Abstract
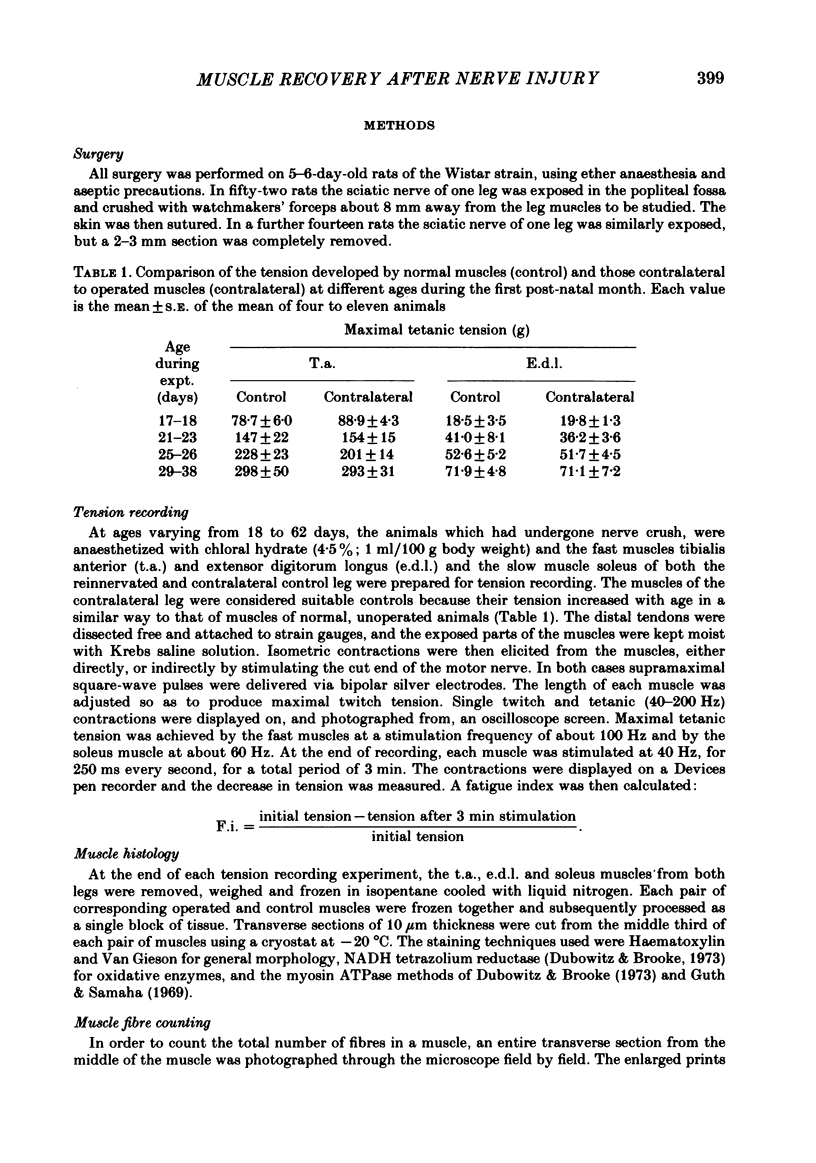
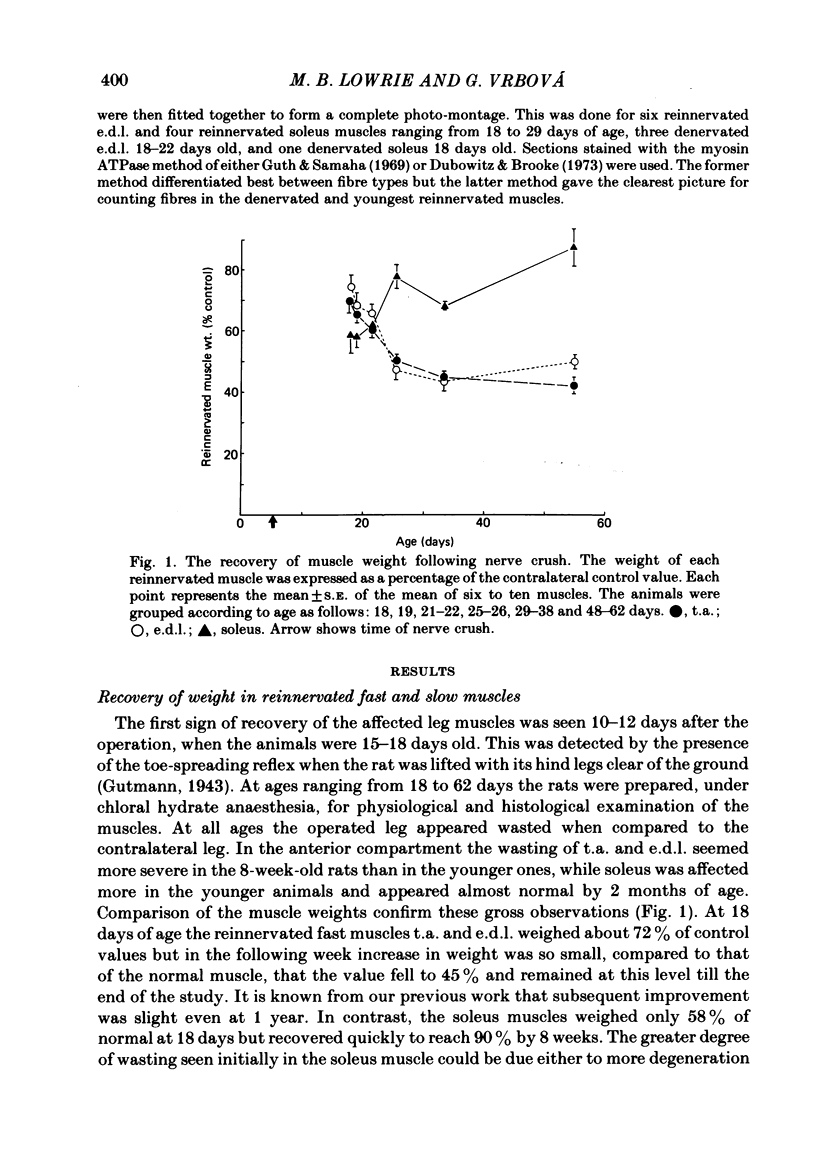
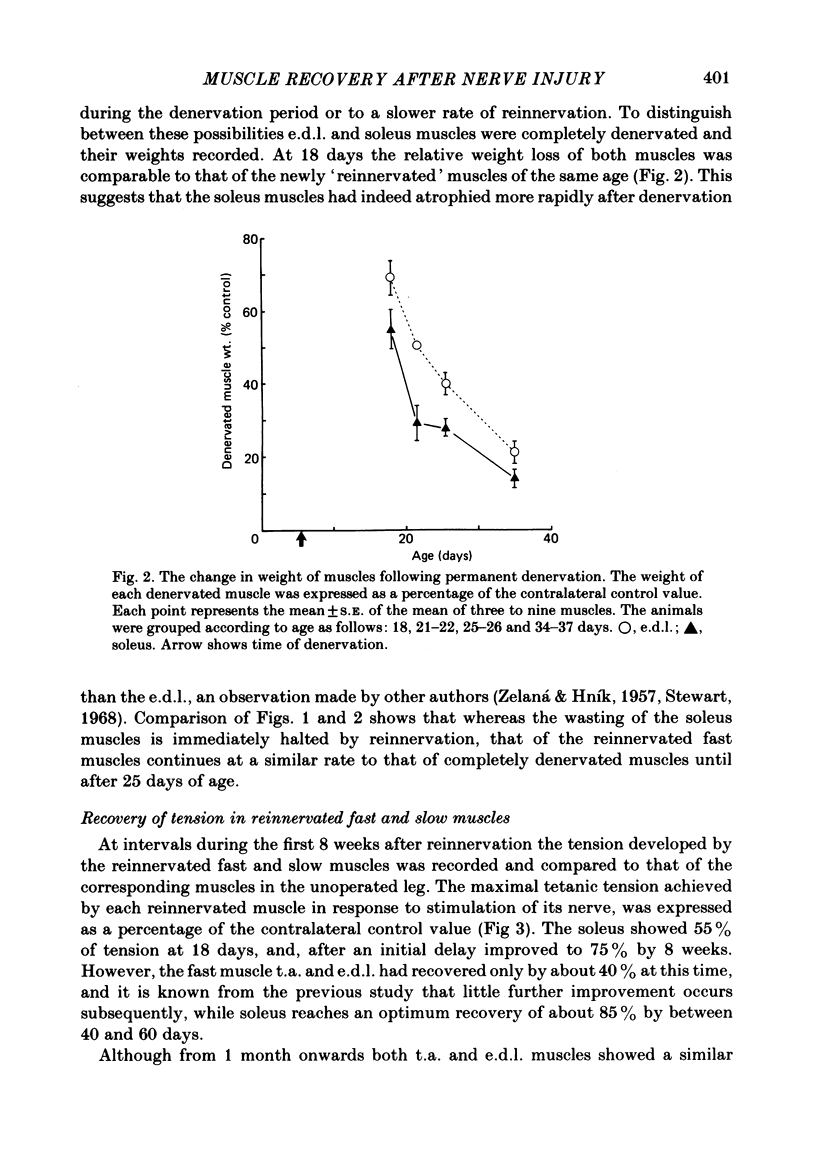
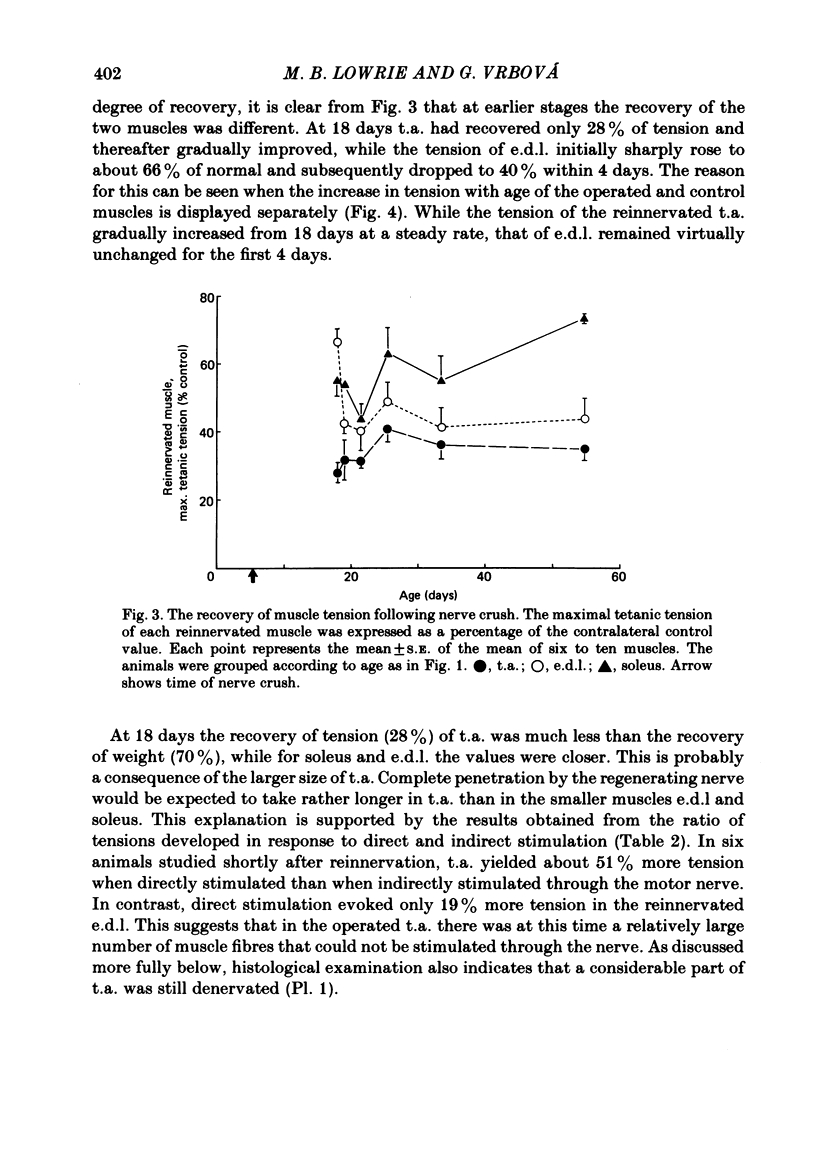
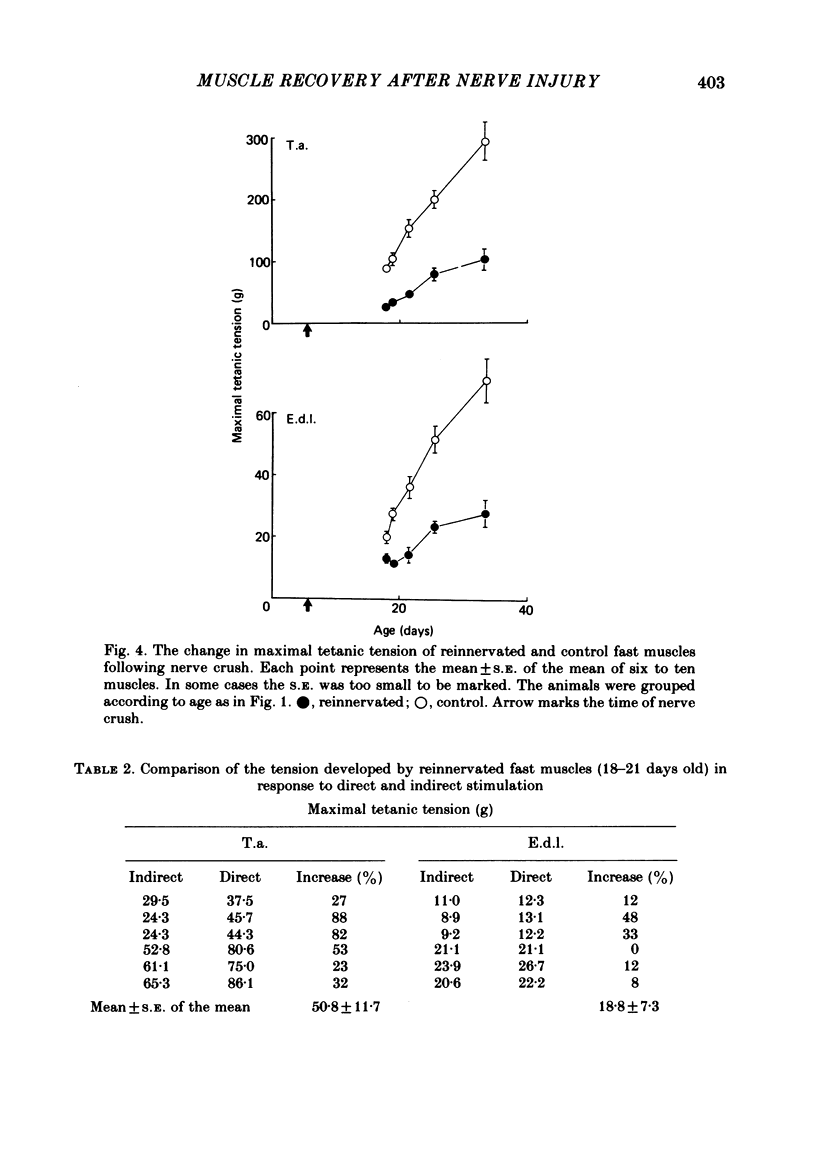
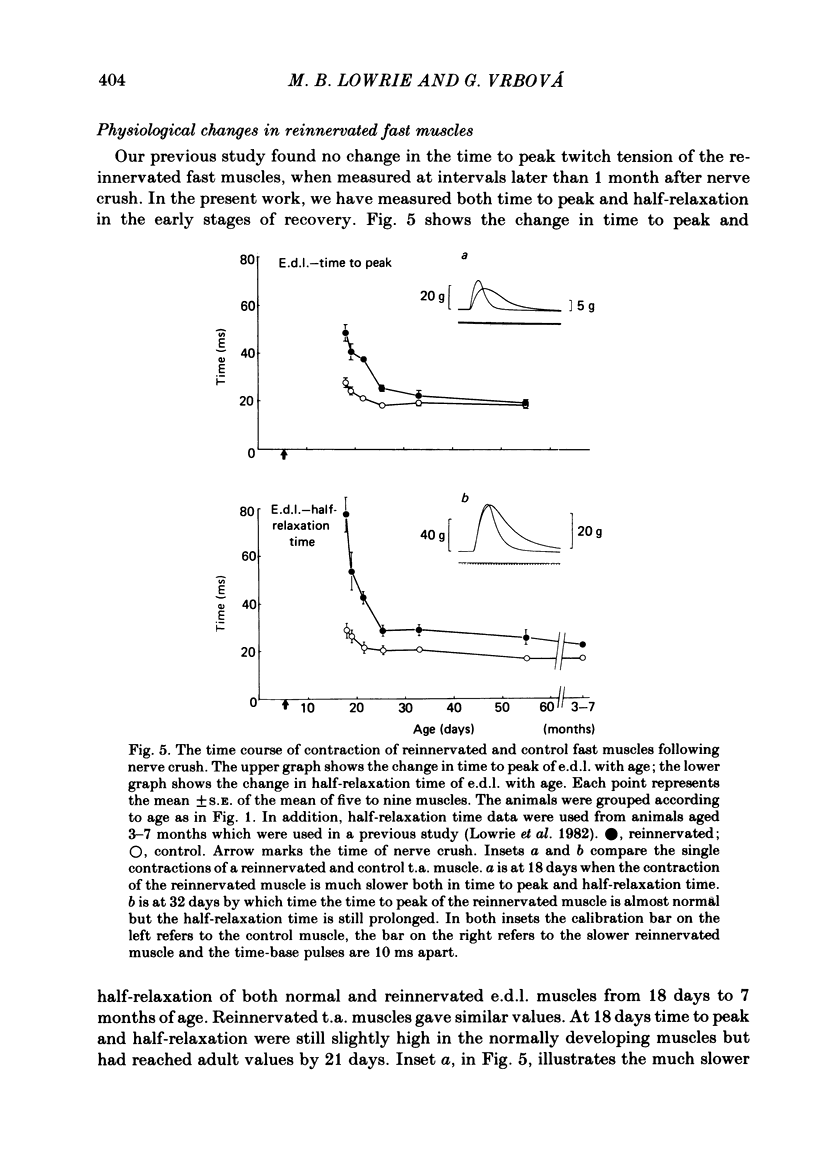
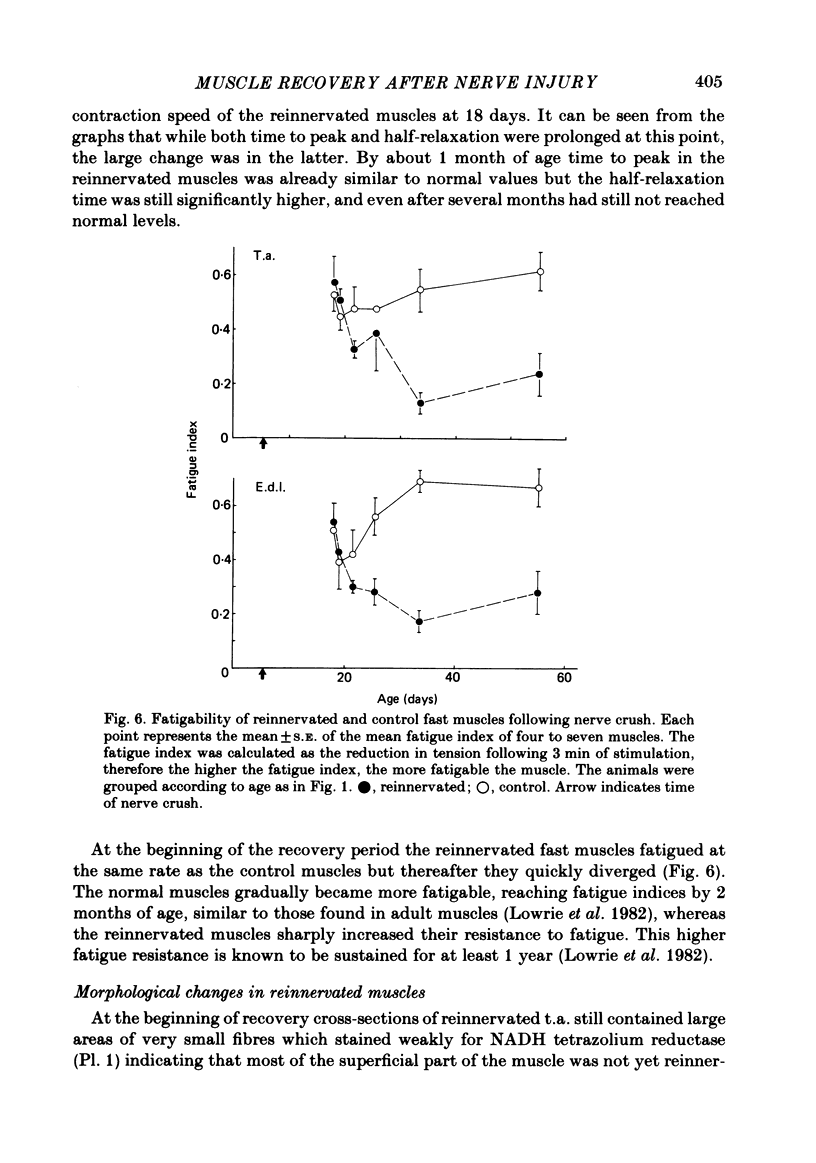
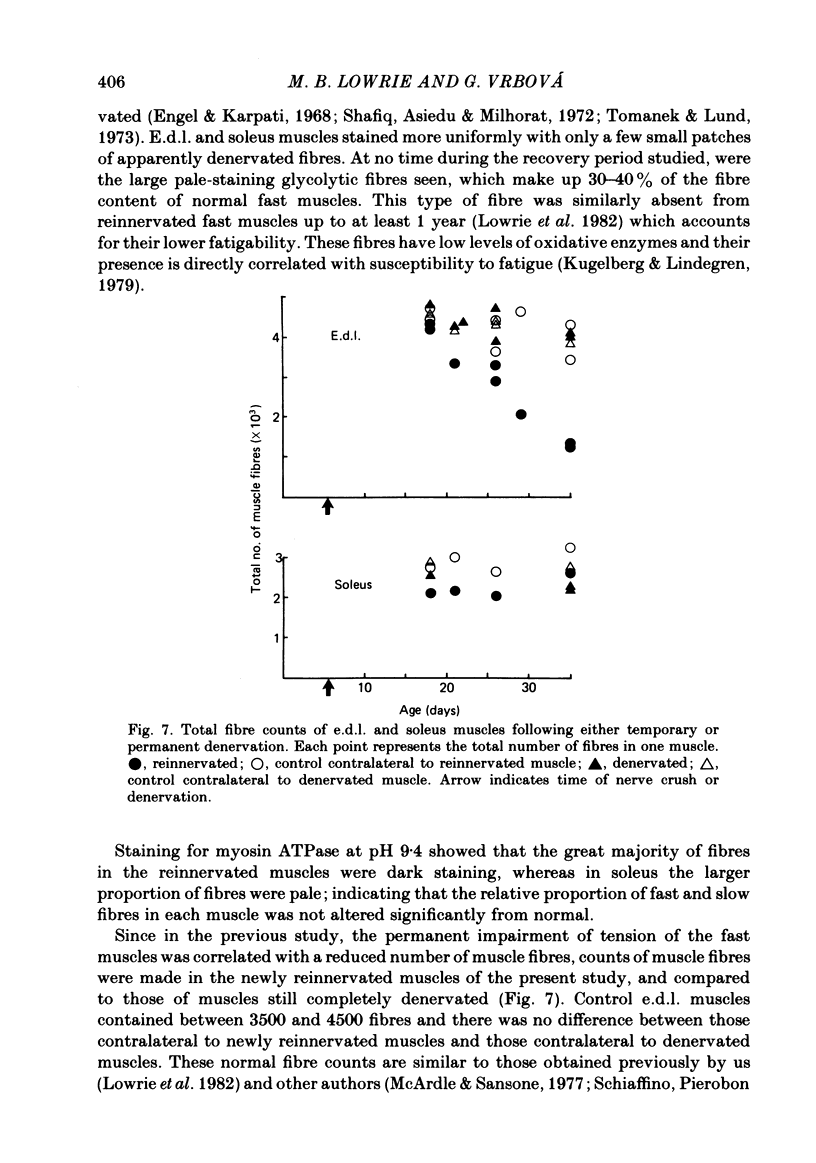
The sciatic nerve was crushed in 5-6-day-old rats and the time course of recovery and changes in physiological and morphological properties of reinnervated fast and slow muscles was compared. The maximal tetanic tension developed by the reinnervated muscles was recorded at different times from about 18 days of age, when functional recovery was first seen, until 2 months. The maximal indirectly elicited tetanic tension of the reinnervated slow soleus muscle gradually increased from 55% of normal at 18 days to 75% of