Abstract
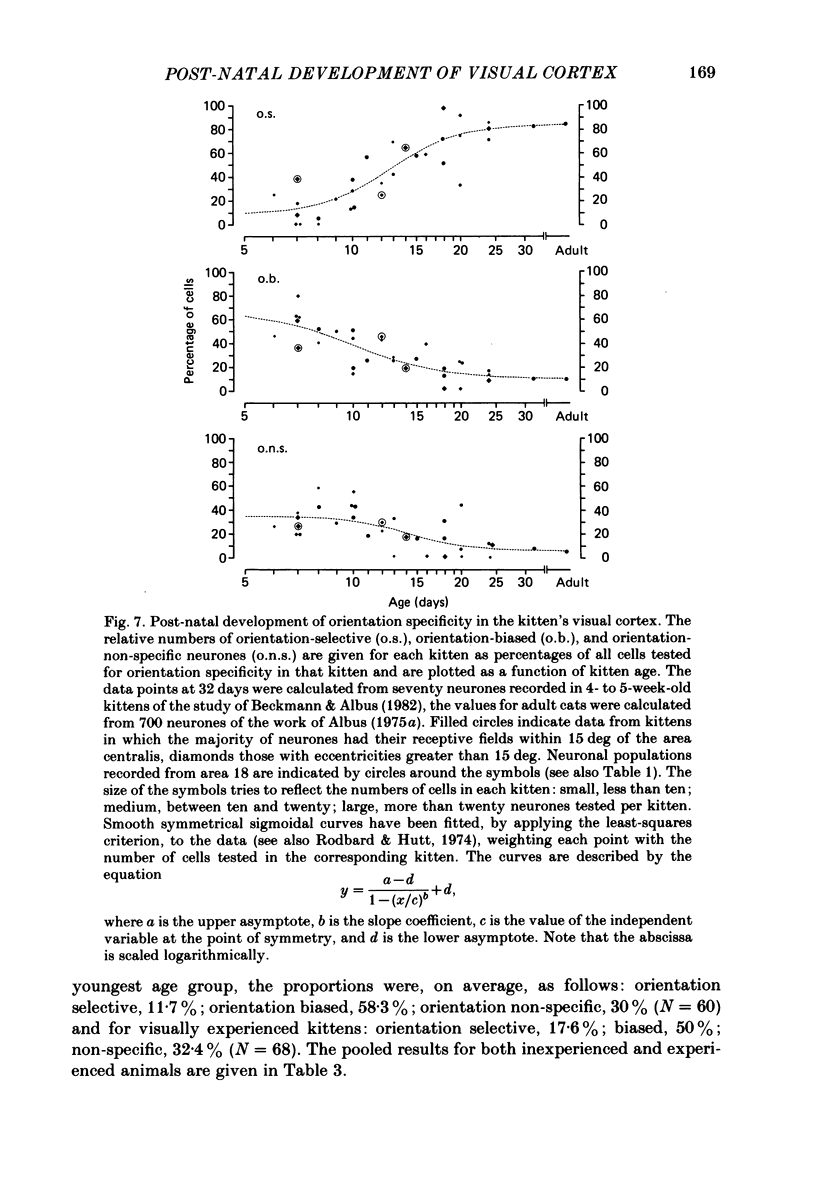
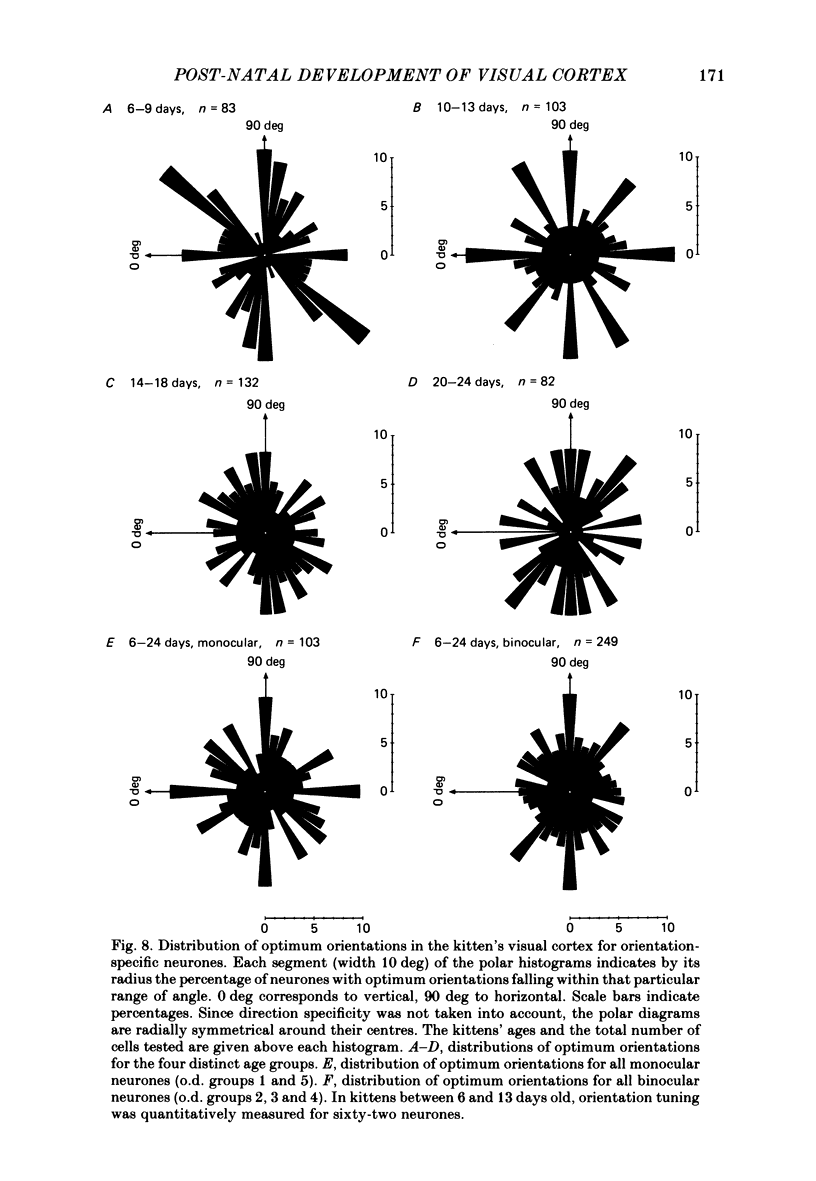
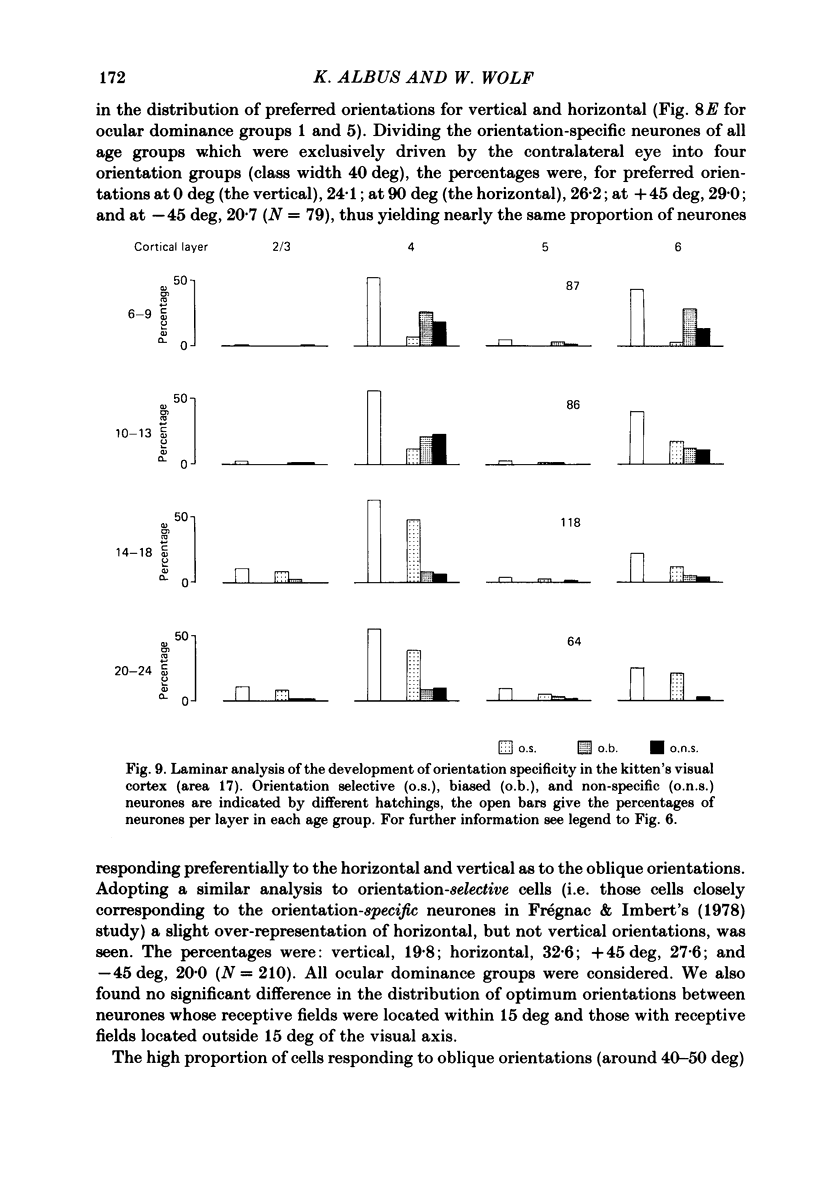
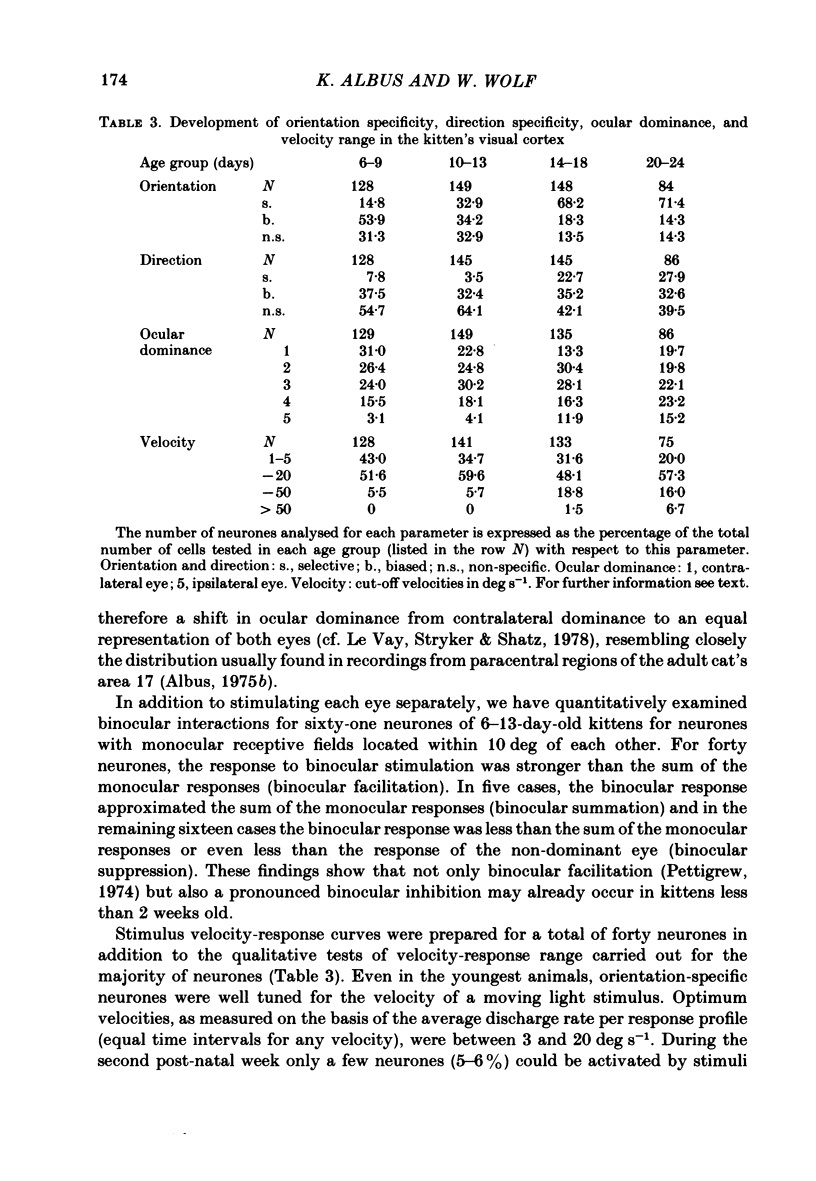
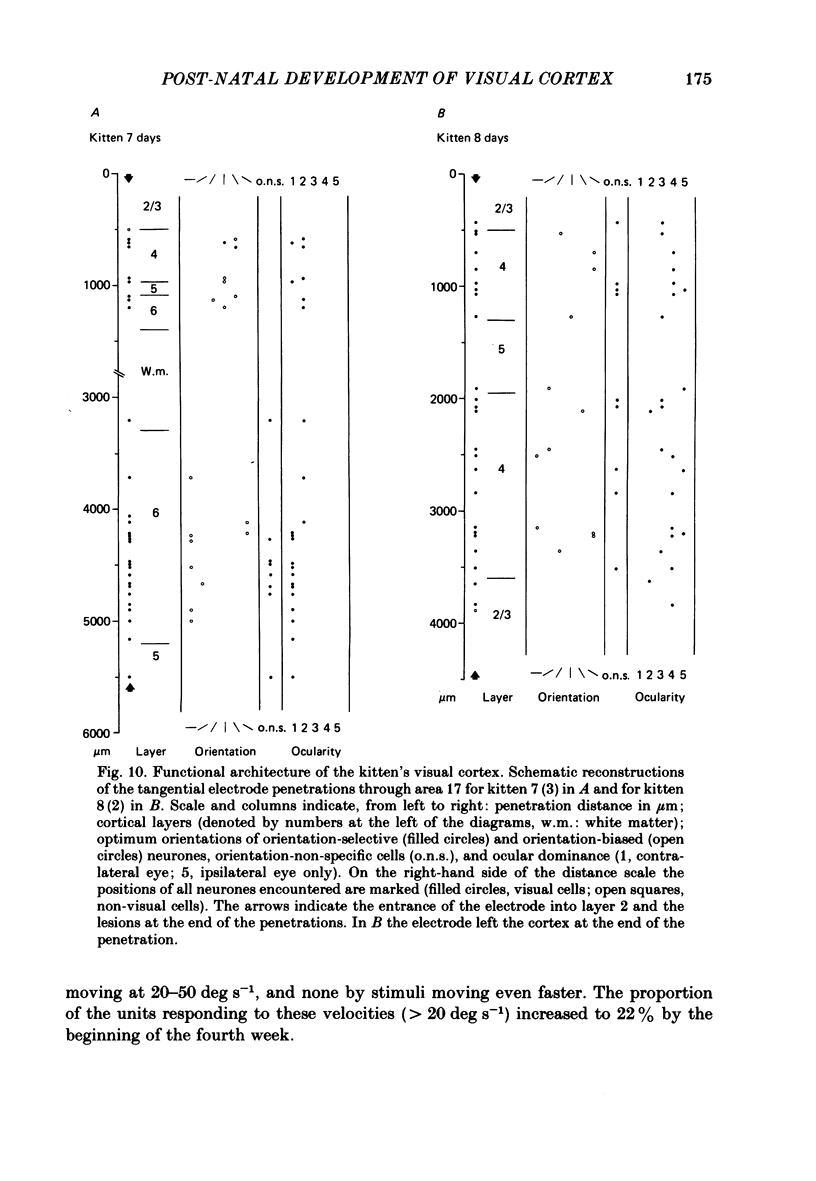
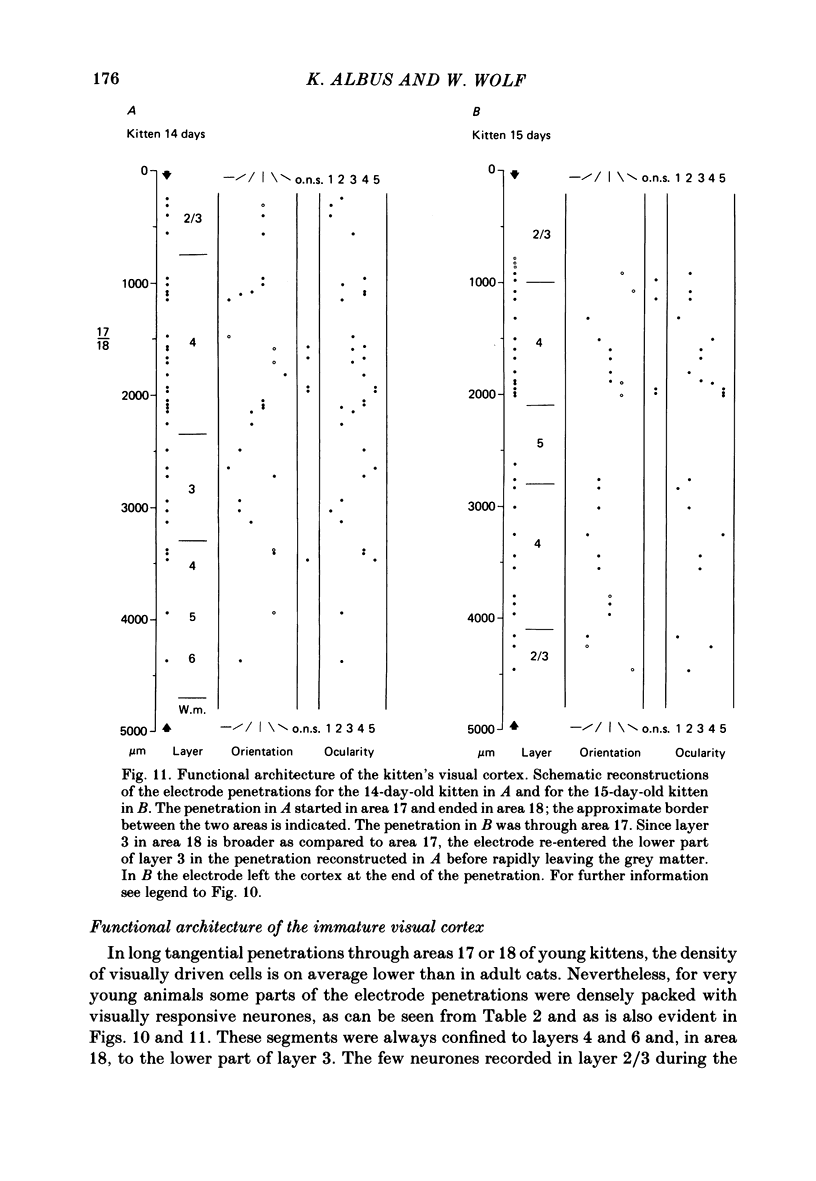
The normal post-natal development of visual cortical functions was studied by recording extracellularly from 612 single neurones in the striate and parastriate cortex of anaesthetized and paralysed kittens, ranging in age from 6 to 24 days. Analyses have been made of laminar differences in the developmental trends of receptive field properties such as orientation specificity and spatial organization of 'on' and 'off' zones. At the beginning of the second post-natal week the majority of neurones (76%) only respond to light 'off' (unimodal 'off' neurones). Only later does the frequency of occurrence of unimodal 'on' neurones and of bimodal or multimodal neurones (with spatially segregated 'on' and 'off' zones arranged side by side) increase so that, by the middle of the fourth week, about equal numbers of these three receptive field types are found. The proportion of 'on-off' neurones (with spatially coincident 'on' and 'off' zones) remains low (between 9% and 12%) during the early post-natal period. In layers 4 and 6 of areas 17 and 18 the frequency of occurrence of visual neurones is quite normal even in the youngest kittens, whereas the probability of recording neurones in layers 2/3 and 5 in kittens less than 14 days old is remarkably low and only gradually improves up to the middle of the fourth week. A very rudimentary order in the