Abstract
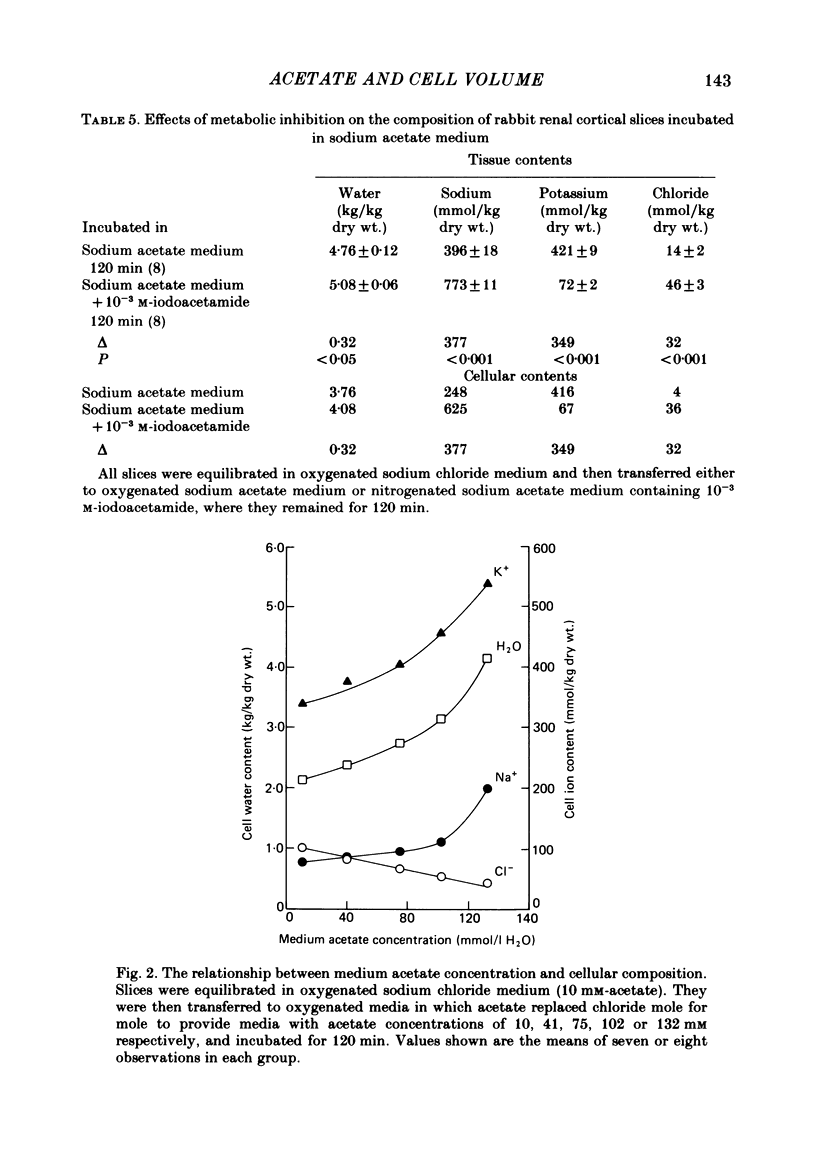
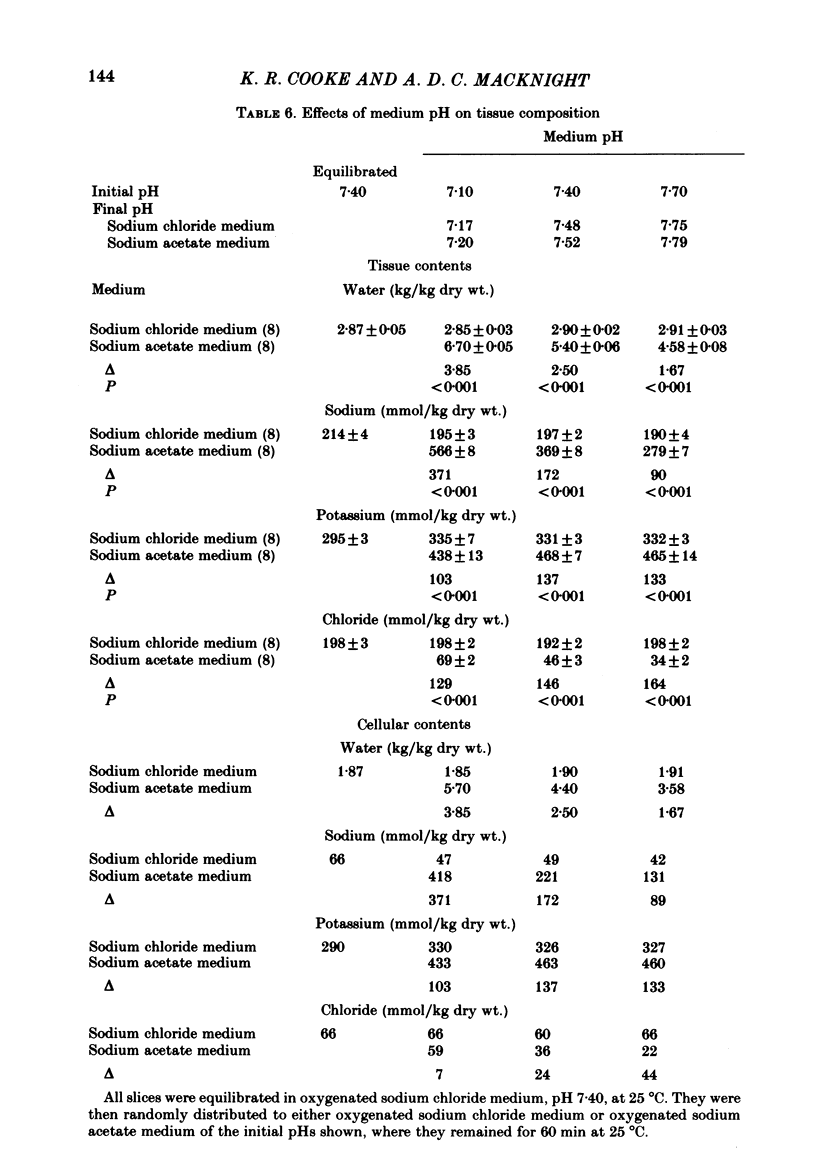
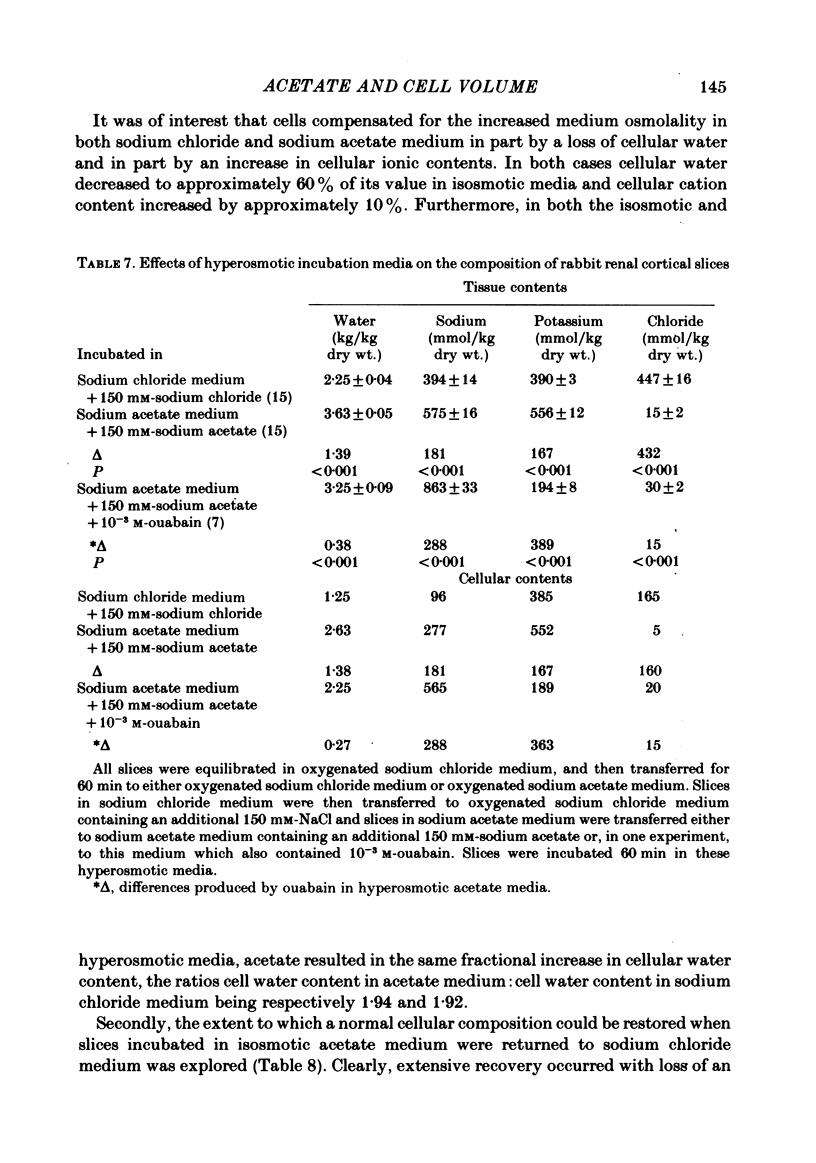
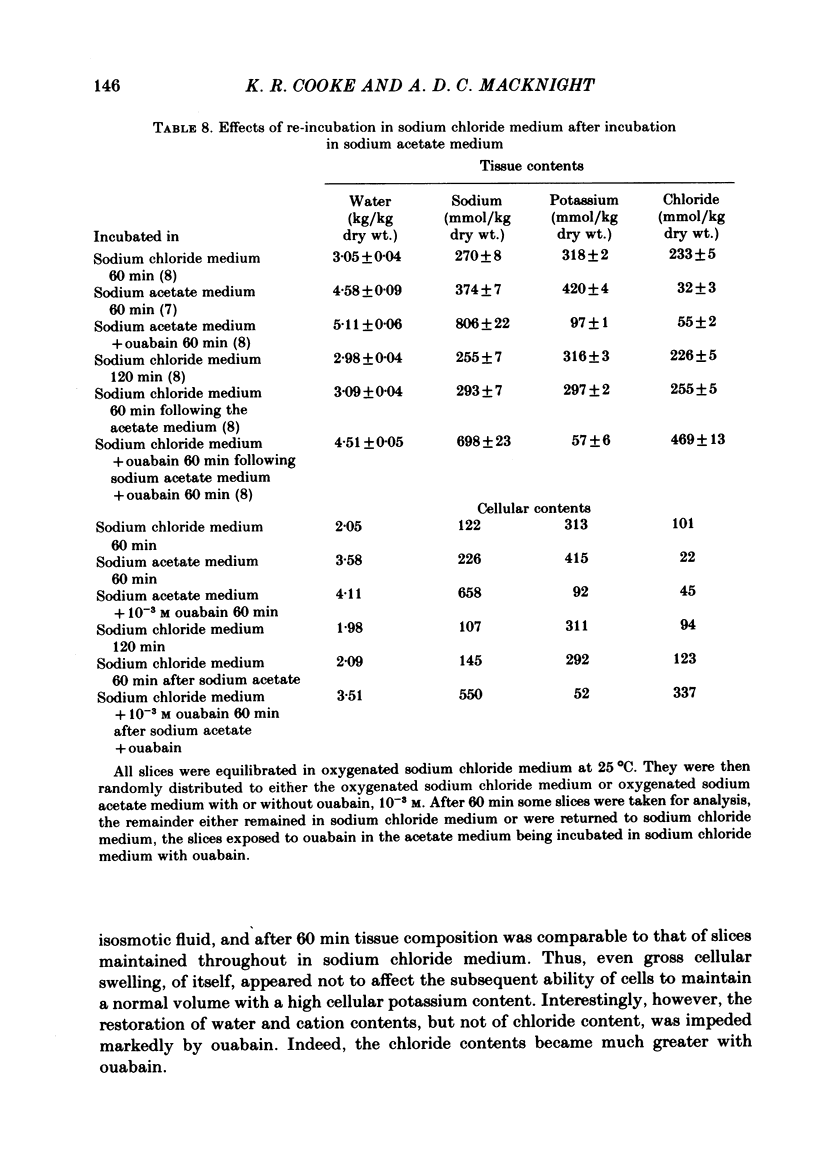
Slices of rabbit renal cortex were incubated at 25 degrees C in media in which acetate replaced chloride. There was gross cellular swelling in isosmotic 132 mM-acetate medium, and this swelling was unique in that, with a normal medium potassium concentration, it was accompanied by a substantial increase in cellular potassium content. This accumulation of potassium, but not the cellular swelling, was dependent upon metabolism and inhibited by ouabain. This accumulation of potassium was not dependent upon the cellular swelling. It also occurred in a hyperosmotic acetate medium in which swelling was minimized. In isosmotic media, the cellular swelling was proportional to medium