normal at 2 months. In contrast, the tension of the reinnervated fast muscle extensor digitorum longus (e.d.l.) fell sharply from 70% of normal at 18 days to 40% at 21 days and remained at that level till the end of the study. The total number of muscle fibres in control, reinnervated and denervated e.d.l. muscles was counted. At 18 days the number of fibres in the reinnervated e.d.l. was similar to normal but by 1 month it had fallen to one-third. This decrease did not take place in permanently denervated muscles until at least 35 days. Loss of fibres in the reinnervated soleus was small. During the early stages of reinnervation the contraction and relaxation of the fast muscles was very prolonged. By 1 month the time taken to reach peak twitch tension had decreased to normal values but the relaxation was still slower and remained so for several months. The study of fatigue resistance showed that at 18 days the reinnervated fast muscles were as fatigable as normal muscles from animals of the same age. The fatigability of normal muscles increased with age to adult levels, but the reinnervated muscles became more fatigue resistant and remained so. Our findings suggest that fast muscles become selectively impaired after nerve injury at 6 days because they lose a large number of fibres after reinnervation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUEKER E. K., MEYERS C. E. The maturity of peripheral nerves at the time of injury as a factor in nerve regeneration. Anat Rec. 1951 Apr;109(4):723–743. doi: 10.1002/ar.1091090409. [DOI] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The effects of partial denervation at birth on the development of muscle fibres and motor units in rat lumbrical muscle. J Physiol. 