spatial arrangement of orientation-specific neurones and ocular dominance distribution is observed even in very young kittens. This order improves rapidly and reaches adult levels during the fourth post-natal week. In visually inexperienced kittens, on average 11% of all responsive neurones are selective for the orientation of elongated visual stimuli, and 58% are biased. The proportion of orientation-selective cells begins to increase rapidly about two days after lid opening, and proportions of orientation-selective cells similar to that in the adult are reached by the end of the fourth post-natal week. Orientation-selective neurones in kittens less than 10 days old are only found in layers 4 and 6 and the lower part of layer 3. In layers 2/3 and 5 they are first seen in larger proportions by the beginning of the third post-natal week. Our results show that, during the first post-natal month, the time course of the functional development of visual cortical neurones depends on receptive field type and on intracortical location.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albus K. A quantitative study of the projection area of the central and the paracentral visual field in area 17 of the cat. II. The spatial organization of the orientation domain. Exp Brain Res. 1975 Dec 22;24(2):181–202. doi: 10.1007/BF00234062. [DOI] [PubMed] [Google Scholar]
- Albus K. Predominance of monocularly driven cells in the projection area of the central visual field in cat's striate cortex. Brain Res. 1975 May 23;89(2):341–347. doi: 10.1016/0006-8993(75)90725-8. [DOI] [PubMed] [Google Scholar]