acetate concentration and was also affected markedly by medium pH, being greatest at an initial medium pH of 7.1 and least at pH 7.7. The swelling was reversed and cellular composition restored when tissue was re-incubated in NaCl medium. Ouabain (10(-3)M) largely prevented this recovery in volume. The results are consistent with plasma-membrane-based theories, on the assumption that membranes are much more permeable to undissociated acetic acid than they are to the acetate ion. They are inconsistent with the expectations of an alternative hypothesis (the association--induction hypothesis) which ascribes the maintenance of cellular composition to properties of cellular proteins and cellular water rather than to those of the plasma membrane. The results do not favour the suggestion that cellular swelling itself results in irreversible cellular damage. The results are consistent with the hypothesis that the ouabain-inhibitable Na-K-ATPase plays a major role in the regulation of cellular volume. No alternative metabolically dependent volume regulating mechanism need be postulated to explain them.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boron W. F., Sackin H. Measurement of intracellular ionic composition and activities in renal tubules. Annu Rev Physiol. 1983;45:483–496. doi: 10.1146/annurev.ph.45.030183.002411. [DOI] [PubMed] [Google Scholar]
- COTLOVE E., TRANTHAM H. V., BOWMAN R. L. An instrument and method for automatic, rapid, accurate, and sensitive titration of chloride in biologic samples. J Lab Clin Med. 1958 Mar;51(3):461–468. [PubMed] [Google Scholar]
