Abstract
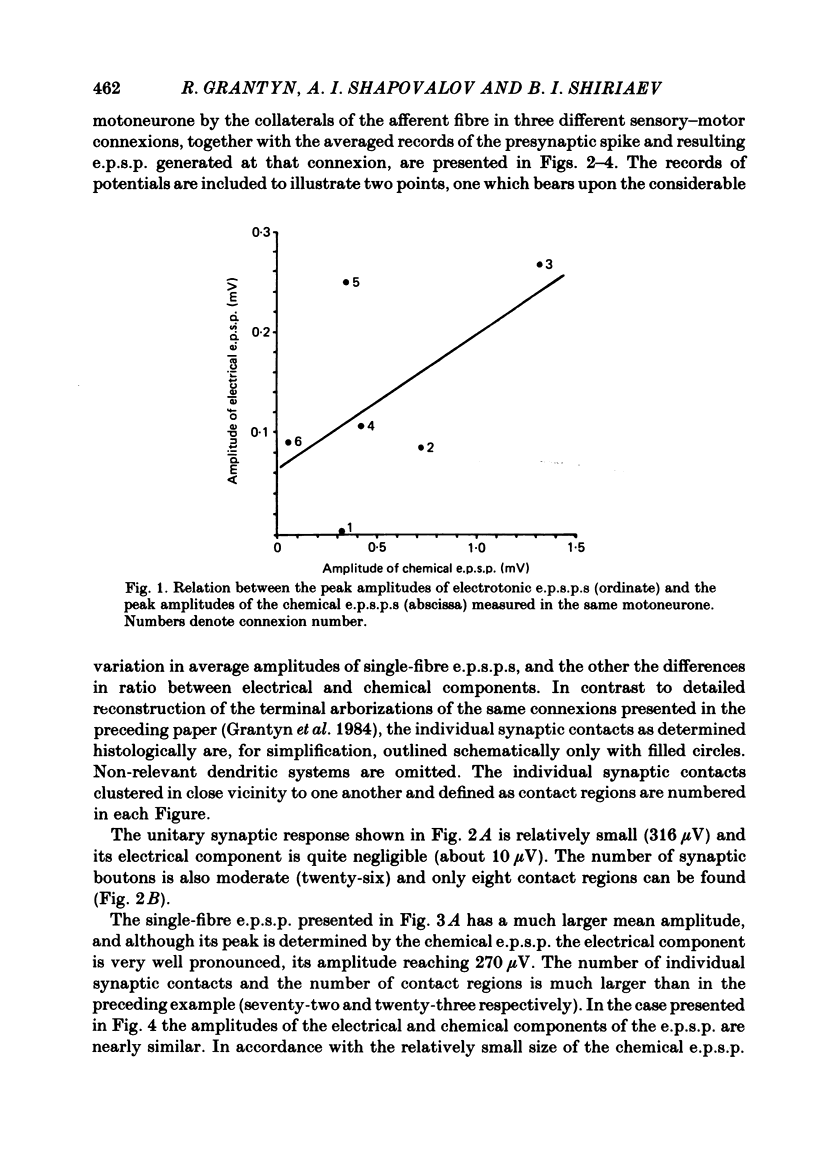
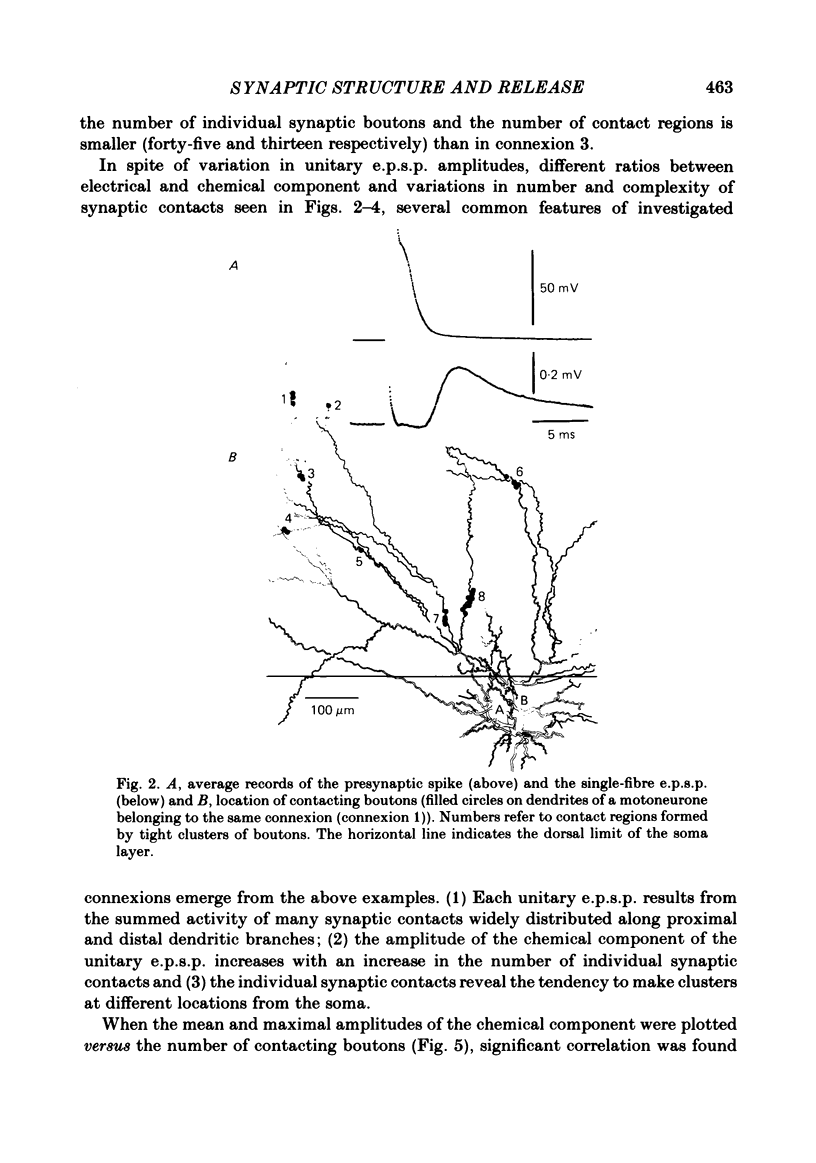
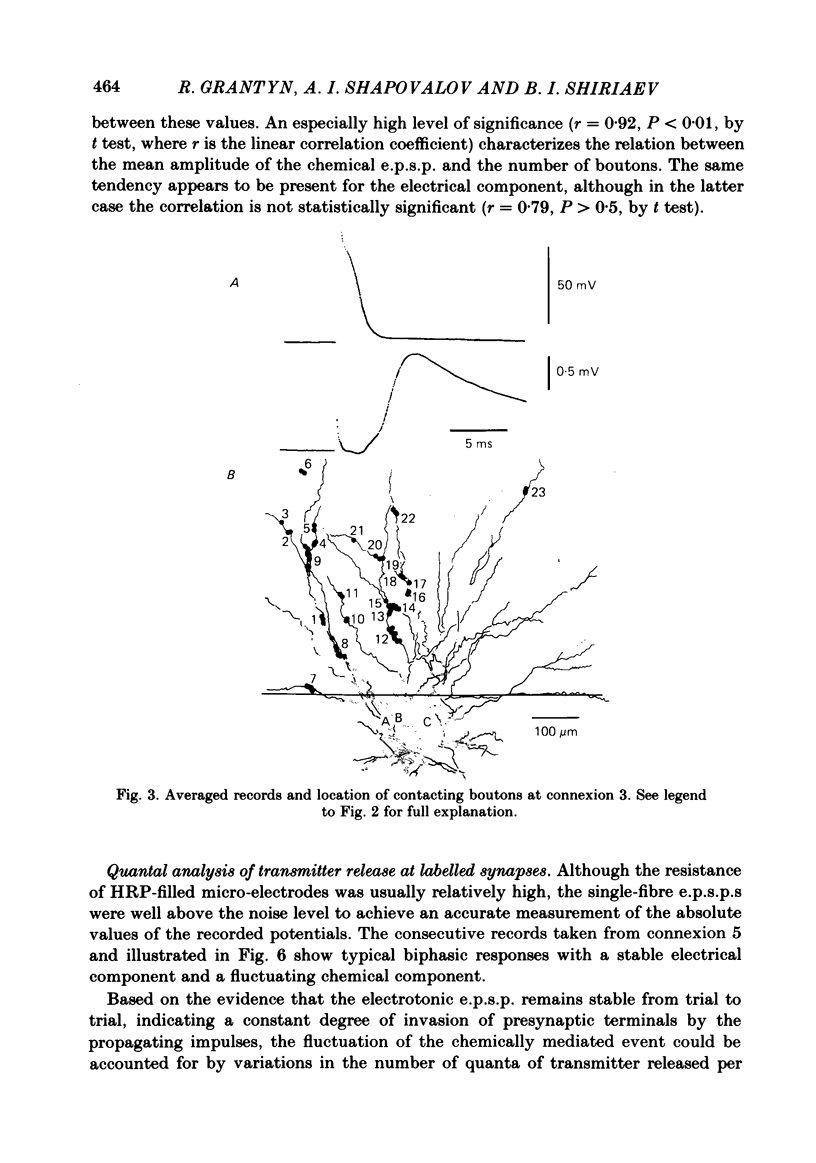
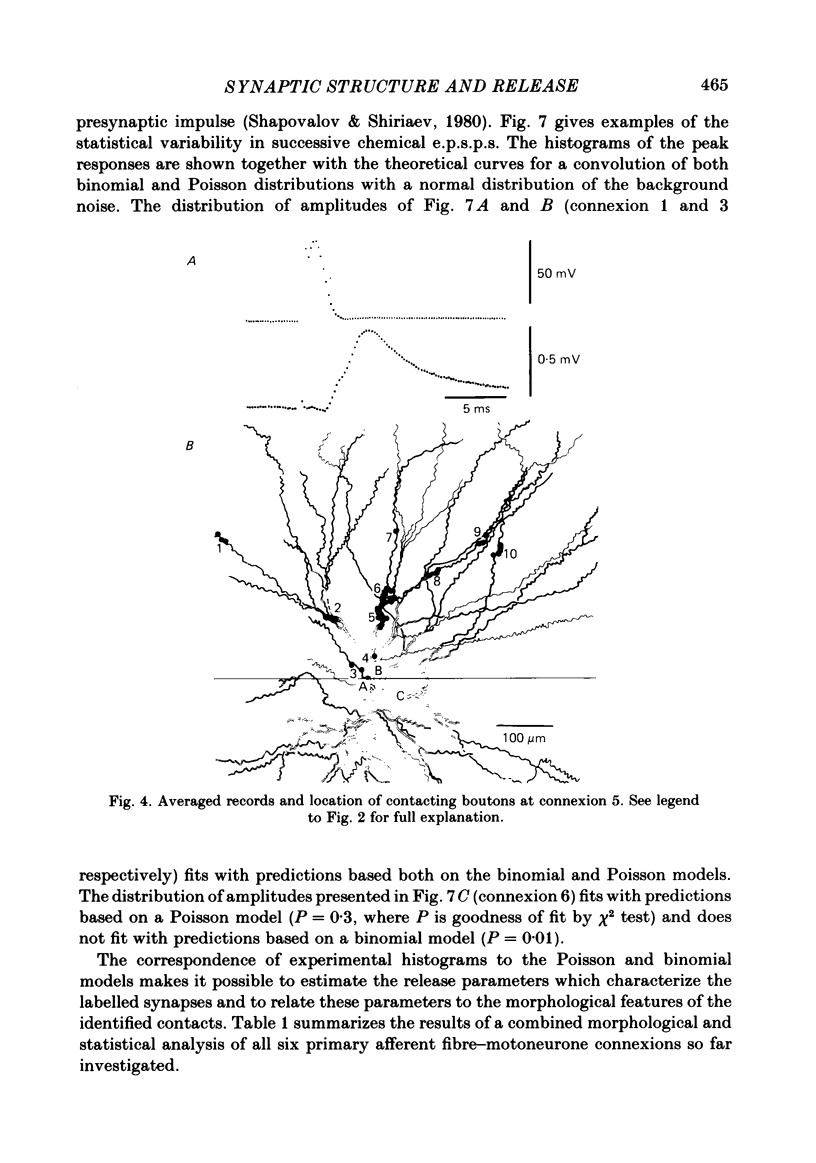
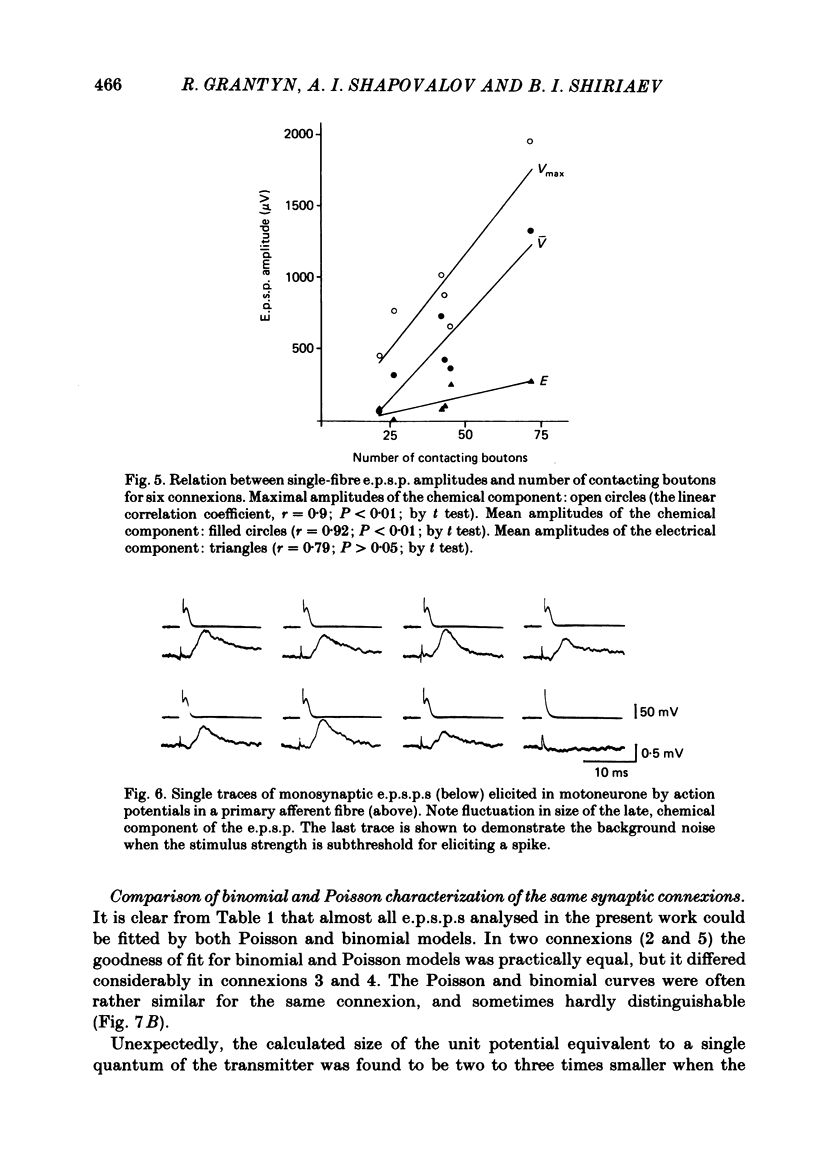
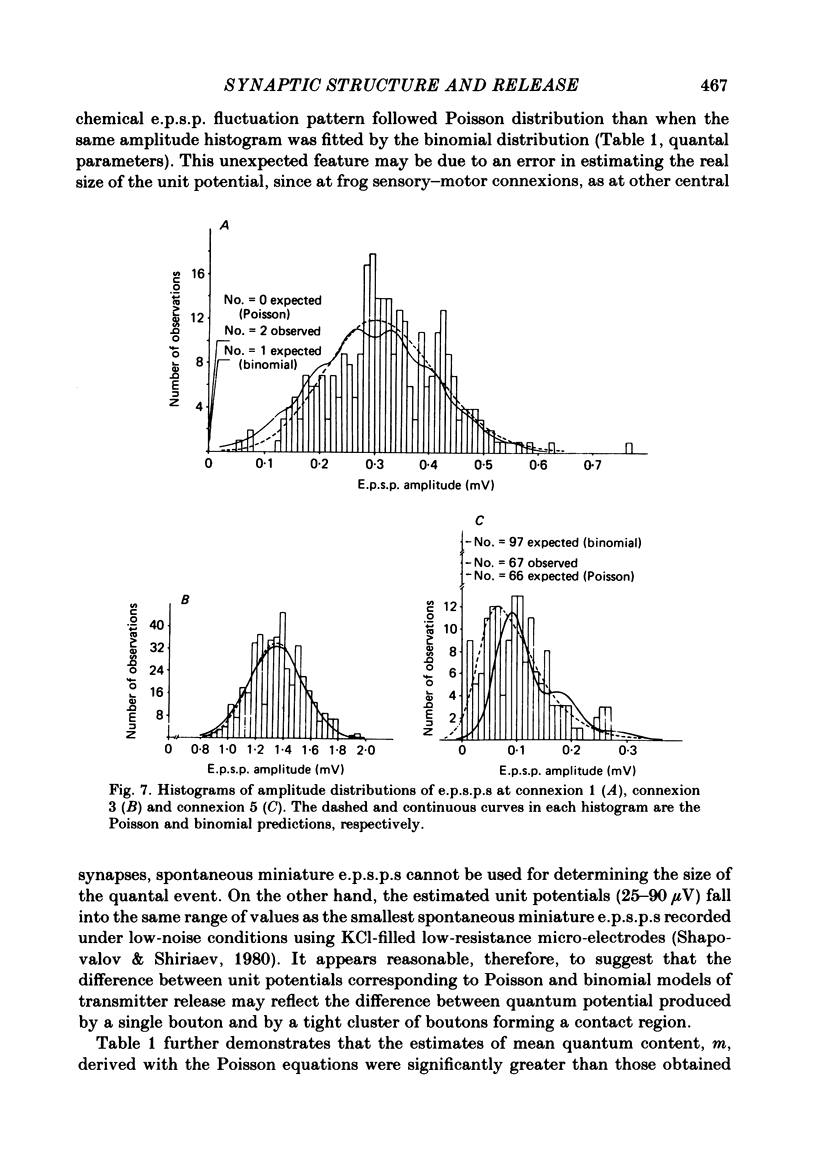
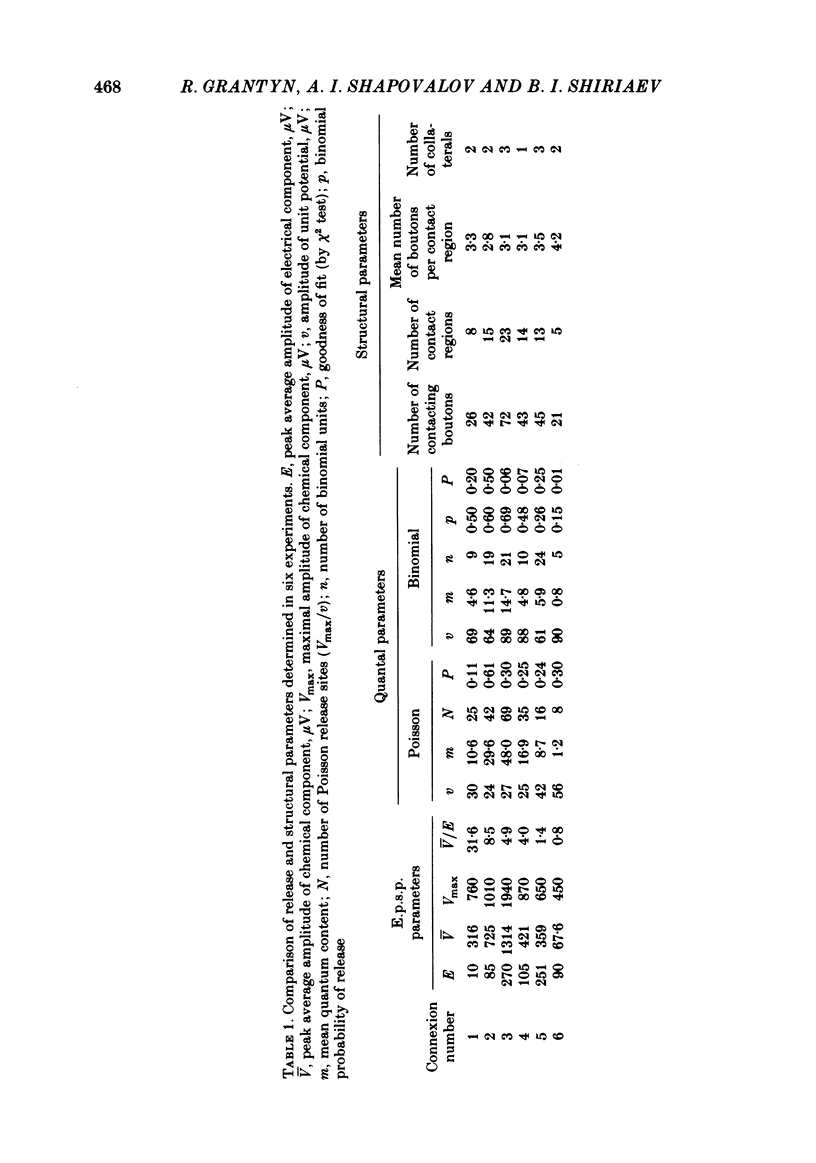
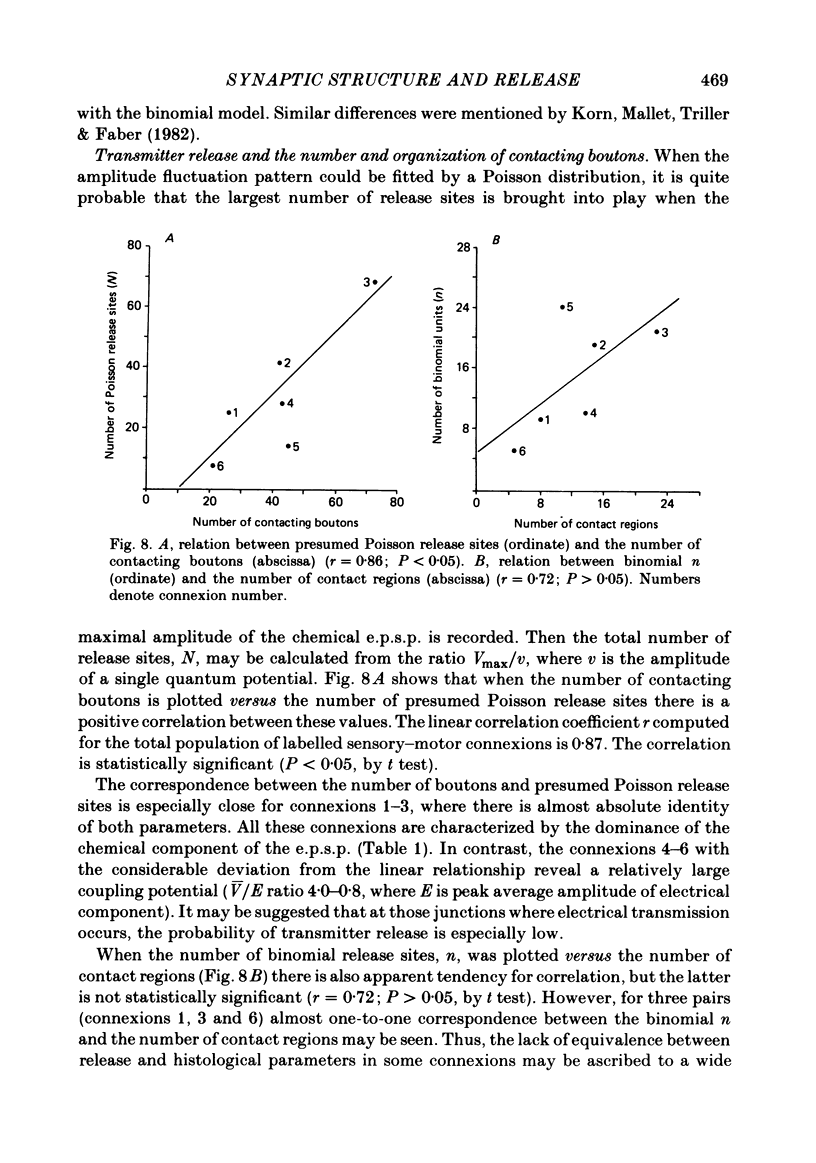
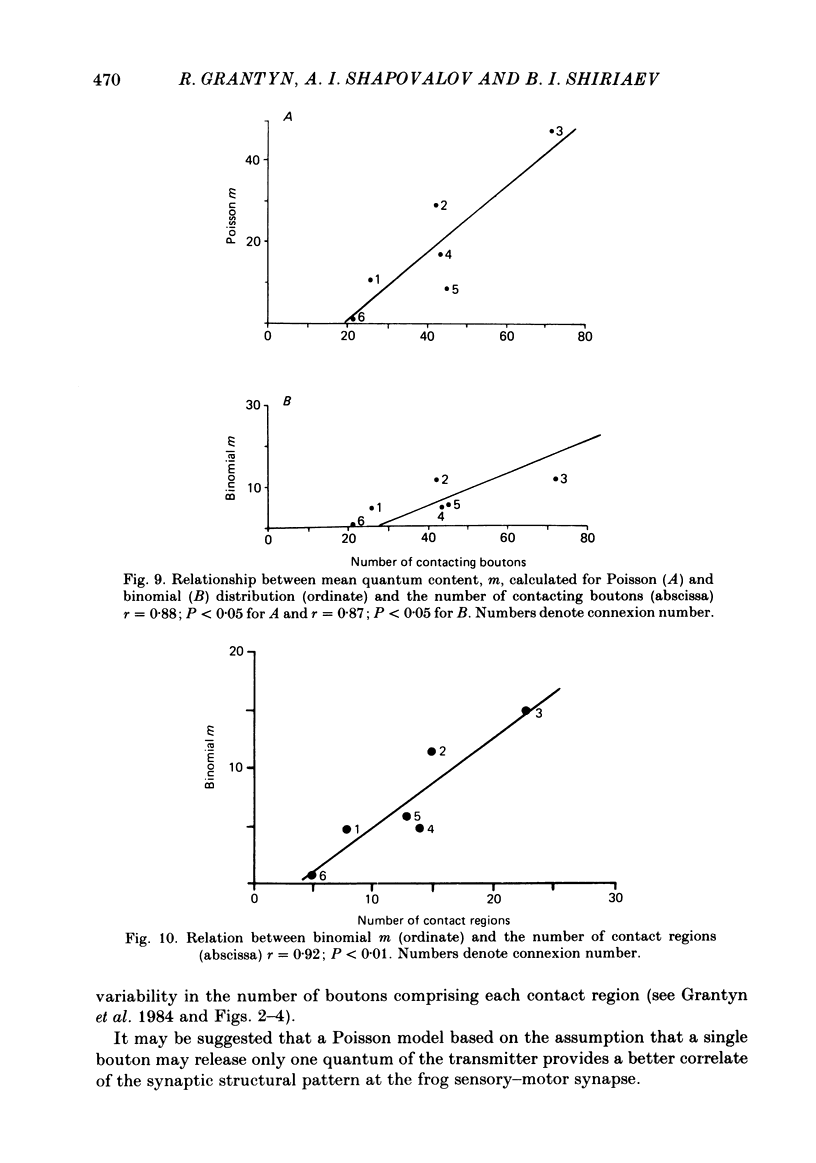
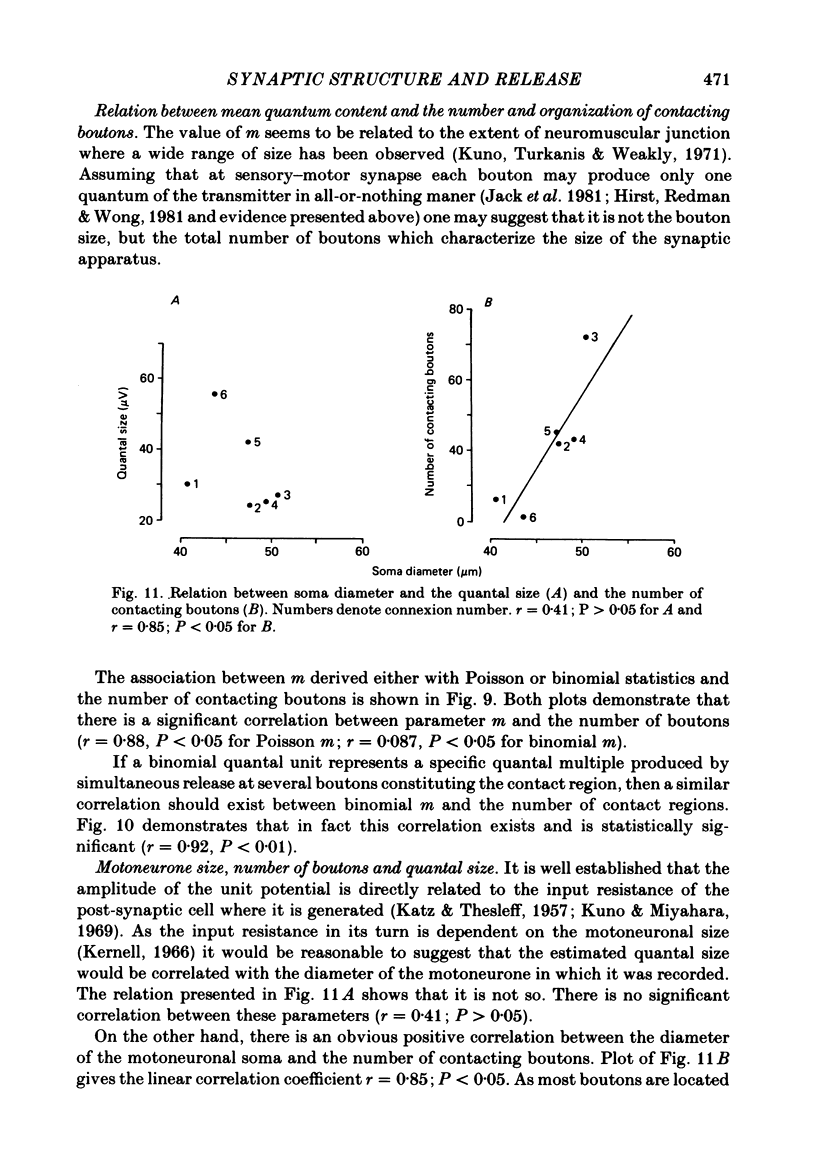
The sensory-motor synaptic connexions in the frog lumbar cord have been used to examine the relationship between the statistical characteristics of the unitary excitatory post-synaptic potential (e.p.s.p.) and the number and organization of synaptic contacts determined when the primary afferent fibre used in evoking the e.p.s.p., and a motoneurone in which it was recorded, were both labelled with horseradish peroxidase (HRP). A significant correlation is found between the number of contacting boutons and the amplitude of the chemical component of the unitary e.p.s.p.s generated at the same connexions. The