1980 Jun;303:265–279. doi: 10.1113/jphysiol.1980.sp013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodensteiner J. B., Engel A. G. Intracellular calcium accumulation in Duchenne dystrophy and other myopathies: a study of 567,000 muscle fibers in 114 biopsies. Neurology. 1978 May;28(5):439–446. doi: 10.1212/wnl.28.5.439. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Hopkins W. G., Keynes R. J. Short- and long-term effects of paralysis on the motor innervation of two different neonatal mouse muscles. J Physiol. 1982 Aug;329:439–450. doi: 10.1113/jphysiol.1982.sp014312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel W. K., Karpati G. Impaired skeletal muscle maturation following neonatal neurectomy. Dev Biol. 1968 Jun;17(6):713–723. doi: 10.1016/0012-1606(68)90015-8. [DOI] [PubMed] [Google Scholar]
- Frank E., Jansen J. K., Lomo T., Westgaard R. H. The interaction between foreign and original motor nerves innervating the soleus muscle of rats. J Physiol. 1975 Jun;247(3):725–743. doi: 10.1113/jphysiol.1975.sp010954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T., Perry R., Tuffery A. R., Vrbová G G G. Possible mechanisms determining synapse formation in developing skeletal muscles of the chick. Cell Tissue Res. 1974;155(1):13–25. doi: 10.1007/BF00220281. [DOI] [PubMed] [Google Scholar]
- Guth L., Samaha F. J. Qualitative differences between actomyosin ATPase of slow and fast mammalian muscle. Exp Neurol. 1969 Sep;25(1):138–152. doi: 10.1016/0014-4886(69)90077-6. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Adaptive transformation of rat soleus motor units during growth. J Neurol Sci. 1976 Mar;27(3):269–289. doi: 