Abstract
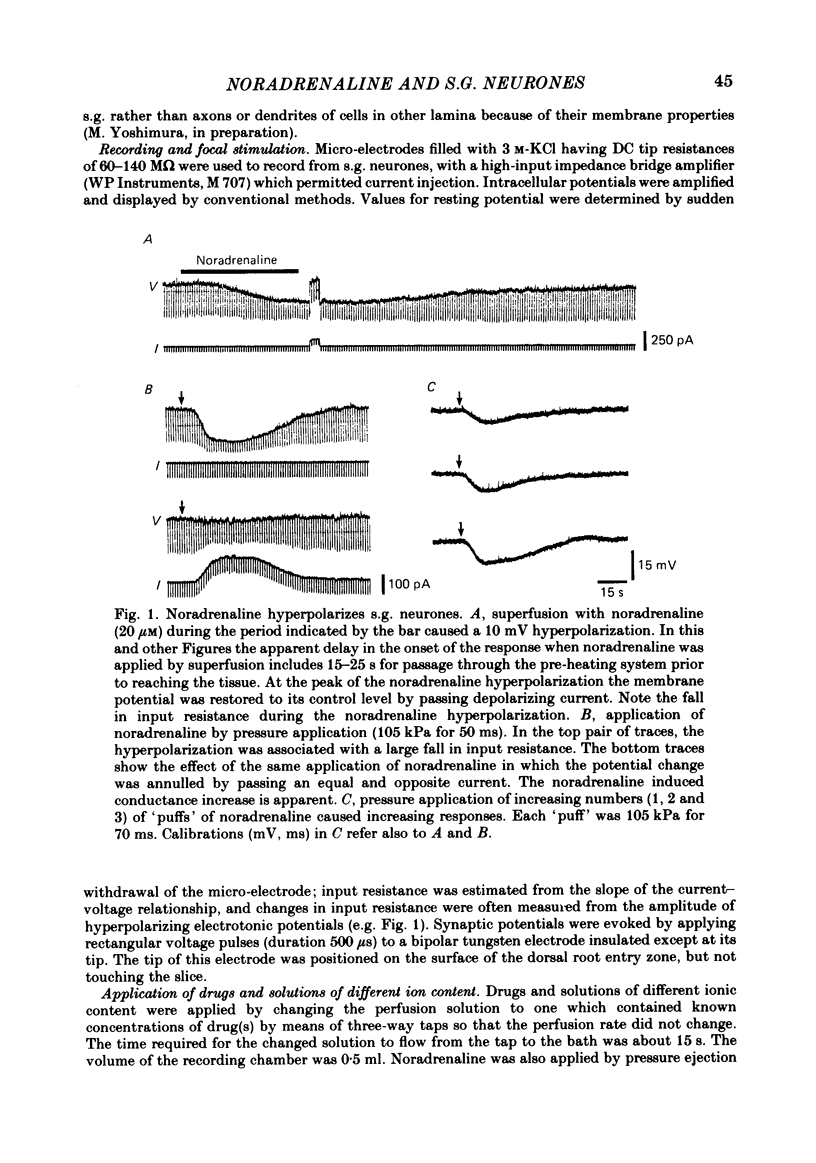
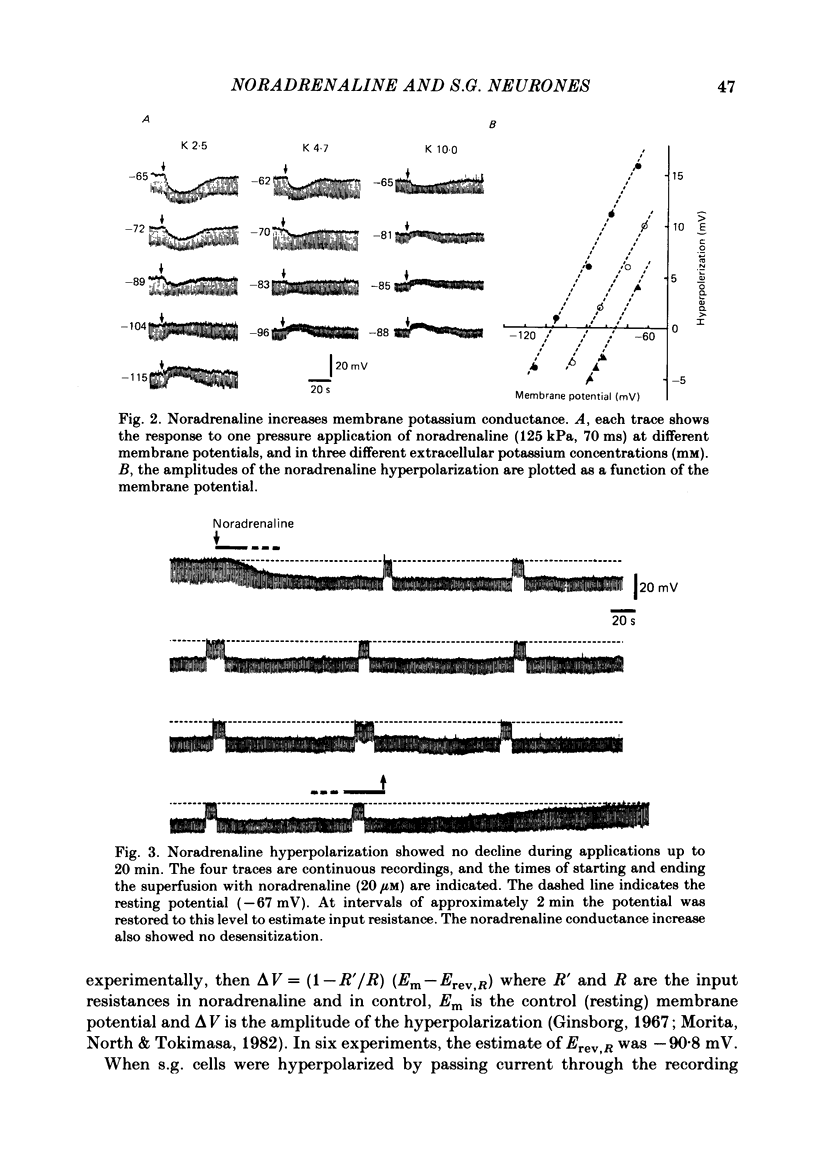
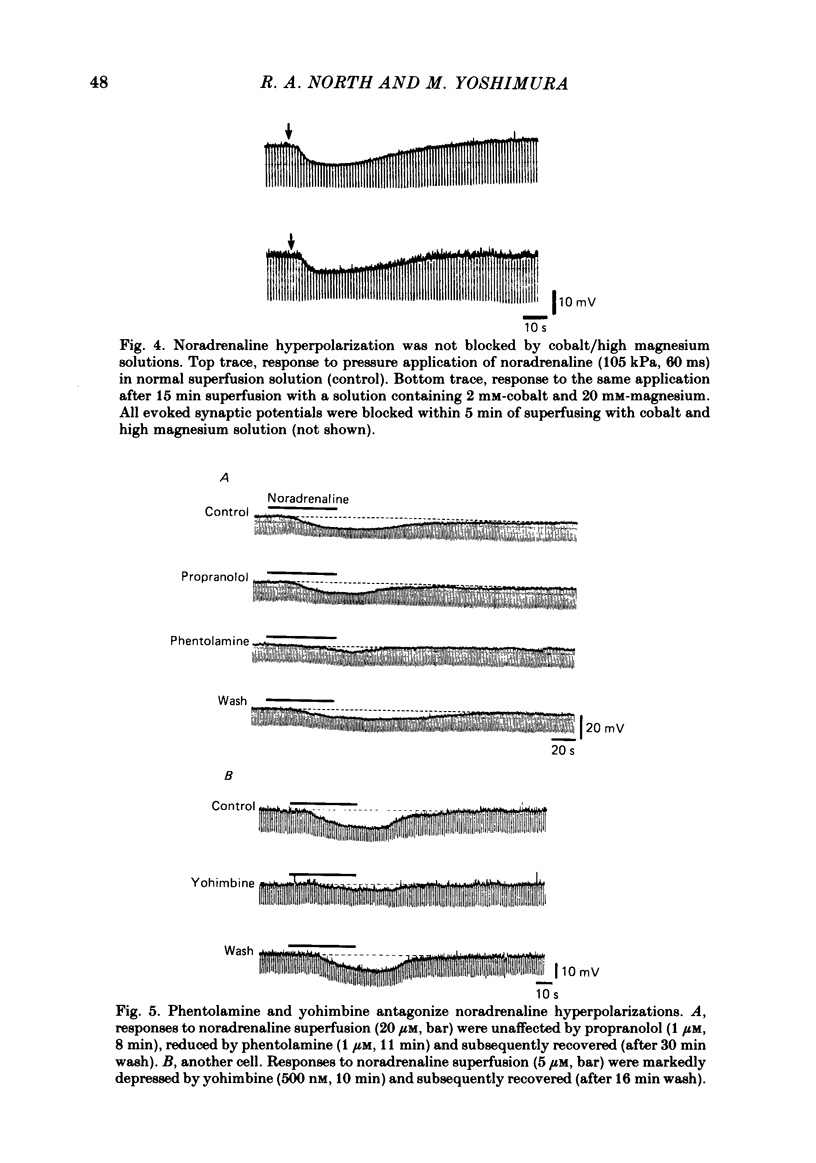
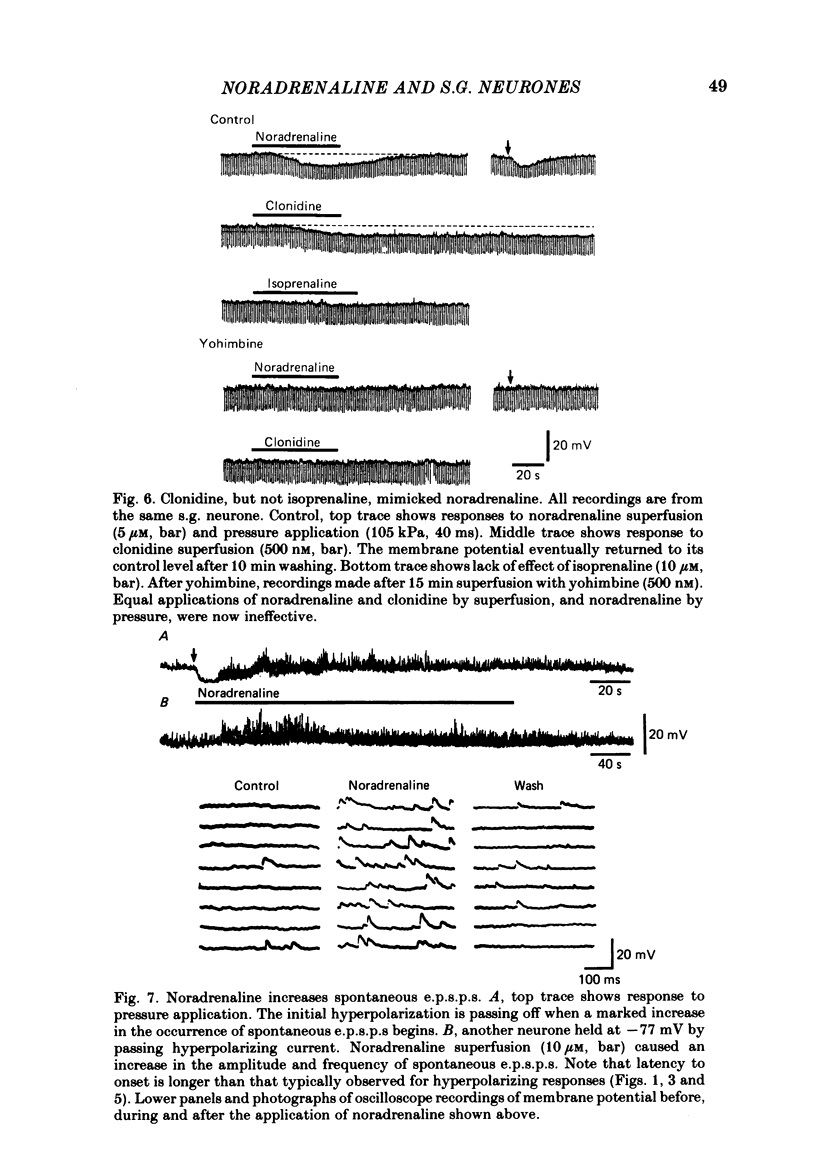
Intracellular recordings were made from substantia gelatinosa (s.g.) neurones in slices cut from adult rat spinal cord and maintained in vitro. Noradrenaline applied by superfusion (1-50 microM), or by brief pressure ejection from a micropipette, reversibly hyperpolarized 80% of the s.g. cells. The noradrenaline induced hyperpolarization was associated with an increase in conductance and it reversed in polarity at -88 mV. The reversal potential changed when the external potassium concentration was changed, as predicted by the Nernst equation. The noradrenaline hyperpolarization was antagonized by phentolamine and yohimbine but not by propranolol and prazosin. The hyperpolarization was probably a direct action on the impaled cell and not due to release or block of release of other transmitters, because the effects persisted during a perfusion with a low calcium/high magnesium solution or in a solution containing cobalt and high magnesium. In 35 of 148 cells, noradrenaline caused a dose-related increase of spontaneous excitatory post-synaptic potentials (e.p.s.p.s). This effect was blocked by tetrodotoxin. The noradrenaline induced increase in e.p.s.p.s was blocked by phentolamine and prazosin but not by the alpha 