Abstract
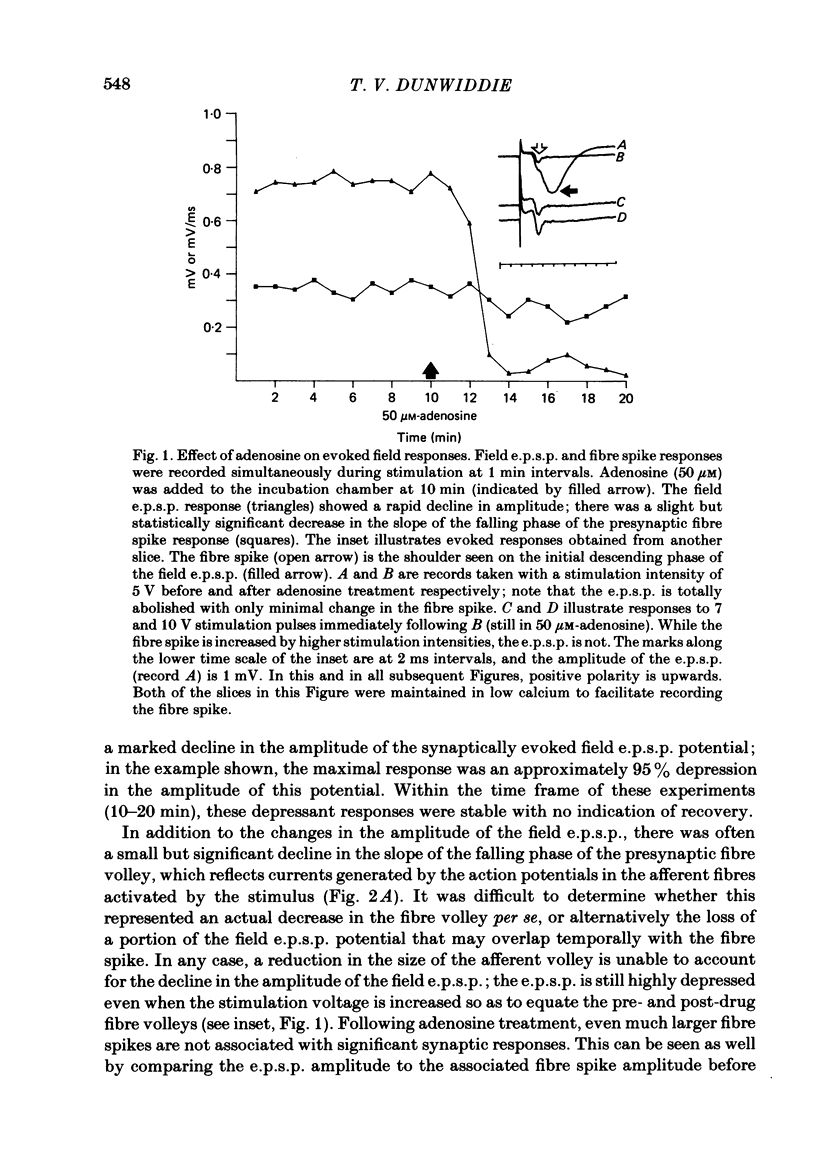
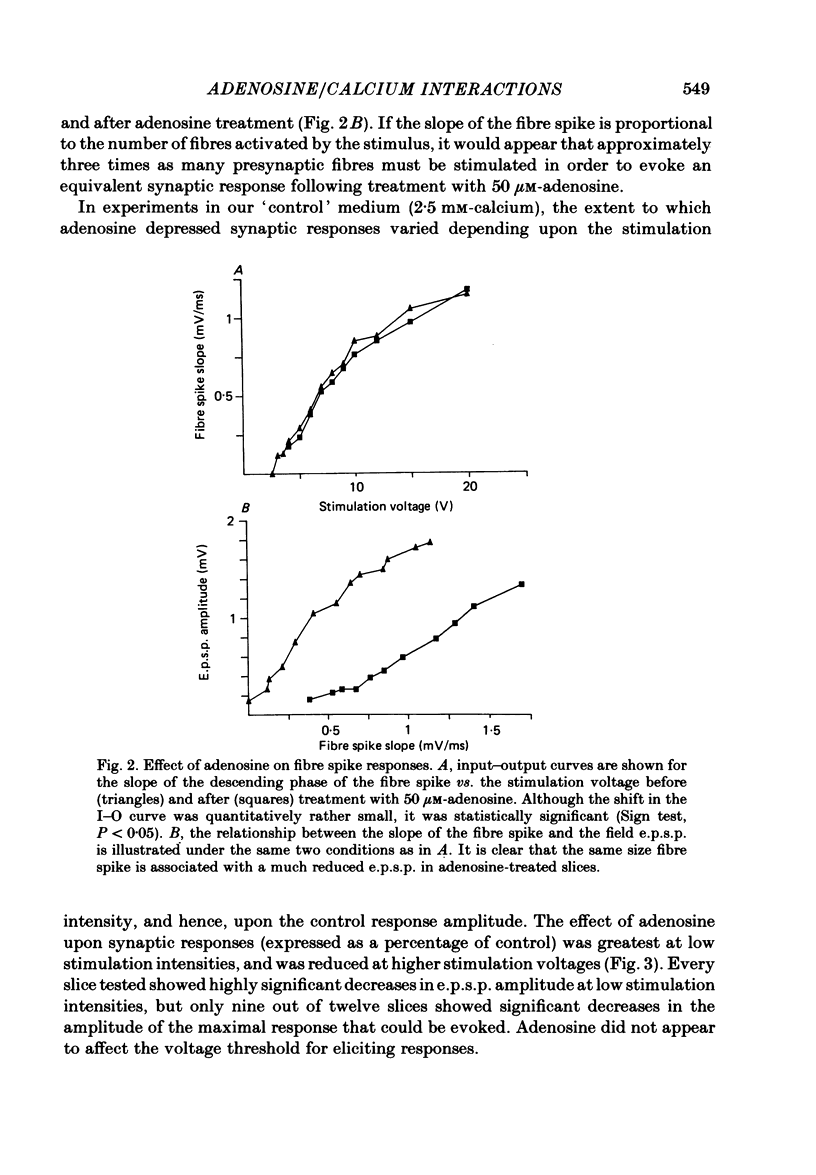
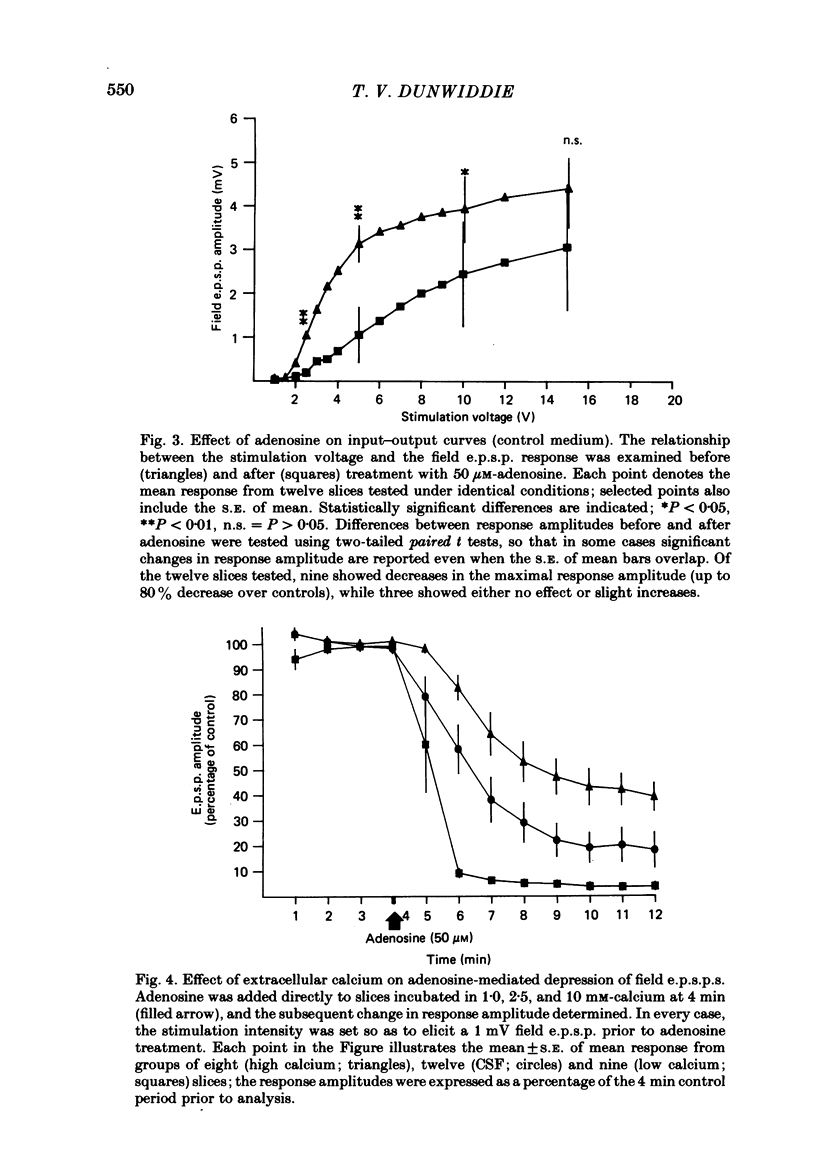
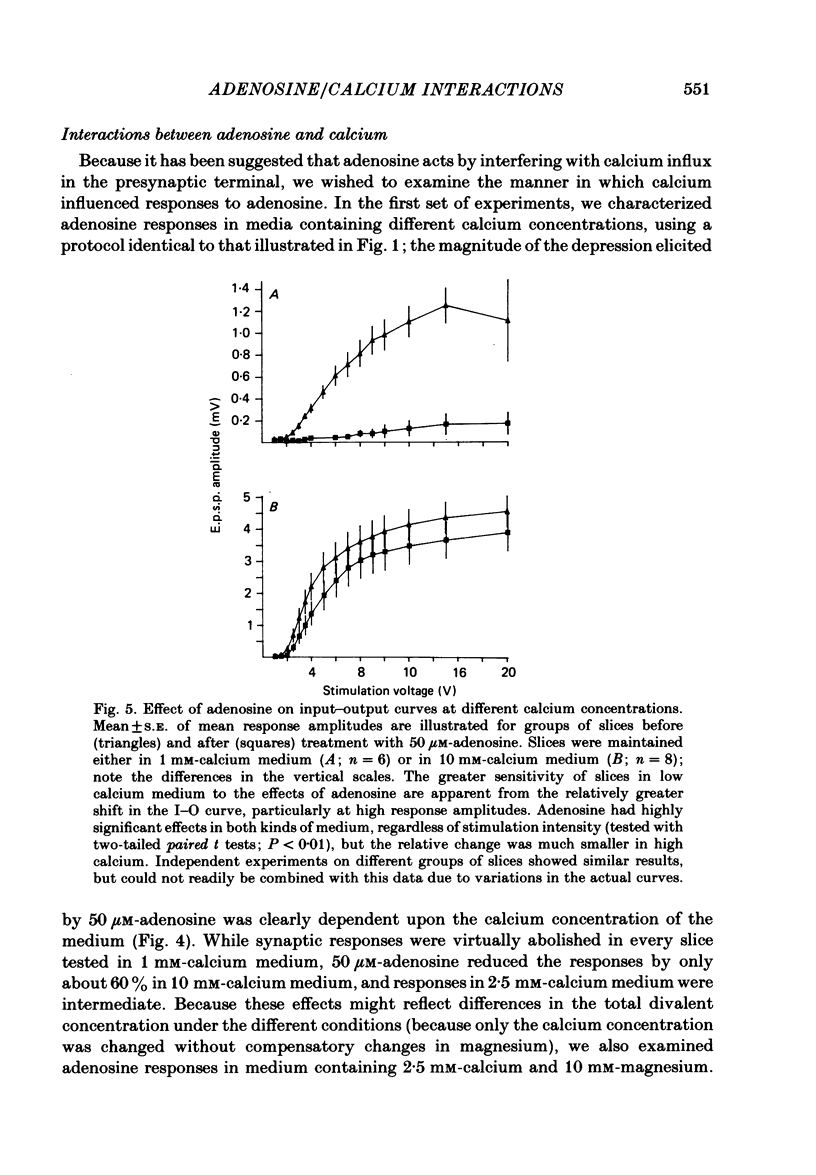
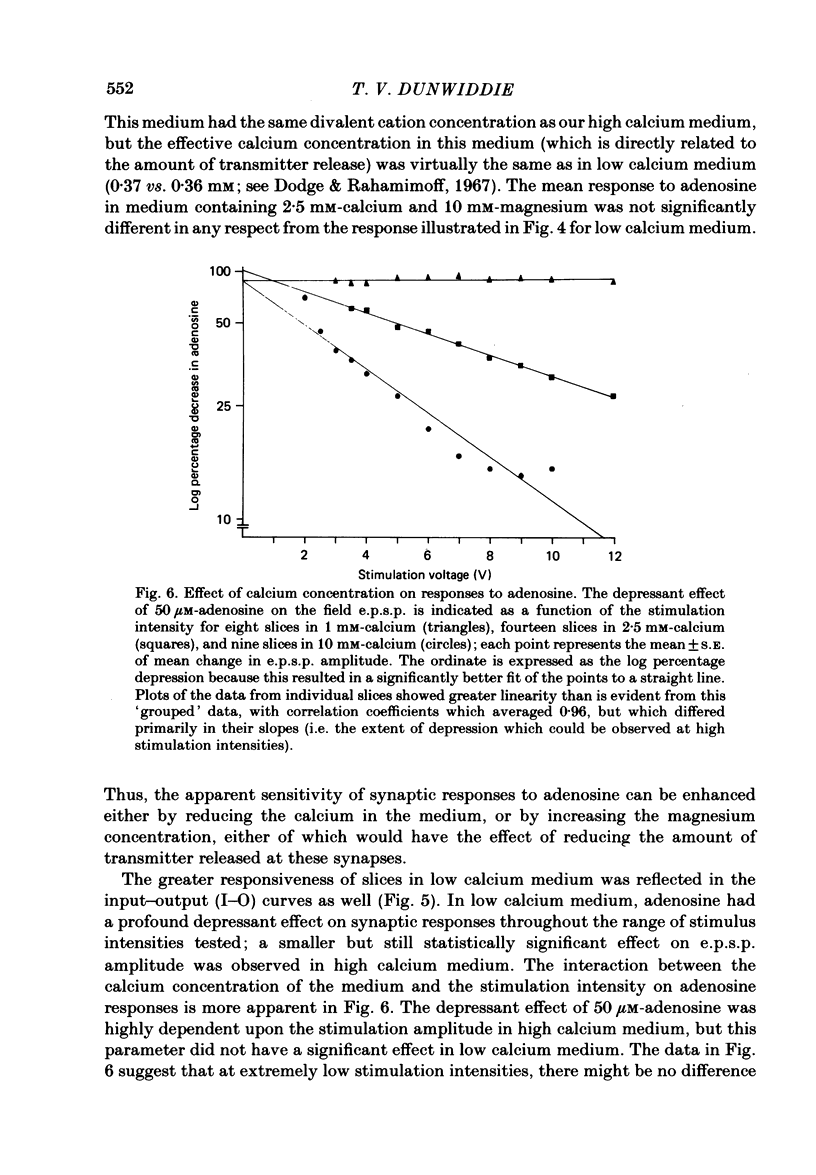
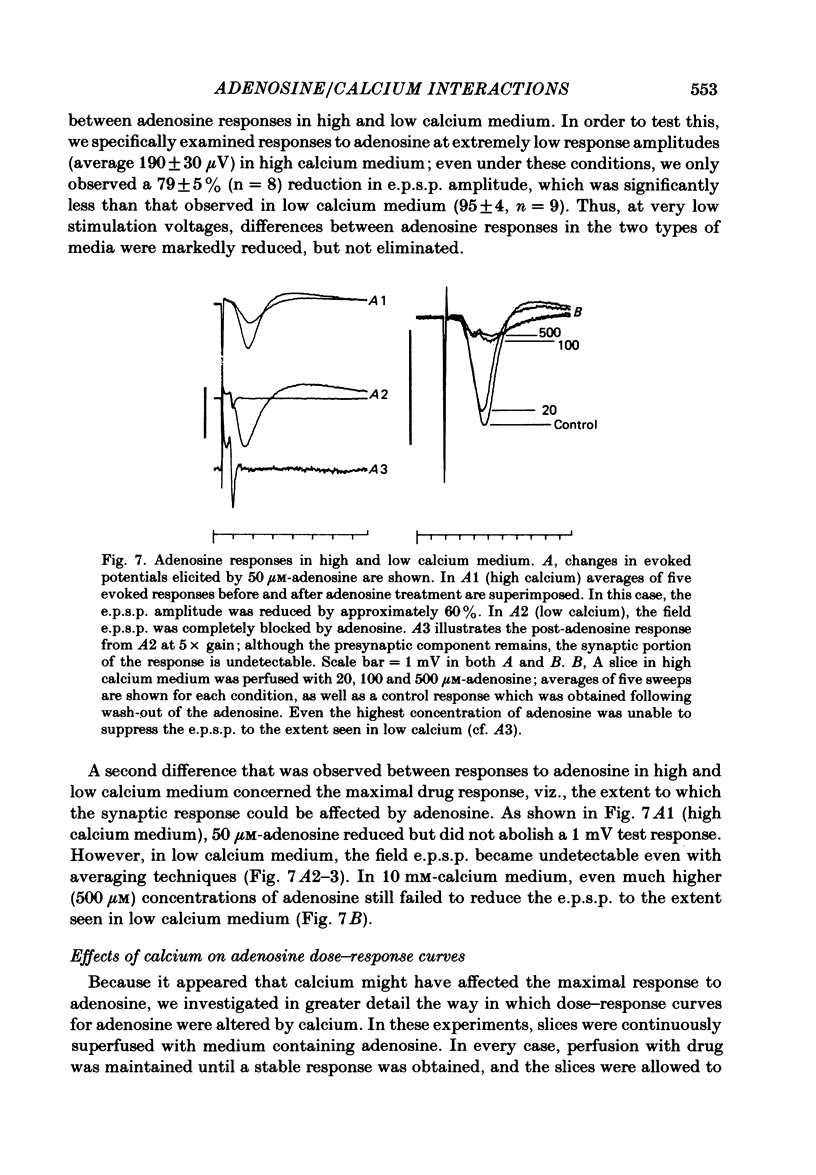
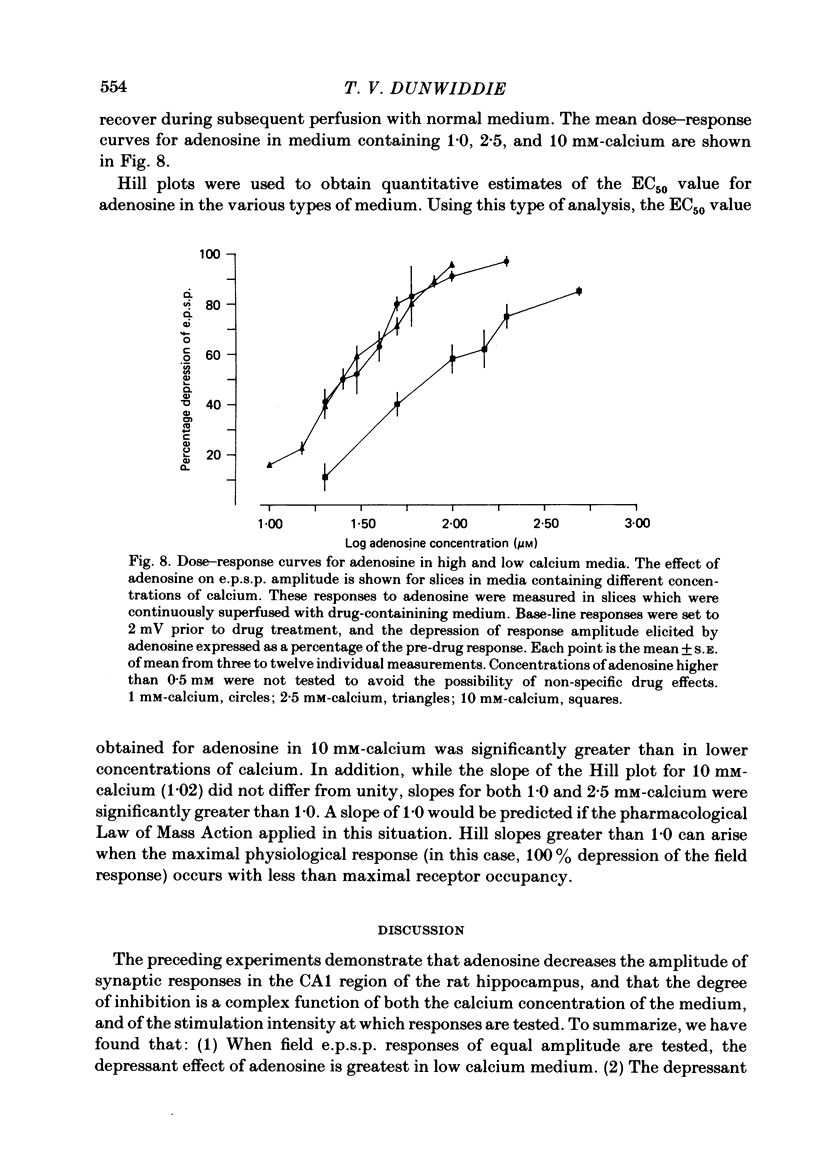
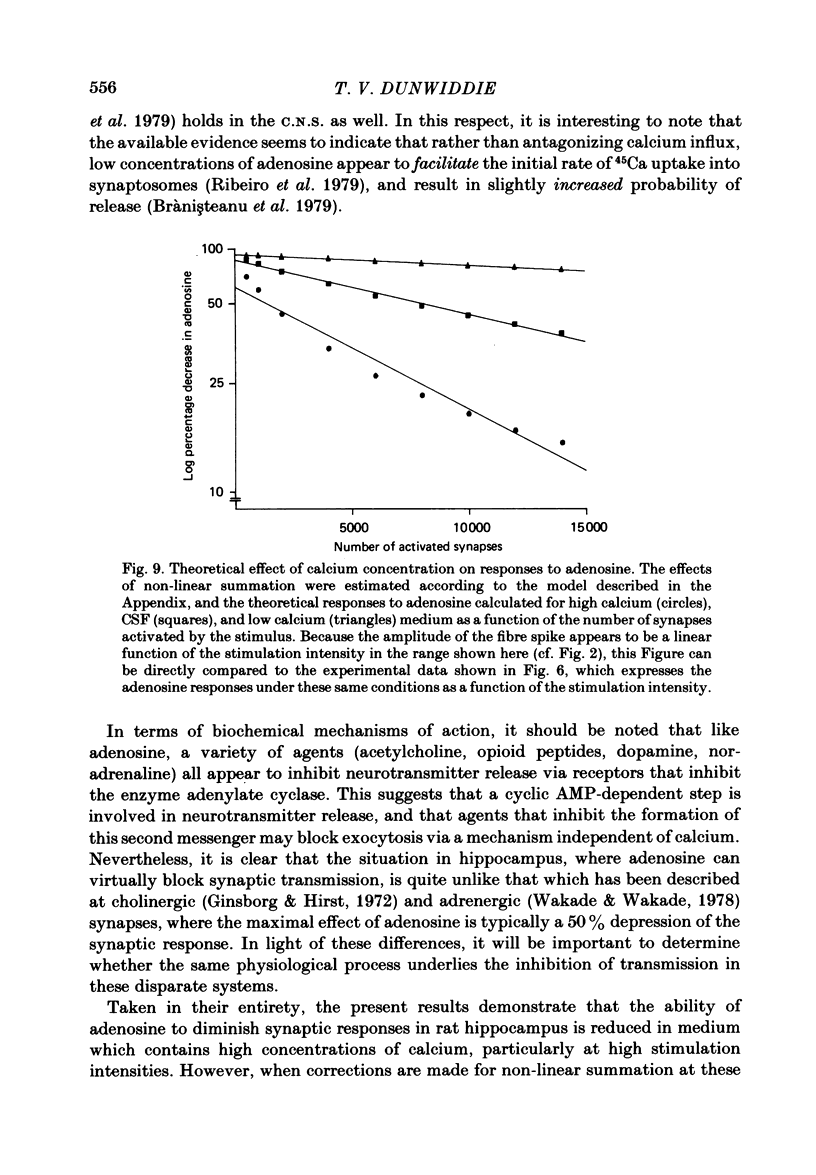
The effect of adenosine on synaptic responses in the in vitro rat hippocampus was examined. As has been previously described, adenosine had a profound depressant effect on synaptic transmission at excitatory synapses on the CA1 pyramidal cells. Although adenosine also produced small decreases in the amplitude of the presynaptic fibre spike, this was unable to account for the relatively much greater decrease in the amplitude of the extracellularly recorded field excitatory post-synaptic potential (e.p.s.p.). The effect of the