- Albus K. The detection of movement direction and effects of contrast reversal in the cat's striate cortex. Vision Res. 1980;20(4):289–293. doi: 10.1016/0042-6989(80)90015-2. [DOI] [PubMed] [Google Scholar]
- Anker R. L. The prenatal development of some of the visual pathways in the cat. J Comp Neurol. 1977 May 1;173(1):185–204. doi: 10.1002/cne.901730111. [DOI] [PubMed] [Google Scholar]
- Barlow H. B., Blakemore C., Pettigrew J. D. The neural mechanism of binocular depth discrimination. J Physiol. 1967 Nov;193(2):327–342. doi: 10.1113/jphysiol.1967.sp008360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann R., Albus K. The geniculocortical system in the early postnatal kitten: an electrophysiological investigation. Exp Brain Res. 1982;47(1):49–56. doi: 10.1007/BF00235885. [DOI] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Receptive fields of simple cells in the cat striate cortex. J Physiol. 1973 May;231(1):31–60. doi: 10.1113/jphysiol.1973.sp010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C. Genetic instructions and developmental plasticity in the kitten's visual cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):425–434. doi: 10.1098/rstb.1977.0052. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Van Sluyters R. C. Innate and environmental factors in the development of the kitten's visual cortex. J Physiol. 1975 Jul;248(3):663–716. doi: 10.1113/jphysiol.1975.sp010995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisseret P., Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J Physiol. 1976 Feb;255(2):511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullier J., Henry G. H. Laminar distribution of first-order neurons and afferent terminals in cat striate cortex. J Neurophysiol. 1979 Sep;42(5):1271–1281. doi: 10.1152/jn.1979.42.5.1271. [DOI] [PubMed] [Google Scholar]