2-blockers yohimbine and RX 781094. A few cells were depolarized by noradrenaline, and this was blocked by prazosin but not by yohimbine. It is suggested that noradrenaline may inhibit nociceptive input to the spinal cord by increasing the potassium conductance of s.g. neurones.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., VanderMaelen C. P. alpha 2-adrenoceptor-mediated hyperpolarization of locus coeruleus neurons: intracellular studies in vivo. Science. 1982 Mar 12;215(4538):1394–1396. doi: 10.1126/science.6278591. [DOI] [PubMed] [Google Scholar]
- Belcher G., Ryall R. W., Schaffner R. The differential effects of 5-hydroxytryptamine, noradrenaline and raphe stimulation on nociceptive and non-nociceptive dorsal horn interneurones in the cat. Brain Res. 1978 Aug 4;151(2):307–321. doi: 10.1016/0006-8993(78)90887-9. [DOI] [PubMed] [Google Scholar]
- Bennett G. J., Hayashi H., Abdelmoumene M., Dubner R. Physiological properties of stalked cells of the substantia gelatinosa intracellularly stained with horseradish peroxidase. Brain Res. 1979 Mar 23;164:285–289. doi: 10.1016/0006-8993(79)90022-2. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Caulfield M. P. Hyperpolarizing 'alpha 2'-adrenoceptors in rat sympathetic ganglia. Br J Pharmacol. 