Abstract
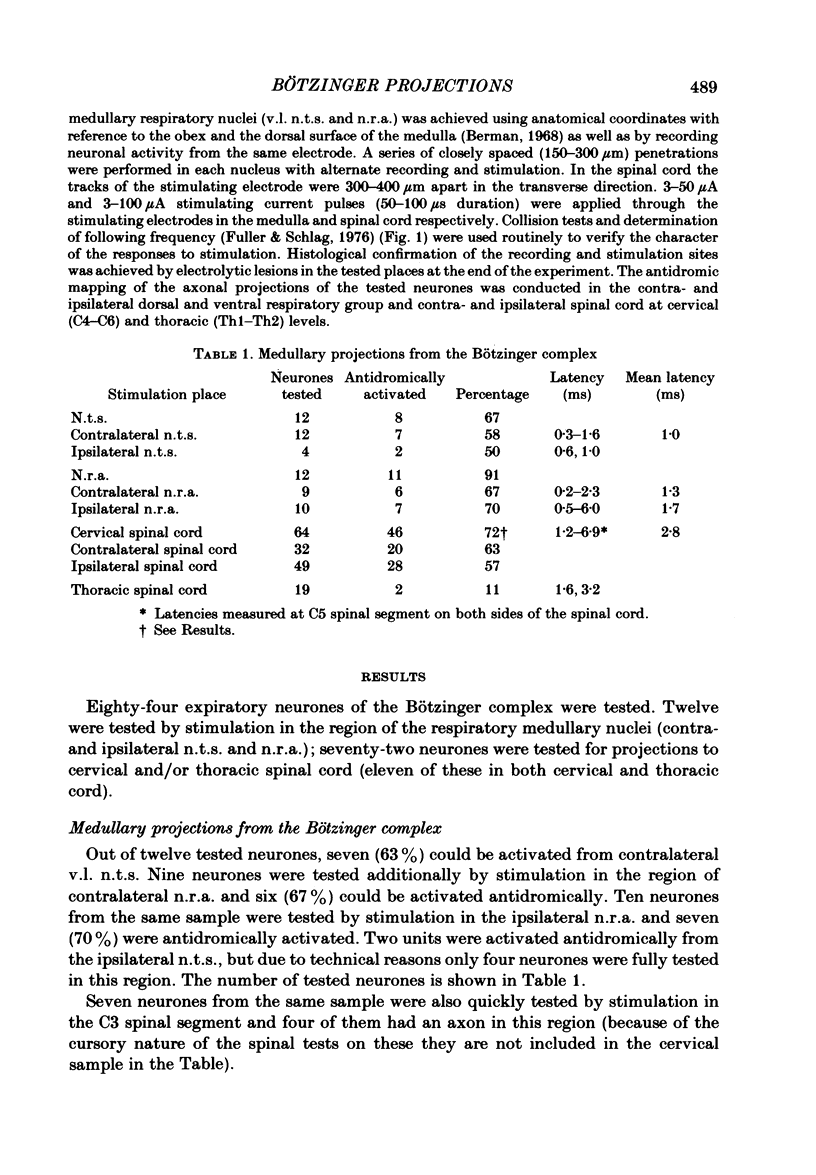
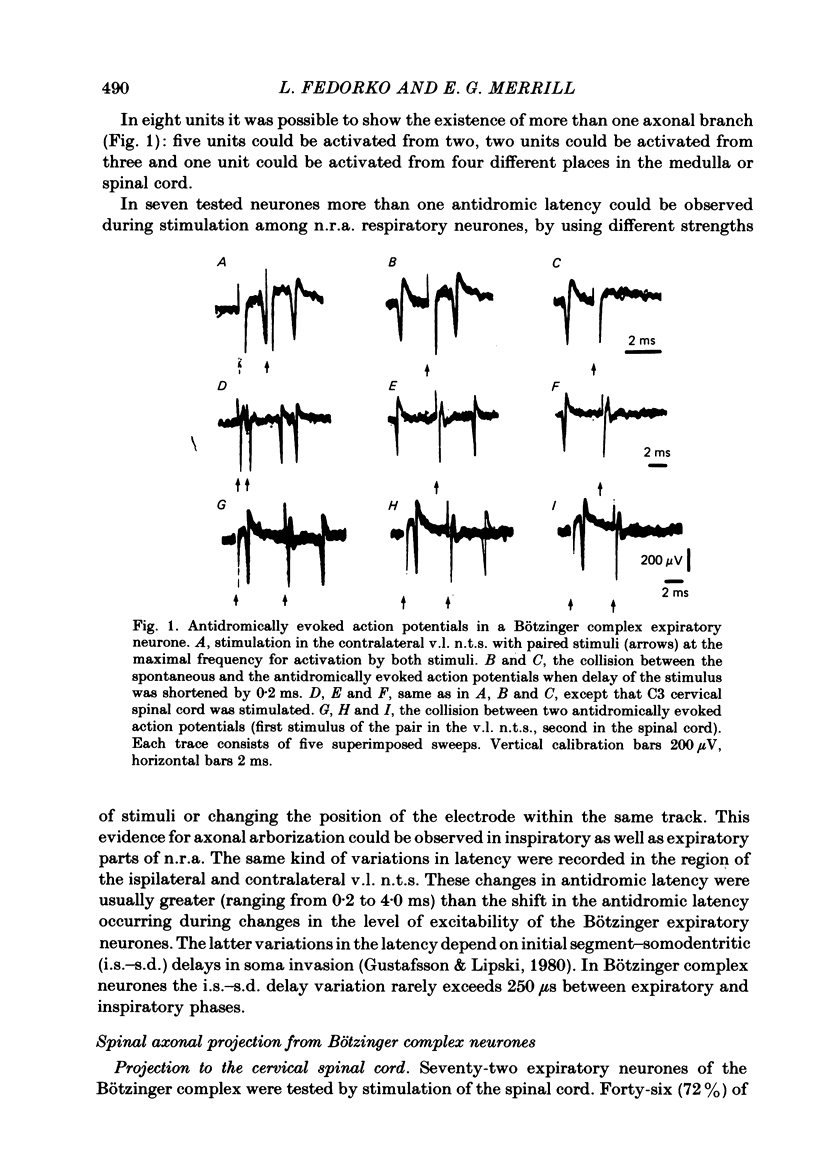
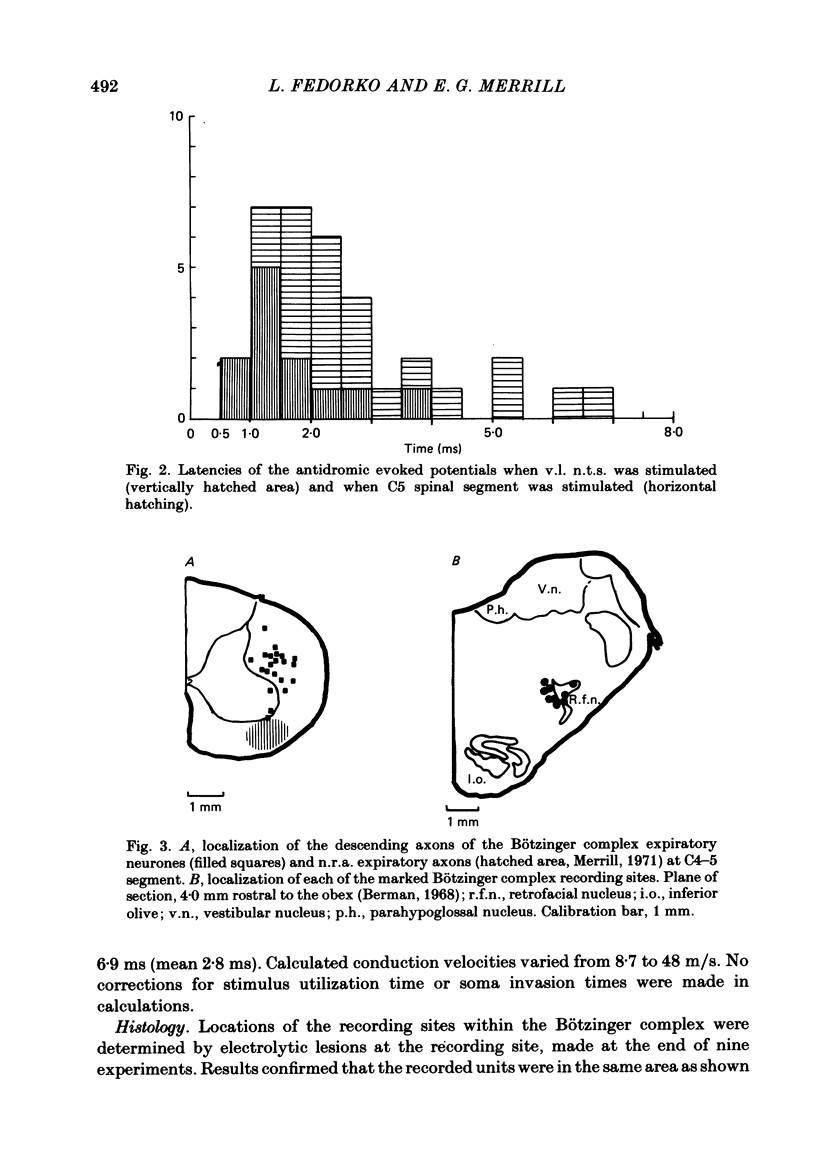
Axonal projections of eighty-four rostral medullary expiratory neurones of the Bötzinger complex were tested using antidromic mapping techniques in anaesthetized cats. A projection to the ventral respiratory neurones of the medulla (n.r.a.) was shown in eleven out of twelve tested neurones. Also a spinal projection to the C5-C6 cervical segments was evident in more than 72% of tested neurones; probably near 100% project to cervical cord. These axonal projections were found bilaterally in both brain stem and spinal cord. The majority of Bötzinger complex expiratory neurones were seen to have two to four axonal collaterals to the ventro-lateral (v.l.) nucleus of the solitary tract (n.t.s.) and/or the n.r.a. and/or the spinal cord. In eight out of twelve of the tested neurones, electrophysiological evidence of axonal arborization in more than one of n.r.a. inspiratory, n.r.a. expiratory or v.l. n.t.s. regions was obtained. Similar evidence for the terminal arborization was found for 26% of tested neurones in the phrenic motor nucleus. The descending spinal expiratory axons of the Bötzinger complex neurones are located in the dorsal and medial parts of the lateral funiculus in C4 and C5 segments. Conduction velocity measurements indicate that these are large myelinated axons. We propose that the Bötzinger complex expiratory neurones are a source of synaptic inhibition for n.r.a. inspiratory neurones and phrenic motoneurones.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger A. J. Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol. 1979 Jan;42(1 Pt 1):76–90. doi: 10.1152/jn.1979.42.1.76. [DOI] [PubMed] [Google Scholar]