- Cooke K. R. Ouabain and regulation of cellular volume in slices of mammalian renal cortex. J Physiol. 1981 Nov;320:319–332. doi: 10.1113/jphysiol.1981.sp013952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaister D., Kerly M. The oxygen consumption and carbohydrate metabolism of the retractor muscle of the foot of Mytilus edulis. J Physiol. 1936 Jun 10;87(1):56–66. doi: 10.1113/jphysiol.1936.sp003388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P. M., Macknight A. D. Effects of replacing medium sodium by choline, caesium, or rubidium, on water and ion contents of renal cortical slices. J Physiol. 1977 May;267(1):113–136. doi: 10.1113/jphysiol.1977.sp011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P. M., Macknight D. C. The regulation of cellular volume in renal cortical slices incubated in hyposmotic medium. J Physiol. 1976 May;257(1):137–154. doi: 10.1113/jphysiol.1976.sp011360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEINZELLER A., KNOTKOVA A. THE EFFECT OF OUABAIN ON THE ELECTROLYTE AND WATER TRANSPORT IN KIDNEY CORTEX AND LIVER SLICES. J Physiol. 1964 Dec;175:172–192. doi: 10.1113/jphysiol.1964.sp007510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella J. L., Aronson P. S. Amiloride inhibition of the Na+-H+ exchanger in renal microvillus membrane vesicles. Am J Physiol. 1981 Oct;241(4):F374–F379. doi: 10.1152/ajprenal.1981.241.4.F374. [DOI] [PubMed] [Google Scholar]
- LEAF A. On the mechanism of fluid exchange of tissues in vitro. Biochem J. 1956 Feb;62(2):241–248. doi: 10.1042/bj0620241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLE J. R. DETERMINATION OF WATER AND ELECTROLYTES IN TISSUE SLICES. Anal Biochem. 1964 Jan;7:87–95. doi: 10.1016/0003-2697(64)90122-8. [DOI] [PubMed] [Google Scholar]
