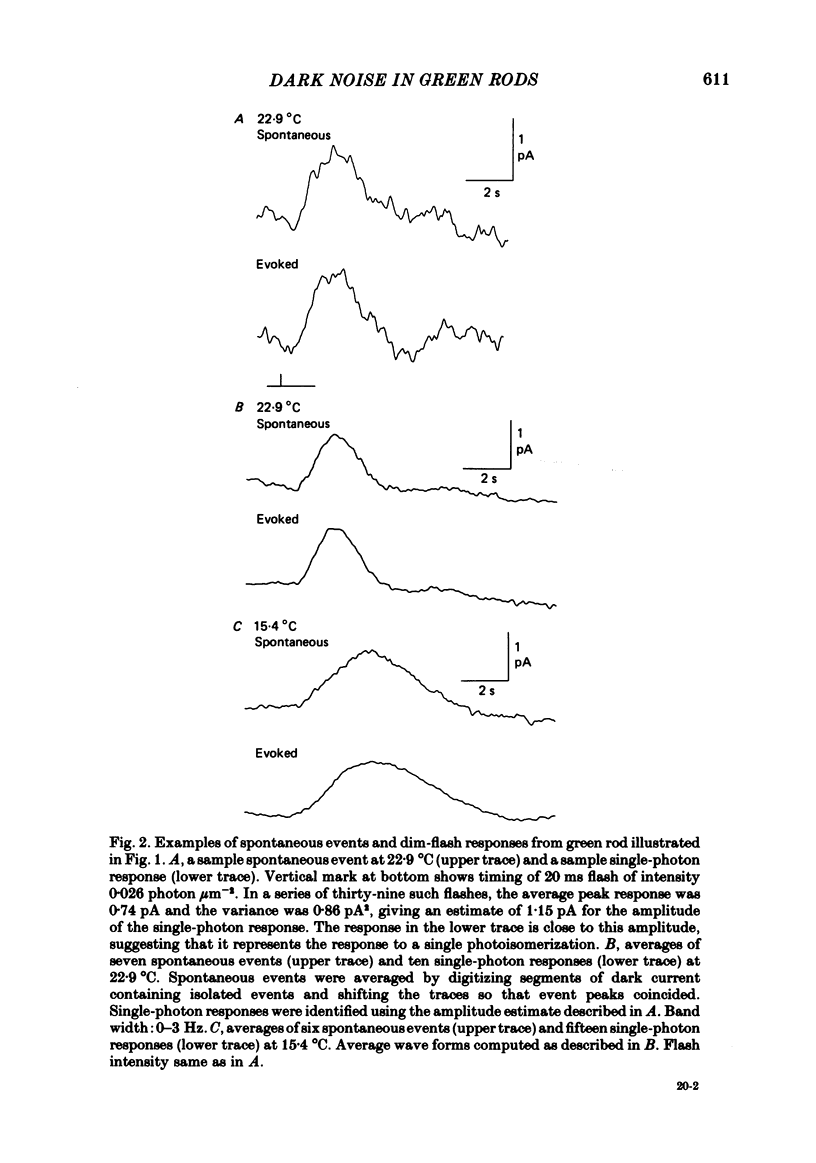
Abstract
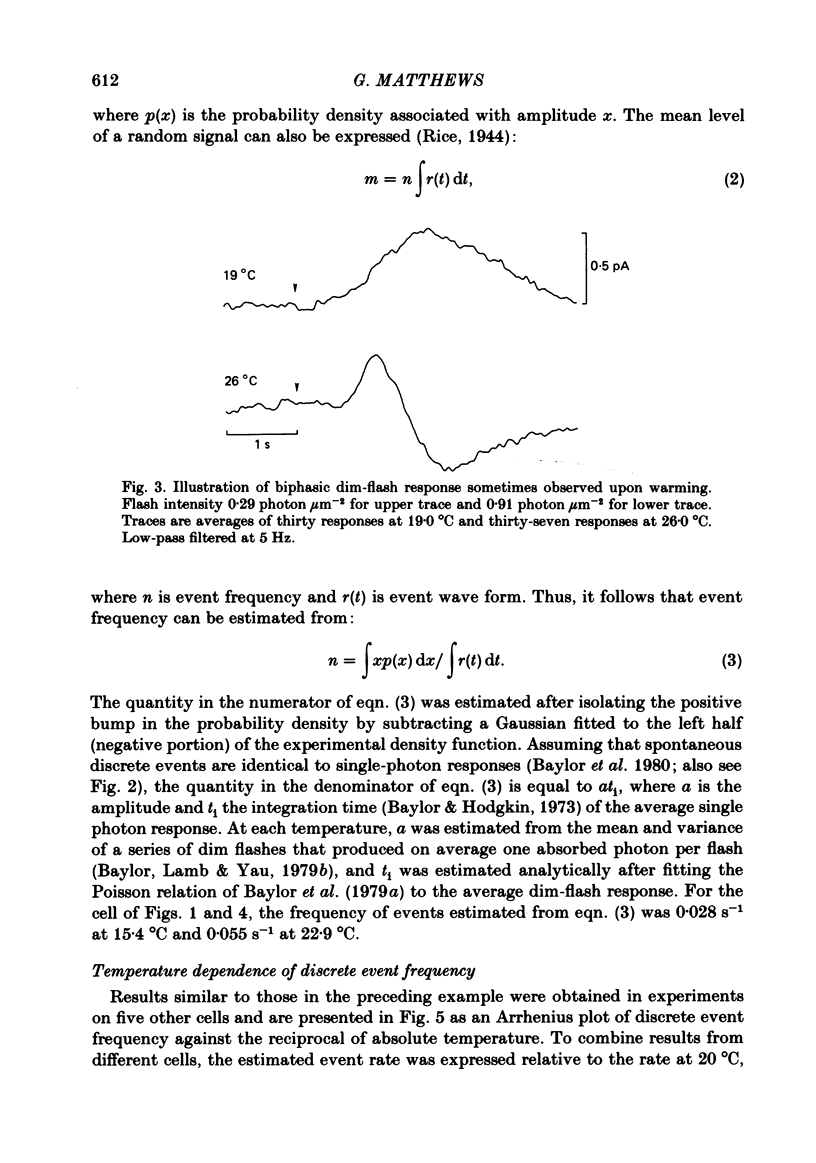
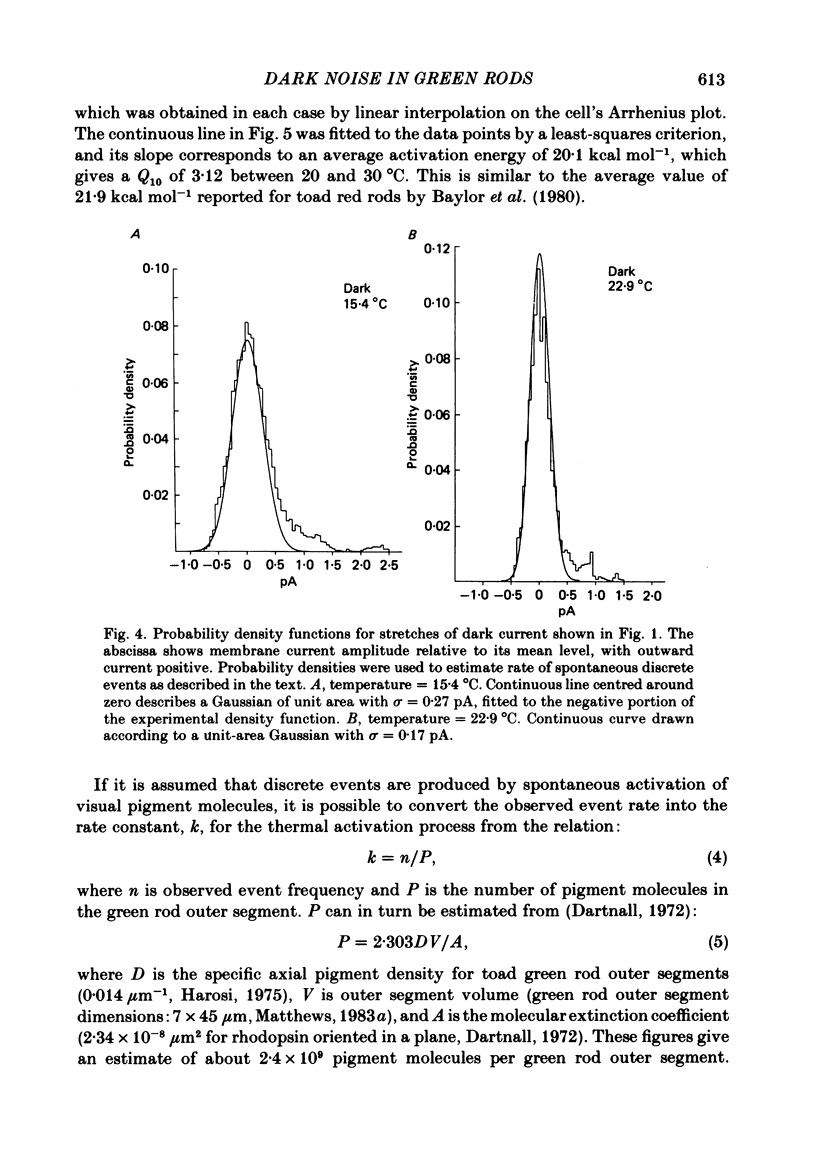
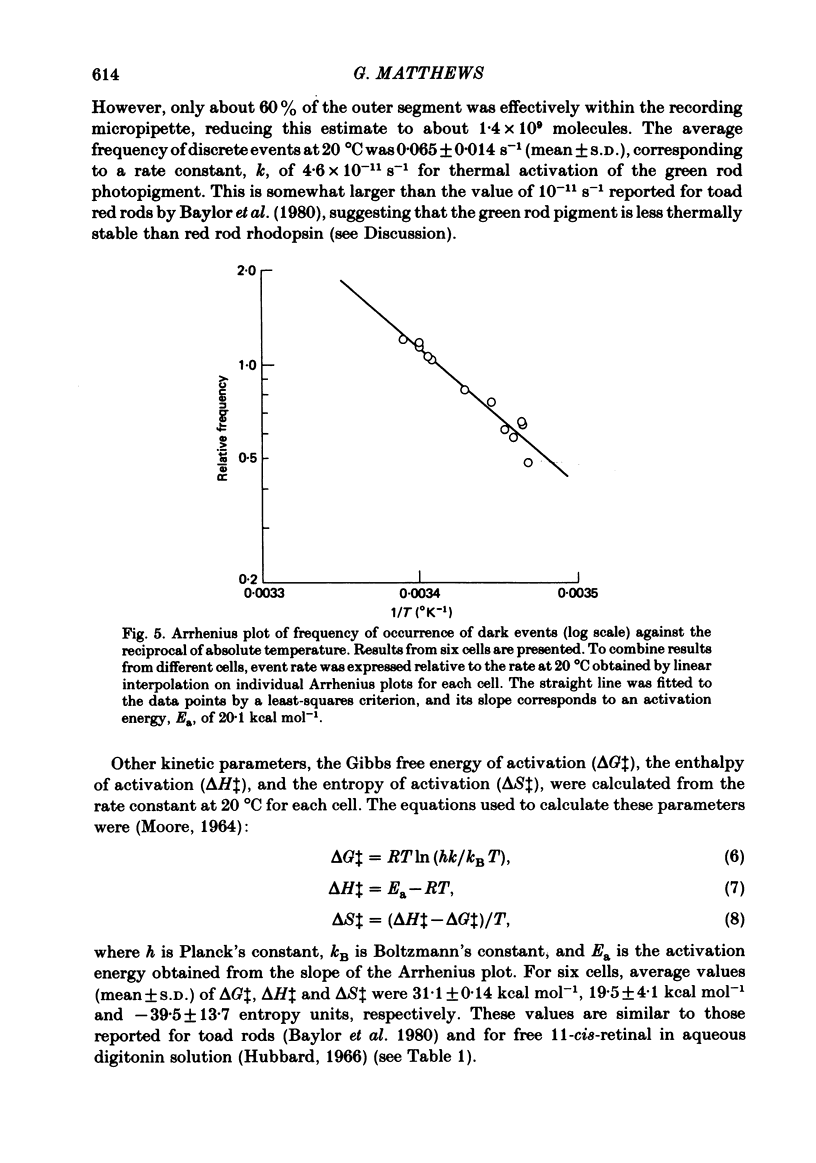
The outer segment membrane current of single green rods from toad retina was recorded with a suction electrode, and the rate of spontaneous noise events similar to single-photon responses was measured at different temperatures. The activation energy, Gibbs free energy of activation, and entropy of activation for the process producing spontaneous events were similar to the values reported for thermal isomerization of free 11-cis-retinal (Hubbard, 1966), suggesting that thermal isomerization of the chromophore may be the trigger for the spontaneous events. The apparent rate constant for thermal activation of the green rod photopigment was about 4 times higher than in red rods but about 1000 times lower than for free 11-cis-retinal. Thus, both red and green