- Bianchi A. L., Barillot J. C. Respiratory neurons in the region of the retrofacial nucleus: pontile, medullary, spinal and vagal projections. Neurosci Lett. 1982 Aug 31;31(3):277–282. doi: 10.1016/0304-3940(82)90033-7. [DOI] [PubMed] [Google Scholar]
- Bystrzycka E. K. Afferent projections to the dorsal and ventral respiratory nuclei in the medulla oblongata of the cat studied by the horseradish peroxidase technique. Brain Res. 1980 Mar 3;185(1):59–66. doi: 10.1016/0006-8993(80)90670-8. [DOI] [PubMed] [Google Scholar]
- Cameron W. E., Averill D. B., Berger A. J. Morphology of cat phrenic motoneurons as revealed by intracellular injection of horseradish peroxidase. J Comp Neurol. 1983 Sep 1;219(1):70–80. doi: 10.1002/cne.902190107. [DOI] [PubMed] [Google Scholar]
- Cohen M. I. Neurogenesis of respiratory rhythm in the mammal. Physiol Rev. 1979 Oct;59(4):1105–1173. doi: 10.1152/physrev.1979.59.4.1105. [DOI] [PubMed] [Google Scholar]
- Fuller J. H., Schlag J. D. Determination of antidromic excitation by the collision test: problems of interpretation. Brain Res. 1976 Aug 13;112(2):283–298. doi: 10.1016/0006-8993(76)90284-5. [DOI] [PubMed] [Google Scholar]
- GILL P. K., KUNO M. EXCITATORY AND INHIBITORY ACTIONS ON PHRENIC MOTONEURONES. J Physiol. 1963 Sep;168:274–289. doi: 10.1113/jphysiol.1963.sp007192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B., Lipski J. Effect of membrane polarization and synaptic activity on the timing of antidromic invasion. Brain Res. 1980 Jan 6;181(1):61–74. doi: 10.1016/0006-8993(80)91259-7. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. An electrophysiological demonstration of the axonal projections of single spinal interneurones in the cat. J Physiol. 1972 May;222(3):597–622. doi: 10.1113/jphysiol.1972.sp009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia M. P. Anatomical organization of central respiratory neurons. Annu Rev Physiol. 1981;43:105–120. doi: 10.1146/annurev.ph.43.030181.000541. [DOI] [PubMed] [Google Scholar]
- Kalia M., Feldman J. L., Cohen M. I. Afferent projections to the inspiratory neuronal region of the ventrolateral nucleus of the tractus solitarius in the cat. Brain Res. 1979 Jul 27;171(1):135–141. doi: 10.1016/0006-8993(79)90739-x. [DOI] [PubMed] [Google Scholar]
- Karczewski W. A., Gromysz H. The significance of species differences in respiratory neurophysiology-the split-brainstem preparation. Experientia. 1982 Jul 15;38(7):826–827. doi: 10.1007/BF01972295. [DOI] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Proceedings: Monosynaptic excitation of thoracic expiratory motoneurones from lateral respiratory neurones in the medulla of the cat. J Physiol. 1973 Oct;234(2):87P–89P. [PubMed] [Google Scholar]
- Lipski J., Merrill E. G. Electrophysiological demonstration of the projection from expiratory neurones in rostral medulla to contralateral dorsal respiratory group. Brain Res. 1980 Sep 22;197(2):521–524. doi: 10.1016/0006-8993(80)91140-3. [DOI] [PubMed] [Google Scholar]
- Merrill E. G., Lipski J., Kubin L., Fedorko L. Origin of the expiratory inhibition of nucleus tractus solitarius inspiratory neurones. Brain Res. 1983 Mar 14;263(1):43–50. doi: 10.1016/0006-8993(83)91198-8. [DOI] [PubMed] [Google Scholar]