1979 Mar;65(3):435–445. doi: 10.1111/j.1476-5381.1979.tb07848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero F., Iggo A., Molony V. An electrophysiological study of neurones in the Substantia Gelatinosa Rolandi of the cat's spinal cord. Q J Exp Physiol Cogn Med Sci. 1979 Oct;64(4):297–314. doi: 10.1113/expphysiol.1979.sp002484. [DOI] [PubMed] [Google Scholar]
- Duggan A. W., Griersmith B. T. Inhibition of the spinal transmission of nociceptive information by supraspinal stimulation in the cat. Pain. 1979 Apr;6(2):149–161. doi: 10.1016/0304-3959(79)90122-2. [DOI] [PubMed] [Google Scholar]
- Egan T. M., Henderson G., North R. A., Williams J. T. Noradrenaline-mediated synaptic inhibition in rat locus coeruleus neurones. J Physiol. 1983 Dec;345:477–488. doi: 10.1113/jphysiol.1983.sp014990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L. Ion movements in junctional transmission. Pharmacol Rev. 1967 Sep;19(3):289–316. [PubMed] [Google Scholar]
- Headley P. M., Duggan A. W., Griersmith B. T. Selective reduction by noradrenaline and 5-hydroxytryptamine of nociceptive responses of cat dorsal horn neurones. Brain Res. 1978 Apr 21;145(1):185–189. doi: 10.1016/0006-8993(78)90809-0. [DOI] [PubMed] [Google Scholar]
- Hodge C. J., Jr, Apkarian A. V., Stevens R., Vogelsang G., Wisnicki H. J. Locus coeruleus modulation of dorsal horn unit responses to cutaneous stimulation. Brain Res. 1981 Jan 12;204(2):415–420. doi: 10.1016/0006-8993(81)90600-4. [DOI] [PubMed] [Google Scholar]