- Bullier J., Henry G. H. Ordinal position of neurons in cat striate cortex. J Neurophysiol. 1979 Sep;42(5):1251–1263. doi: 10.1152/jn.1979.42.5.1251. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. The development of synapses in kitten visual cortex during visual deprivation. Exp Neurol. 1975 Mar;46(3):445–451. doi: 10.1016/0014-4886(75)90118-1. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. The development of synapses in the visual system of the cat. J Comp Neurol. 1975 Mar 15;160(2):147–166. doi: 10.1002/cne.901600202. [DOI] [PubMed] [Google Scholar]
- Daniels J. D., Pettigrew J. D., Norman J. L. Development of single-neuron responses in kitten's lateral geniculate nucleus. J Neurophysiol. 1978 Nov;41(6):1373–1393. doi: 10.1152/jn.1978.41.6.1373. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Fuchs A. F. The development of spatial-frequency selectivity in kitten striate cortex. J Physiol. 1981 Jul;316:1–10. doi: 10.1113/jphysiol.1981.sp013767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frégnac Y., Imbert M. Early development of visual cortical cells in normal and dark-reared kittens: relationship between orientation selectivity and ocular dominance. J Physiol. 1978 May;278:27–44. doi: 10.1113/jphysiol.1978.sp012290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979 Jul 12;280(5718):120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS OF CELLS IN STRIATE CORTEX OF VERY YOUNG, VISUALLY INEXPERIENCED KITTENS. J Neurophysiol. 