calcium concentration of the bathing medium on responses to adenosine was also examined. Adenosine generally had a greater depressant effect on field e.p.s.p.s from slices maintained in low concentrations of calcium (1 mM) than in high (10 mM-calcium). However, at low response amplitudes, the effect of adenosine in the two kinds of medium was quite similar. When corrections were made for non-linear summation of e.p.s.p.s, it was found that the effects of adenosine appeared to be independent of the calcium concentration of the medium. These data suggest that adenosine inhibits synaptic transmission by a calcium-independent regulatory mechanism, and that adenosine does not interfere with calcium influx or calcium levels in the presynaptic terminal. Comparisons of dose-response relationships in media containing different concentrations of calcium suggest that a maximal drug response can occur with less than maximal receptor occupancy at physiological calcium concentrations (less than 2.5 mM).
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brănişteanu D. D., Haulică I. D., Proca B., Nhue B. G. Adenosine effects upon transmitter release parameters in the Mg2+-paralyzed neuro-muscular junction of frog. Naunyn Schmiedebergs Arch Pharmacol. 1979 Sep;308(3):273–279. doi: 10.1007/BF00501393. [DOI] [PubMed] [Google Scholar]
- Daum P. R., Hill S. J., Young J. M. Histamine H1-agonist potentiation of adenosine-stimulated cyclic AMP accumulation in slices of guinea-pig cerebral cortex: comparison of response and binding parameters. Br J Pharmacol. 1982 Oct;77(2):347–357. doi: 10.1111/j.1476-5381.1982.tb09304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T. V., Basile A. S., Palmer M. R. Electrophysiological