- Hoffer B. J., Siggins G. R., Oliver A. P., Bloom F. E. Activation of the pathway from locus coeruleus to rat cerebellar Purkinje neurons: pharmacological evidence of noradrenergic central inhibition. J Pharmacol Exp Ther. 1973 Mar;184(3):553–569. [PubMed] [Google Scholar]
- Light A. R., Trevino D. L., Perl E. R. Morphological features of functionally defined neurons in the marginal zone and substantia gelatinosa of the spinal dorsal horn. J Comp Neurol. 1979 Jul 15;186(2):151–171. doi: 10.1002/cne.901860204. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature. 1982 Oct 14;299(5884):636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- Morita K., North R. A. Clonidine activates membrane potassium conductance in myenteric neurones. Br J Pharmacol. 1981 Oct;74(2):419–428. doi: 10.1111/j.1476-5381.1981.tb09987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., North R. A., Tokimasa T. The calcium-activated potassium conductance in guinea-pig myenteric neurones. J Physiol. 1982 Aug;329:341–354. doi: 10.1113/jphysiol.1982.sp014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REXED B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952 Jun;96(3):414–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]
- Reddy S. V., Maderdrut J. L., Yaksh T. L. Spinal cord pharmacology of adrenergic agonist-mediated antinociception. J Pharmacol Exp Ther. 1980 Jun;213(3):525–533. [PubMed] [Google Scholar]