rod opsin appear to stabilize 11-cis-retinal against thermal isomerization, but green rod opsin is somewhat less effective. The speed of the average dim-flash response increased as temperature was raised, as reported previously in both cone and rod photoreceptors. The reciprocal of the time-to-peak of the dim-flash response had an average Q10 of 3.3 between 20 and 30 degrees C. Changing temperature shifted the time scale of the response without altering response wave form, suggesting that all delay stages shaping the light response were approximately equally affected by temperature. At temperatures greater than 25 degrees C, flash responses were sometimes biphasic, i.e. inward dark current first decreased after a flash, then transiently increased beyond the resting dark level.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashmore J. F., Falk G. Dark noise in retinal bipolar cells and stability of rhodopsin in rods. Nature. 1977 Nov 3;270(5632):69–71. doi: 10.1038/270069a0. [DOI] [PubMed] [Google Scholar]
- BARLOW H. B. Increment thresholds at low intensities considered as signal/noise discriminations. J Physiol. 1957 May 23;136(3):469–488. doi: 10.1113/jphysiol.1957.sp005774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. The electrical response of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):685–727. doi: 10.1113/jphysiol.1974.sp010731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Temperature effects on the membrane current of retinal rods of the toad. J Physiol. 1983 Apr;337:723–734. doi: 10.1113/jphysiol.1983.sp014651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. Energy uptake in the first step of visual excitation. Nature. 1979 Nov 29;282(5738):531–533. doi: 10.1038/282531a0. [DOI] [PubMed] [Google Scholar]
- HUBBARD R. The thermal stability of rhodopsin and opsin. J Gen Physiol. 1958 Nov 20;42(2):259–280. doi: 10.1085/jgp.42.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R. The stereoisomerization of 11-cis-retinal. J Biol Chem. 1966 Apr 25;241(8):1814–1818. [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Visual pigments of frog and tadpole (Rana pipiens). Vision Res. 1968 Jul;8(7):761–775. doi: 10.1016/0042-6989(68)90128-4. [DOI] [PubMed] [Google Scholar]
- Matthews G. Physiological characteristics of single green rod photoreceptors from toad retina. J Physiol. 1983 Sep;342:347–359. doi: 10.1113/jphysiol.1983.sp014855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papermaster D. S., Schneider B. G., Zorn M. A., Kraehenbuhl J. P. Immunocytochemical localization of opsin in outer segments and Golgi zones of frog photoreceptor cells. An electron microscope analysis of cross-linked albumin-embedded retinas. J Cell Biol. 1978 Apr;77(1):196–210. doi: 10.1083/jcb.77.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