amplitude fluctuation patterns of the single-fibre e.p.s.p.s could be fitted by both Poisson and binomial distribution. The number of presumed Poisson release sites as estimated from the ratio Vmax/v (where Vmax is the maximal amplitude of the chemical component of e.p.s.p. and v is quantal size) is always less than or equal to the total number of boutons observed histologically. In three connexions there was a close correspondence between the number of binomial release units, n, and the number of contact regions formed by the tight clusters of contacting boutons. The unit potential amplitude estimated from the Poisson distribution is found to be two to three times smaller than the quantal size calculated from binomial distribution. A similar numerical relationship was found between the number of contacting boutons and the number of contact regions. It is suggested that at a single bouton, transmission results in release of a single quantum of transmitter, whereas the binomial quantum probably reflects the multi-quantal release occurring simultaneously at boutons comprising a contact region. A significant correlation is found between the mean quantum content estimated either from Poisson or binomial distribution and the number of contacting boutons and contact regions respectively, indicating the dependence of quantal release on the magnitude of synaptic surface. No correlation is found between the motoneuronal soma diameter and the quantal size, although the former is significantly correlated with the number of contacting boutons.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adanina V. O., Shapovalov A. I. Smeshannye sinapsy pervichnykh afferentnykh volokon na motoneironakh liagushki, identifitsirovannye s pomoshch'iu neiroplaz-maticheskogo transporta peroksidazy khrena i élektronnoi mikroskopii. Dokl Akad Nauk SSSR. 1983;269(1):231–232. [PubMed] [Google Scholar]
- Bennet M. R., Lavidis N. A. Variation in quantal secretion at different release sites along developing and mature motor terminal branches. Brain Res. 1982 Sep;281(1):1–9. doi: 10.1016/0165-3806(82)90107-9. [DOI] [PubMed] [Google Scholar]