responses to adenosine analogs in rat hippocampus and cerebellum: evidence for mediation by adenosine receptors of the A1 subtype. Life Sci. 1984 Jan 2;34(1):37–47. doi: 10.1016/0024-3205(84)90328-x. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T. V., Hoffer B. J. Adenine nucleotides and synaptic transmission in the in vitro rat hippocampus. Br J Pharmacol. 1980 May;69(1):59–68. doi: 10.1111/j.1476-5381.1980.tb10883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T., Lynch G. Long-term potentiation and depression of synaptic responses in the rat hippocampus: localization and frequency dependency. J Physiol. 1978 Mar;276:353–367. doi: 10.1113/jphysiol.1978.sp012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie T., Mueller A., Palmer M., Stewart J., Hoffer B. Electrophysiological interactions of enkephalins with neuronal circuitry in the rat hippocampus. I. Effects on pyramidal cell activity. Brain Res. 1980 Feb 24;184(2):311–330. doi: 10.1016/0006-8993(80)90801-x. [DOI] [PubMed] [Google Scholar]
- Fredholm B. B., Hedqvist P. Modulation of neurotransmission by purine nucleotides and nucleosides. Biochem Pharmacol. 1980 Jun 15;29(12):1635–1643. doi: 10.1016/0006-2952(80)90117-3. [DOI] [PubMed] [Google Scholar]
- Ginsborg B. L., Hirst G. D. The effect of adenosine on the release of the transmitter from the phrenic nerve of the rat. J Physiol. 1972 Aug;224(3):629–645. doi: 10.1113/jphysiol.1972.sp009916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda Y. Physiological roles of adenosine derivatives which are released during neurotransmission in mammalian brain. J Physiol (Paris) 1978;74(5):463–470. [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton B. L., Barnes C. A., Andersen P. Synaptic efficacy and EPSP summation in granule cells of rat fascia dentata studied in vitro. J Neurophysiol. 