1963 Nov;26:994–1002. doi: 10.1152/jn.1963.26.6.994. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggelund P., Albus K. Response variability and orientation discrimination of single cells in striate cortex of cat. Exp Brain Res. 1978 Jun 19;32(2):197–211. doi: 10.1007/BF00239727. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Bishop P. O., Tupper R. M., Dreher B. Orientation specificity and response variability of cells in the striate cortex. Vision Res. 1973 Sep;13(9):1771–1779. doi: 10.1016/0042-6989(73)90094-1. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. P., Cynader M. Functional aspects of plasticity in the visual system of adult cats after early monocular deprivation. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):411–424. doi: 10.1098/rstb.1977.0051. [DOI] [PubMed] [Google Scholar]
- Kato H., Bishop P. O., Orban G. A. Hypercomplex and simple/complex cell classifications in cat striate cortex. J Neurophysiol. 1978 Sep;41(5):1071–1095. doi: 10.1152/jn.1978.41.5.1071. [DOI] [PubMed] [Google Scholar]
- LeVay S., Gilbert C. D. Laminar patterns of geniculocortical projection in the cat. Brain Res. 1976 Aug 20;113(1):1–19. doi: 10.1016/0006-8993(76)90002-0. [DOI] [PubMed] [Google Scholar]
- LeVay S., Stryker M. P., Shatz C. J. Ocular dominance columns and their development in layer IV of the cat's visual cortex: a quantitative study. J Comp Neurol. 1978 May 1;179(1):223–244. doi: 10.1002/cne.901790113. [DOI] [PubMed] [Google Scholar]