- Bennett M. R., Lavidis N. A. The effect of calcium ions on the secretion of quanta evoked by an impulse at nerve terminal release sites. J Gen Physiol. 1979 Oct;74(4):429–456. doi: 10.1085/jgp.74.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Non-quantal fluctuations and transmission failures in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):689–704. doi: 10.1113/jphysiol.1976.sp011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Statistical fluctuations in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):665–688. doi: 10.1113/jphysiol.1976.sp011488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. An analysis of the relationship between the current and potential generated by a quantum of acetylcholine in muscle fibers without transverse tubules. J Membr Biol. 1973;12(3):247–272. doi: 10.1007/BF01870004. [DOI] [PubMed] [Google Scholar]
- Grantyn R., Shapovalov A. I., Shiriaev B. I. Combined morphological and electrophysiological description of connections between single primary afferent fibres and individual motoneurons in the frog spinal cord. Exp Brain Res. 1982;48(3):459–462. doi: 10.1007/BF00238624. [DOI] [PubMed] [Google Scholar]
- Grantyn R., Shapovalov A. I., Shiriaev B. I. Tracing of frog sensory-motor synapses by intracellular injection of horseradish peroxidase. J Physiol. 1984 Apr;349:441–458. doi: 10.1113/jphysiol.1984.sp015166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Redman S. J., Wong K. Post-tetanic potentiation and facilitation of synaptic potentials evoked in cat spinal motoneurones. J Physiol. 1981 Dec;321:97–109. doi: 10.1113/jphysiol.1981.sp013973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. J., Redman S. J., Wong K. The components of synaptic potentials evoked in cat spinal motoneurones by impulses in single group Ia afferents. J Physiol. 1981 Dec;321:65–96. doi: 10.1113/jphysiol.1981.sp013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. A STUDY OF SPONTANEOUS MINIATURE POTENTIALS IN SPINAL MOTONEURONES. J Physiol. 