- Leaf A. Cell swelling. A factor in ischemic tissue injury. Circulation. 1973 Sep;48(3):455–458. doi: 10.1161/01.cir.48.3.455. [DOI] [PubMed] [Google Scholar]
- Ling G. N., Peterson K. A theory of cell swelling in high concentrations of Kc1 and other chloride salts. Bull Math Biol. 1977;39(6):721–741. doi: 10.1007/BF02461780. [DOI] [PubMed] [Google Scholar]
- MUDGE G. H. Electrolyte and water metabolism of rabbit kidney slices; effect of metabolic inhibitors. Am J Physiol. 1951 Oct;167(1):206–223. doi: 10.1152/ajplegacy.1951.167.1.206. [DOI] [PubMed] [Google Scholar]
- Macknight A. D. Comparison of analytic techniques: chemical, isotopic, and microprobe analyses. Fed Proc. 1980 Sep;39(11):2881–2887. [PubMed] [Google Scholar]
- Macknight A. D., Leaf A. Regulation of cellular volume. Physiol Rev. 1977 Jul;57(3):510–573. doi: 10.1152/physrev.1977.57.3.510. [DOI] [PubMed] [Google Scholar]
- Macknight A. D. Water and electrolyte contents of rat renal cortical slices incubated in medium containing p-chloromercuri-benzoic acid or p-chloromercuribenzoic acid and ouabain. Biochim Biophys Acta. 1968 Dec 10;163(4):500–505. doi: 10.1016/0005-2736(68)90079-5. [DOI] [PubMed] [Google Scholar]
- Macknight A. D. Water and electrolyte contents of rat renal cortical slices incubated in potassium-free media and media containing ouabain. Biochim Biophys Acta. 1968 Mar 1;150(2):263–270. doi: 10.1016/0005-2736(68)90169-7. [DOI] [PubMed] [Google Scholar]
- McIver D. J., Macknight A. D. Extracellular space in some isolated tissues. J Physiol. 1974 May;239(1):31–49. doi: 10.1113/jphysiol.1974.sp010554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negendank W. Studies of ions and water in human lymphocytes. Biochim Biophys Acta. 1982 Oct 20;694(2):123–161. doi: 10.1016/0304-4157(82)90022-3. [DOI] [PubMed] [Google Scholar]
