peak inward sodium current in both intact and CsF-perfused axons. Intracellular application of OTMA+ at 0.8 mM to CsF-perfused axons causes a reversible 50% suppression of peak inward sodium current. The inhibition of peak inward current by internal OTMA+ arises largely from a shift of the steady-state activation parameter (m infinity) in the depolarizing direction along the voltage axis. There is little use dependence of the current suppression by OTMA+ OA applied either internally or externally is more effective at suppressing peak inward sodium current at pH 6.0 than at pH 7.4. At pH 6.0 external application of 5 mM-OA to perfused axons causes approximately 60% suppression. This is associated with a depolarizing shift of m infinity of about 13 mV and a hyperpolarizing shift of the steady-state inactivation (h infinity) curve of about 4 mV. The effects of internal and external OA are broadly similar except that the h infinity shift is not seen with internal application. OS- at concentrations above 2.0 mM produces complete irreversible loss of sodium current. At 2.0 mM, OS- produces 10% current suppression and a small depolarizing shift of the m infinity curve. Internal and external applications of OS- differ little except that external OS- causes a 25% increase in the time constant of activation (tau m). The possible origins of these effects are discussed. It is proposed that the shift of m infinity caused by internal OTMA+ is due to a diminution of the lipid dipole potential at the internal surface of the membrane caused by OTMA+ adsorption. This effect could also account for the m infinity shift caused by OA. The results showing that OA produces shifts of opposite sign in the voltage dependence of m infinity and h infinity are discussed with respect to their implications for models of sodium channel gating.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER P. F., HODGKIN A. L., MEVES H. THE EFFECT OF DILUTING THE INTERNAL SOLUTION ON THE ELECTRICAL PROPERTIES OF A PERFUSED GIANT AXON. J Physiol. 1964 Apr;170:541–560. doi: 10.1113/jphysiol.1964.sp007348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Shrager P., Goldstein D. A. Modification of sodium and potassium channel gating kinetics by ether and halothane. J Gen Physiol. 1981 Mar;77(3):233–253. doi: 10.1085/jgp.77.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalan M. D. Local anesthetic block of sodium channels in normal and pronase-treated squid giant axons. Biophys J. 1978 Aug;23(2):285–311. doi: 10.1016/S0006-3495(78)85449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Testa P. L., Wanke E. Intracellular pH and ionic channels in the Loligo vulgaris giant axon. Biophys J. 1981 Aug;35(2):393–413. doi: 10.1016/S0006-3495(81)84798-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspar D. L., Kirschner D. A. Myelin membrane structure at 10 A resolution. Nat New Biol. 1971 May 12;231(19):46–52. doi: 10.1038/newbio231046a0. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J. R., Haydon D. A. The interaction of n-octanol with black lipid bilayer membranes. Biochim Biophys Acta. 1979 Oct 19;557(1):259–263. doi: 10.1016/0005-2736(79)90108-1. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert D. L., Ehrenstein G. Effect of divalent cations on potassium conductance of squid axons: determination of surface charge. Biophys J. 1969 Mar;9(3):447–463. doi: 10.1016/S0006-3495(69)86396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. I., Meves H. The effect of external potassium on the removal of sodium inactivation in squid giant axons. J Physiol. 1981 Jun;315:493–514. doi: 10.1113/jphysiol.1981.sp013760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A. Functions of the lipid in bilayer ion permeability. Ann N Y Acad Sci. 1975 Dec 30;264:2–16. doi: 10.1111/j.1749-6632.1975.tb31472.x. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M., Levinson S. R., Requena J. Anaesthesia by the n-alkanes. A comparative study of nerve impulse blockage and the properties of black lipid bilayer membranes. Biochim Biophys Acta. 1977 Oct 3;470(1):17–34. doi: 10.1016/0005-2736(77)90058-x. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Kimura J. E. Some effects of n-pentane on the sodium and potassium currents of the squid giant axon. J Physiol. 1981 Mar;312:57–70. doi: 10.1113/jphysiol.1981.sp013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Requena J., Urban B. W. Some effects of aliphatic hydrocarbons on the electrical capacity and ionic currents of the squid giant axon membrane. J Physiol. 1980 Dec;309:229–245. doi: 10.1113/jphysiol.1980.sp013506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of alcohols and other non-ionic surface active substances on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:411–427. doi: 10.1113/jphysiol.1983.sp014813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The action of hydrocarbons and carbon tetrachloride on the sodium current of the squid giant axon. J Physiol. 1983 May;338:435–450. doi: 10.1113/jphysiol.1983.sp014682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Urban B. W. The effects of some inhalation anaesthetics on the sodium current of the squid giant axon. J Physiol. 1983 Aug;341:429–439. doi: 10.1113/jphysiol.1983.sp014814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977 Apr;69(4):497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J. E., Meves H. The effect of temperature on the asymmetrical charge movement in squid giant axons. J Physiol. 1979 Apr;289:479–500. doi: 10.1113/jphysiol.1979.sp012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papahadjopoulos D. Surface properties of acidic phospholipids: interaction of monolayers and hydrated liquid crystals with uni- and bi-valent metal ions. Biochim Biophys Acta. 1968 Sep 17;163(2):240–254. doi: 10.1016/0005-2736(68)90103-x. [DOI] [PubMed] [Google Scholar]
- Reyes J., Latorre R. Effect of the anesthetics benzyl alcohol and chloroform on bilayers made from monolayers. Biophys J. 1979 Nov;28(2):259–279. doi: 10.1016/S0006-3495(79)85175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Rudy B. Destruction of the sodium conductance inactivation by a specific protease in perfused nerve fibres from Loligo. J Physiol. 1976 Nov;262(2):501–531. doi: 10.1113/jphysiol.1976.sp011608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., Stone W. L., Bennett P. B. Can regular solution theory be applied to lipid bilayer membranes? Biochim Biophys Acta. 1979 Jan 5;550(1):38–47. doi: 10.1016/0005-2736(79)90113-5. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Porter E. G., Miller K. W. The solubility of anesthetic gases in lipid bilayers. Biochim Biophys Acta. 1981 Jul 20;645(2):327–338. doi: 10.1016/0005-2736(81)90204-2. [DOI] [PubMed] [Google Scholar]
- Strichartz G. R. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J Gen Physiol. 1973 Jul;62(1):37–57. doi: 10.1085/jgp.62.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G., Eisenman G., McLaughlin S. G., Krasne S. Ionic probes of membrane structures. Ann N Y Acad Sci. 1972 Jun 20;195:273–290. [PubMed] [Google Scholar]
- Wanke E., Carbone E., Testa P. L. The sodium channel and intracellular H+ blockage in squid axons. Nature. 1980 Sep 4;287(5777):62–63. doi: 10.1038/287062a0. [DOI] [PubMed] [Google Scholar]