1963 Sep;168:389–422. doi: 10.1113/jphysiol.1963.sp007199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNO M. QUANTAL COMPONENTS OF EXCITATORY SYNAPTIC POTENTIALS IN SPINAL MOTONEURONES. J Physiol. 1964 Dec;175:81–99. doi: 10.1113/jphysiol.1964.sp007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D. Input resistance, electrical excitability, and size of ventral horn cells in cat spinal cord. Science. 1966 Jun 17;152(3729):1637–1640. doi: 10.1126/science.152.3729.1637. [DOI] [PubMed] [Google Scholar]
- Korn H., Mallet A., Triller A., Faber D. S. Transmission at a central inhibitory synapse. II. Quantal description of release, with a physical correlate for binomial n. J Neurophysiol. 1982 Sep;48(3):679–707. doi: 10.1152/jn.1982.48.3.679. [DOI] [PubMed] [Google Scholar]
- Kuno M., Miyahara J. T. Analysis of synaptic efficacy in spinal motoneurones from 'quantum' aspects. J Physiol. 1969 Apr;201(2):479–493. doi: 10.1113/jphysiol.1969.sp008768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno M., Turkanis S. A., Weakly J. N. Correlation between nerve terminal size and transmitter release at the neuromuscular junction of the frog. J Physiol. 1971 Mar;213(3):545–556. doi: 10.1113/jphysiol.1971.sp009399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. How the size of motoneurones determines their susceptibility to discharge. Nature. 1979 Dec 20;282(5741):859–861. doi: 10.1038/282859a0. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I. Interneuronal synapses with electrical, dual and chemical mode of transmission in vertebrates. Neuroscience. 1980;5(7):1113–1124. doi: 10.1016/0306-4522(80)90190-6. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I. Dual mode of junctional transmission at synapses between single primary afferent fibres and motoneurones in the amphibian. J Physiol. 1980 Sep;306:1–15. doi: 10.1113/jphysiol.1980.sp013381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I. Single-fiber EPSPs in amphibian motoneurons. Brain Res. 1979 Jan 19;160(3):519–523. doi: 10.1016/0006-8993(79)91079-5. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I., Tamarova Z. A. Synaptic activity in motoneurons of the immature cat spinal cord in vitro. Effects of manganese and tetrodotoxin. Brain Res. 1979 Jan 19;160(3):524–528. doi: 10.1016/0006-8993(79)91080-1. [DOI] [PubMed] [Google Scholar]
- Shiriaev B. I. Sinapticheskie éffekty, vyzyvaemye v motoneironakh spinnogo mozga liagushki adekvatnym razdrazheniem myshechnykh vereten. Fiziol Zh SSSR Im I M Sechenova. 1983 Sep;69(9):1157–1163. [PubMed] [Google Scholar]
- Tamarova Z. A., Shapovalov A. I., Shiriaev B. I. Vliianie ionov magniia, margantsa i nedostatka kal'tsiia na sinapticheskuiu peredachu v izolirovannom spinnom mozge krysy. Neirofiziologiia. 1978;10(5):530–533. [PubMed] [Google Scholar]
- Taugner R., Sonnhof U., Richter D. W., Schiller A. Mixed (chemical and electrical) synapses on frog spinal motoneurons. Cell Tissue Res. 1978 Oct 6;193(1):41–59. doi: 10.1007/BF00221600. [DOI] [PubMed] [Google Scholar]