- Leventhal A. G., Hirsch H. V. Receptive-field properties of different classes of neurons in visual cortex of normal and dark-reared cats. J Neurophysiol. 1980 Apr;43(4):1111–1132. doi: 10.1152/jn.1980.43.4.1111. [DOI] [PubMed] [Google Scholar]
- Lund J. S., Henry G. H., MacQueen C. L., Harvey A. R. Anatomical organization of the primary visual cortex (area 17) of the cat. A comparison with area 17 of the macaque monkey. J Comp Neurol. 1979 Apr 15;184(4):599–618. doi: 10.1002/cne.901840402. [DOI] [PubMed] [Google Scholar]
- Molliver M. E., Van der Loos H. The ontogenesis of cortical circuitry: the spatial distribution of synapses in somesthetic cortex of newborn dog. Ergeb Anat Entwicklungsgesch. 1970;42(4):5–53. [PubMed] [Google Scholar]
- Morest D. K. The growth of dendrites in the mammalian brain. Z Anat Entwicklungsgesch. 1969;128(4):290–317. doi: 10.1007/BF00522529. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Van Sluyters R. C. Visual neural development. Annu Rev Psychol. 1981;32:477–522. doi: 10.1146/annurev.ps.32.020181.002401. [DOI] [PubMed] [Google Scholar]
- NOBACK C. R., PURPURA D. P. Postnatal ontogenesis of neurons in cat neocortex. J Comp Neurol. 1961 Dec;117:291–307. doi: 10.1002/cne.901170303. [DOI] [PubMed] [Google Scholar]
- OTSUKA R., HASSLER R. [On the structure and segmentation of the cortical center of vision in the cat]. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1962;203:212–234. doi: 10.1007/BF00352744. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Davis T. L. Comparison of responses to moving and stationary stimuli in cat striate cortex. J Neurophysiol. 