1981 Nov;46(5):952–966. doi: 10.1152/jn.1981.46.5.952. [DOI] [PubMed] [Google Scholar]
- Mueller A. L., Hoffer B. J., Dunwiddie T. V. Noradrenergic responses in rat hippocampus: evidence for medication by alpha and beta receptors in the in vitro slice. Brain Res. 1981 Jun 9;214(1):113–126. doi: 10.1016/0006-8993(81)90442-x. [DOI] [PubMed] [Google Scholar]
- Reddington M., Lee K. S., Schubert P. An A1-adenosine receptor, characterized by [3H] cyclohexyladenosine binding, mediates the depression of evoked potentials in a rat hippocampal slice preparation. Neurosci Lett. 1982 Mar 5;28(3):275–279. doi: 10.1016/0304-3940(82)90070-2. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. A., Sá-Almeida A. M., Namorado J. M. Adenosine and adenosine triphosphate decrease 45Ca uptake by synaptosomes stimulated by potassium. Biochem Pharmacol. 1979 Apr 15;28(8):1297–1300. doi: 10.1016/0006-2952(79)90428-3. [DOI] [PubMed] [Google Scholar]
- Schubert P., Mitzdorf U. Analysis and quantitative evaluation of the depressive effect of adenosine on evoked potentials in hippocampal slices. Brain Res. 1979 Aug 17;172(1):186–190. doi: 10.1016/0006-8993(79)90910-7. [DOI] [PubMed] [Google Scholar]
- Smellie F. W., Daly J. W., Dunwiddie T. V., Hoffer B. J. The dextro and levorotatory isomers of N-phenylisopropyladenosine: stereospecific effects on cyclic AMP-formation and evoked synaptic responses in brain slices. Life Sci. 1979 Nov 12;25(20):1739–1748. doi: 10.1016/0024-3205(79)90477-6. [DOI] [PubMed] [Google Scholar]
- Stone T. W. The effects of 4-aminopyridine on the isolated vas deferens and its effects on the inhibitory properties of adenosine, morphine, noradrenaline and gamma-aminobutyric acid. Br J Pharmacol. 1981 Jul;73(3):791–796. doi: 10.1111/j.1476-5381.1981.tb16817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi E. S., Knoll J. The inhibitory effect of adenosine and related nucleotides on the release of acetylcholine. Neuroscience. 1976;1(5):391–398. doi: 10.1016/0306-4522(76)90132-9. [DOI] [PubMed] [Google Scholar]
- Wakade A. R., Wakade T. D. Inhibition of noradrenaline release by adenosine. J Physiol. 1978 Sep;282:35–49. doi: 10.1113/jphysiol.1978.sp012446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. H., Phillis J. W., Thierry D. L. Adenosine receptor agonists inhibit K+-evoked Ca2+ uptake by rat brain cortical synaptosomes. J Neurochem. 1982 Sep;39(3):700–708. doi: 10.1111/j.1471-4159.1982.tb07949.x. [DOI] [PubMed] [Google Scholar]