- Merrill E. G. The descending pathways from the lateral respiratory neurones in cats. J Physiol. 1971 Oct;218 (Suppl):82P–83P. [PubMed] [Google Scholar]
- Merrill E. G. The lateral respiratory neurones of the medulla: their associations with nucleus ambiguus, nucleus retroambigualis, the spinal accessory nucleus and the spinal cord. Brain Res. 1970 Nov 11;24(1):11–28. doi: 10.1016/0006-8993(70)90271-4. [DOI] [PubMed] [Google Scholar]
- Richter D. W., Camerer H., Meesmann M., Röhrig N. Studies on the synaptic interconnection between bulbar respiratory neurones of cats. Pflugers Arch. 1979 Jul;380(3):245–257. doi: 10.1007/BF00582903. [DOI] [PubMed] [Google Scholar]
- SHAPIRO A., COHEN H. D. THE USE OF MERCURY CAPILLARY LENGTH GAUGES FOR THE MEASUREMENT OF THE VOLUME OF THORACIC AND DIAPHRAGMATIC COMPONENTS OF HUMAN RESPIRATION: A THEORETICAL ANALYSIS AND A PRACTICAL METHOD. Trans N Y Acad Sci. 1965 Apr;27:634–649. doi: 10.1111/j.2164-0947.1965.tb02222.x. [DOI] [PubMed] [Google Scholar]
- St John W. M. Independent brain stem sites for ventilatory neurogenesis. J Appl Physiol Respir Environ Exerc Physiol. 1983 Aug;55(2):433–439. doi: 10.1152/jappl.1983.55.2.433. [DOI] [PubMed] [Google Scholar]
- Wyman R. J. Neural generation of the breathing rhythm. Annu Rev Physiol. 1977;39:417–448. doi: 10.1146/annurev.ph.39.030177.002221. [DOI] [PubMed] [Google Scholar]
- Zielinski A. T., Gebber G. L. Basis for late expiratory spinal inhibition of phrenic nerve discharge. Am J Physiol. 1975 Jun;228(6):1690–1694. doi: 10.1152/ajplegacy.1975.228.6.1690. [DOI] [PubMed] [Google Scholar]