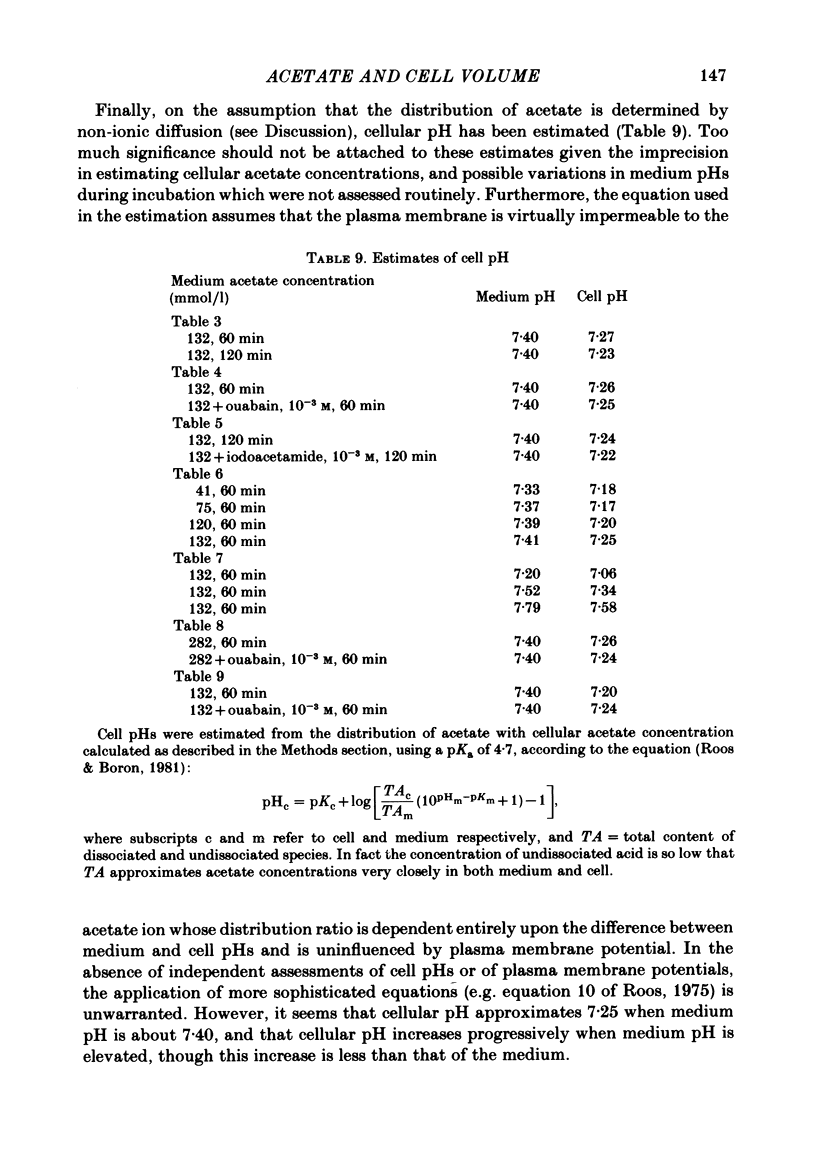
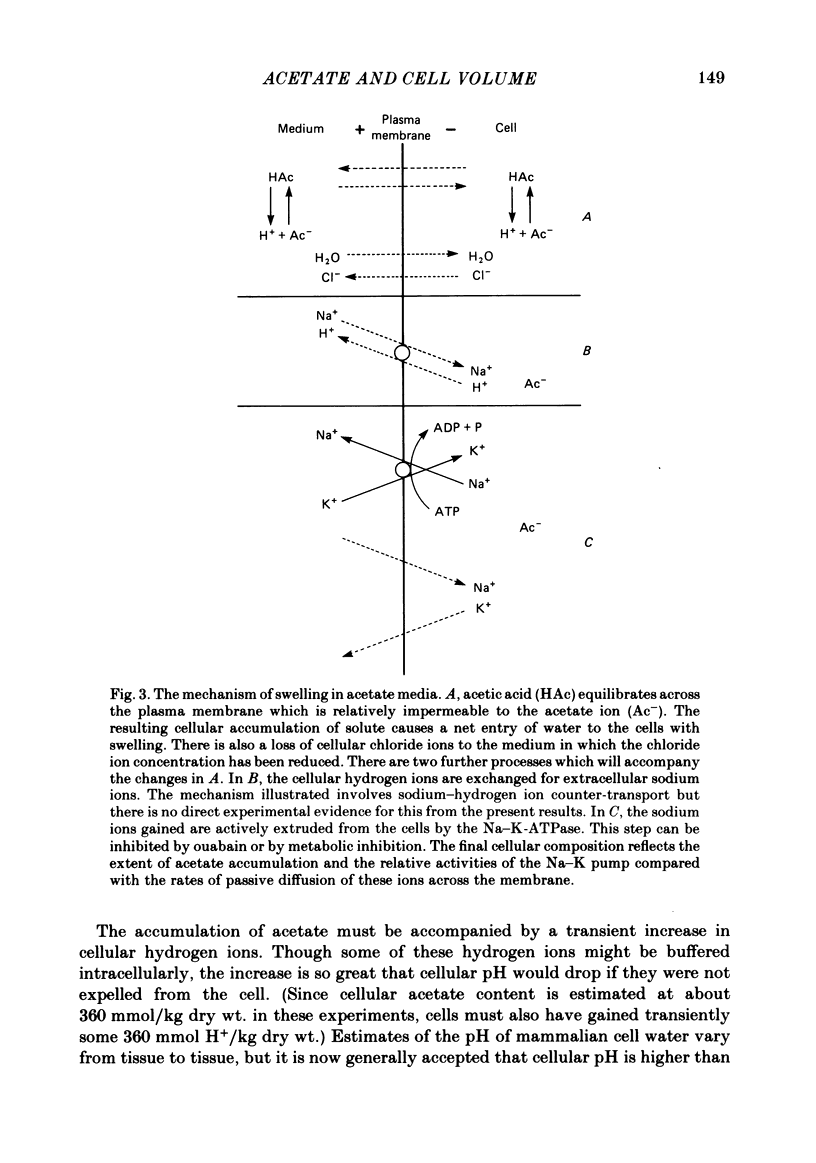
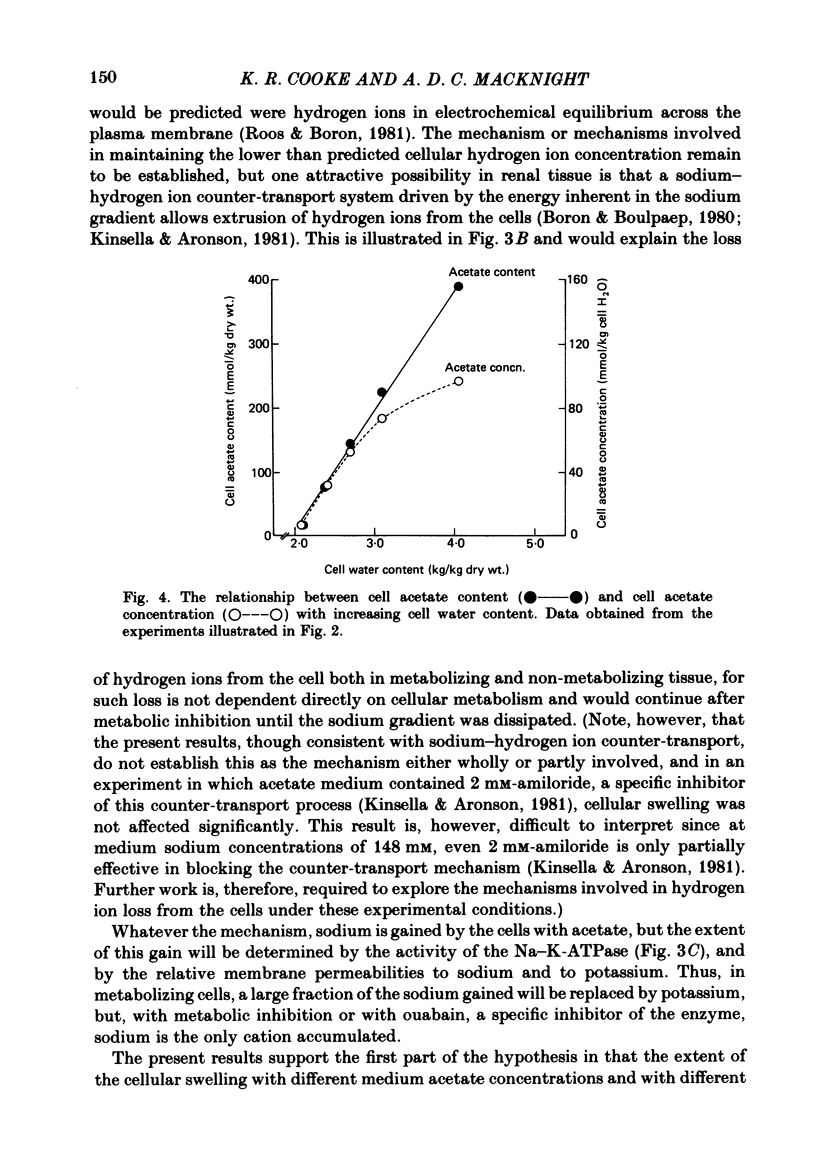
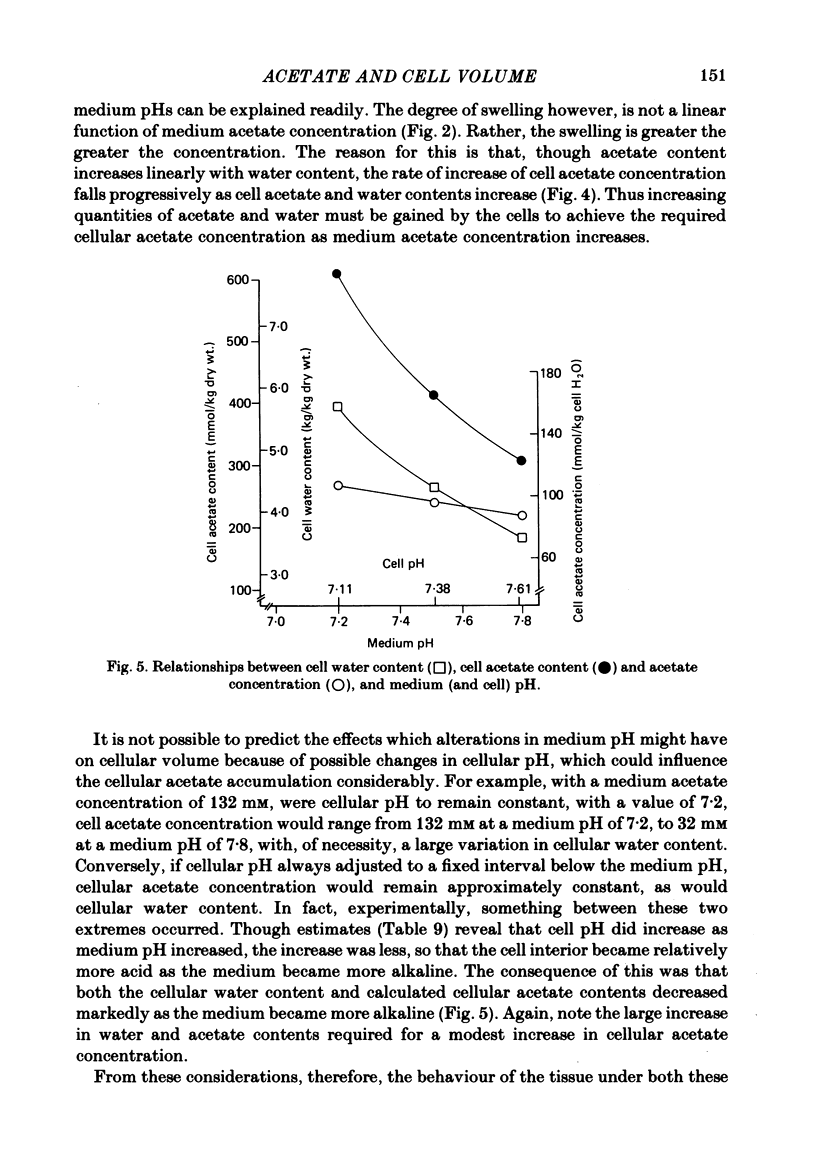
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Roos A. Intracellular pH and distribution of weak acids across cell membranes. A study of D- and L-lactate and of DMO in rat diaphragm. J Physiol. 1975 Jul;249(1):1–25. doi: 10.1113/jphysiol.1975.sp011000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOSTESON D. C., HOFFMAN J. F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol. 1960 Sep;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittembury G., Proverbio F. Two modes of Na extrusion in cells from guinea pig kidney cortex slices. Pflugers Arch. 1970;316(1):1–25. doi: 10.1007/BF00587893. [DOI] [PubMed] [Google Scholar]