- Rogawski M. A., Aghajanian G. K. Modulation of lateral geniculate neurone excitability by noradrenaline microiontophoresis or locus coeruleus stimulation. Nature. 1980 Oct 23;287(5784):731–734. doi: 10.1038/287731a0. [DOI] [PubMed] [Google Scholar]
- Satoh K., Kashiba A., Kimura H., Maeda T. Noradrenergic axon terminals in the substantia gelatinosa of the rat spinal cord: an electron-microscopic study using glyoxylic acid-potassium permanganate fixation. Cell Tissue Res. 1982;222(2):359–378. doi: 10.1007/BF00213218. [DOI] [PubMed] [Google Scholar]
- VanderMaelen C. P., Aghajanian G. K. Intracellular studies showing modulation of facial motoneurone excitability by serotonin. Nature. 1980 Sep 25;287(5780):346–347. doi: 10.1038/287346a0. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., Nishi S. Intracellular recordings from lateral horn cells fo the spinal cord in vitro. J Auton Nerv Syst. 1982 Jul;6(1):5–11. doi: 10.1016/0165-1838(82)90017-0. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., North R. A. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature. 1983 Oct 6;305(5934):529–530. doi: 10.1038/305529a0. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Kuhar M. J. Noradrenergic alpha 1 and alpha 2 receptors: light microscopic autoradiographic localization. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1696–1700. doi: 10.1073/pnas.77.3.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]