1981 Aug;46(2):277–295. doi: 10.1152/jn.1981.46.2.277. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Davis T. L. Receptive-field structure in cat striate cortex. J Neurophysiol. 1981 Aug;46(2):260–276. doi: 10.1152/jn.1981.46.2.260. [DOI] [PubMed] [Google Scholar]
- Pettigrew J. D. The effect of visual experience on the development of stimulus specificity by kitten cortical neurones. J Physiol. 1974 Feb;237(1):49–74. doi: 10.1113/jphysiol.1974.sp010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purpura D. P., Shofer R. J., Scarff T. Properties of synaptic activities and spike potentials of neurons in immature neocortex. J Neurophysiol. 1965 Sep;28(5):925–942. doi: 10.1152/jn.1965.28.5.925. [DOI] [PubMed] [Google Scholar]
- Schoppmann A. Projections from areas 17 and 18 of the visual cortex to the nucleus of the optic tract. Brain Res. 1981 Oct 26;223(1):1–17. doi: 10.1016/0006-8993(81)90802-7. [DOI] [PubMed] [Google Scholar]
- Sherk H., Stryker M. P. Quantitative study of cortical orientation selectivity in visually inexperienced kitten. J Neurophysiol. 1976 Jan;39(1):63–70. doi: 10.1152/jn.1976.39.1.63. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol. 1975 Sep;250(2):305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W., Tretter F., Cynader M. Organization of cat striate cortex: a correlation of receptive-field properties with afferent and efferent connections. J Neurophysiol. 1975 Sep;38(5):1080–1098. doi: 10.1152/jn.1975.38.5.1080. [DOI] [PubMed] [Google Scholar]
- Stein B. E., Gallagher H. L. Maturation of cortical control over superior colliculus cells in cat. Brain Res. 1981 Nov 2;223(2):429–435. doi: 10.1016/0006-8993(81)91160-4. [DOI] [PubMed] [Google Scholar]