10.1016/0022-510x(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Lindegren B. Transmission and contraction fatigue of rat motor units in relation to succinate dehydrogenase activity of motor unit fibres. J Physiol. 1979 Mar;288:285–300. [PMC free article] [PubMed] [Google Scholar]
- Lowrie M. B., Krishnan S., Vrbová G. Recovery of slow and fast muscles following nerve injury during early post-natal development in the rat. J Physiol. 1982 Oct;331:51–66. doi: 10.1113/jphysiol.1982.sp014364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J. J., Sansone F. M. Re-innervation of fast and slow twitch muscle following nerve crush at birth. J Physiol. 1977 Oct;271(3):567–586. doi: 10.1113/jphysiol.1977.sp012015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanes G. J. Motor localization and the effects of nerve injury on the ventral horn cells of the spinal cord. J Anat. 1946 Jul;80(Pt 3):117–131. [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M., Kasprzak H., Feng H., Fertuck H. Endplates after esterase inactivation in vivo: correlation between esterase concentration, functional response and fine structure. J Neurocytol. 1979 Feb;8(1):95–115. doi: 10.1007/BF01206461. [DOI] [PubMed] [Google Scholar]
- Samaha F. J., Gergely J. Biochemical abnormalities of the sarcoplasmic reticulum in muscular dystrophy. N Engl J Med. 1969 Jan 23;280(4):184–188. doi: 10.1056/NEJM196901232800403. [DOI] [PubMed] [Google Scholar]
- Shafiq S. A., Asiedu S. A., Milhorat A. T. Effect of neonatal neurectomy on differentiation of fiber types in rat skeletal muscle. Exp Neurol. 1972 Jun;35(3):529–540. doi: 10.1016/0014-4886(72)90123-9. [DOI] [PubMed] [Google Scholar]
- Stewart D. M. Effect of age on the response of four muscles of the rat to denervation. Am J Physiol. 1968 May;214(5):1139–1146. doi: 10.1152/ajplegacy.1968.214.5.1139. [DOI] [PubMed] [Google Scholar]
- Takagi A., Schotland D. L., Rowland L. P. Sarcoplasmic reticulum in Duchenne muscular dystrophy. Arch Neurol. 1973 Jun;28(6):380–384. doi: 10.1001/archneur.1973.00490240040006. [DOI] [PubMed] [Google Scholar]
- Tomanek R. J., Lund D. D. Degeneration of different types of skeletal muscle fibres. I. Denervation. J Anat. 1973 Dec;116(Pt 3):395–407. [PMC free article] [PubMed] [Google Scholar]
- Tóth L., Karcsú S., Poberai M., Sávay G. A light and electron microscopic histochemical study on the mechanism of DFP-induced acute and subacute myopathy. Neuropathol Appl Neurobiol. 1981 Sep-Oct;7(5):399–410. doi: 10.1111/j.1365-2990.1981.tb00241.x. [DOI] [PubMed] [Google Scholar]