- Thorn F., Gollender M., Erickson P. The development of the kittens visual optics. Vision Res. 1976;16(10):1145–1149. doi: 10.1016/0042-6989(76)90255-8. [DOI] [PubMed] [Google Scholar]
- Toyama K., Kimura M., Tanaka K. Organization of cat visual cortex as investigated by cross-correlation technique. J Neurophysiol. 1981 Aug;46(2):202–214. doi: 10.1152/jn.1981.46.2.202. [DOI] [PubMed] [Google Scholar]
- Tsumoto T., Suda K. Laminar differences in development of afferent innervation to striate cortex neurones in kittens. Exp Brain Res. 1982;45(3):433–446. doi: 10.1007/BF01208604. [DOI] [PubMed] [Google Scholar]
- Van Hof-Van Duin J. Direction preference of optokinetic responses in monocularly tested normal kittens and light deprived cats. Arch Ital Biol. 1978 Sep;116(3-4):471–477. [PubMed] [Google Scholar]
- Vidyasagar T. R., Urbas J. V. Orientation sensitivity of cat LGN neurones with and without inputs from visual cortical areas 17 and 18. Exp Brain Res. 1982;46(2):157–169. doi: 10.1007/BF00237172. [DOI] [PubMed] [Google Scholar]
- Winfield D. A. The postnatal development of synapses in the visual cortex of the cat and the effects of eyelid closure. Brain Res. 1981 Feb 9;206(1):166–171. doi: 10.1016/0006-8993(81)90110-4. [DOI] [PubMed] [Google Scholar]
- Wood C. C., Spear P. D., Braun J. J. Direction-specific deficits in horizontal optokinetic nystagmus following removal of visual cortex in the cat. Brain Res. 1973 Sep 28;60(1):231–237. doi: 10.1016/0006-8993(73)90862-7. [DOI] [PubMed] [Google Scholar]


