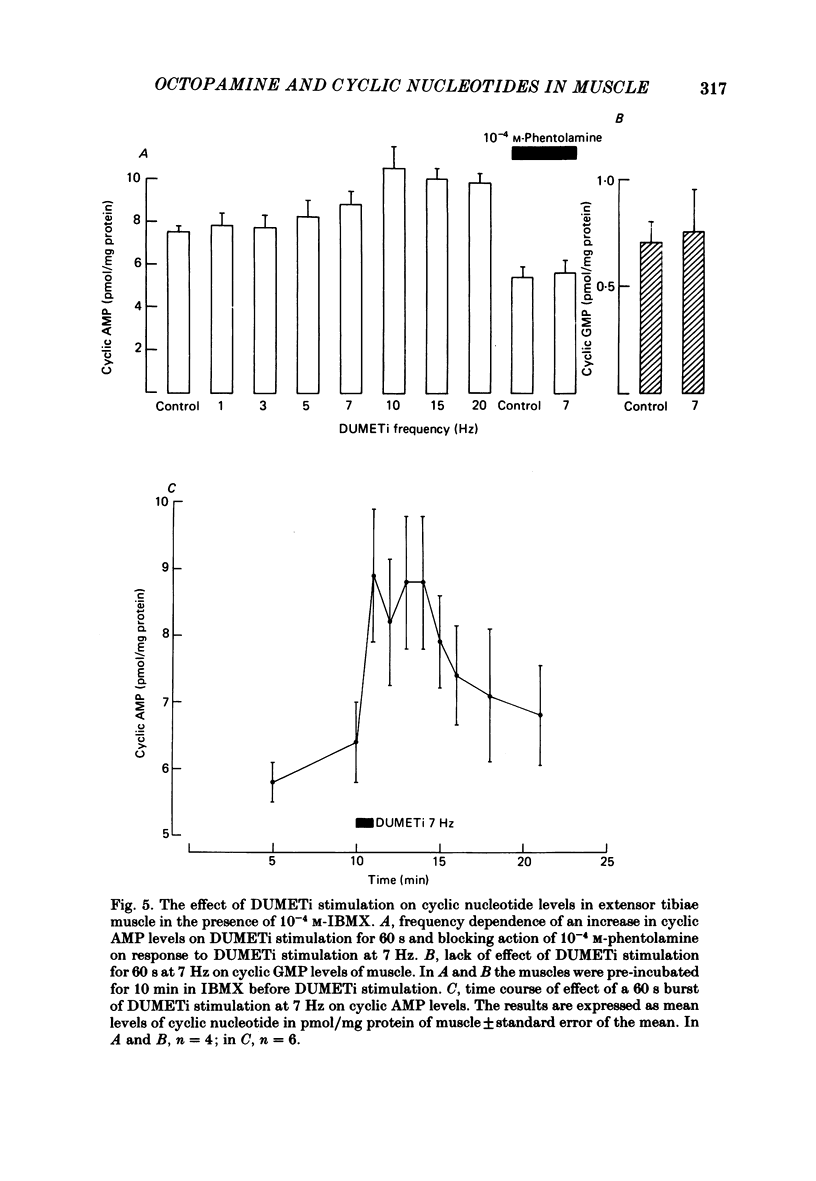
Abstract
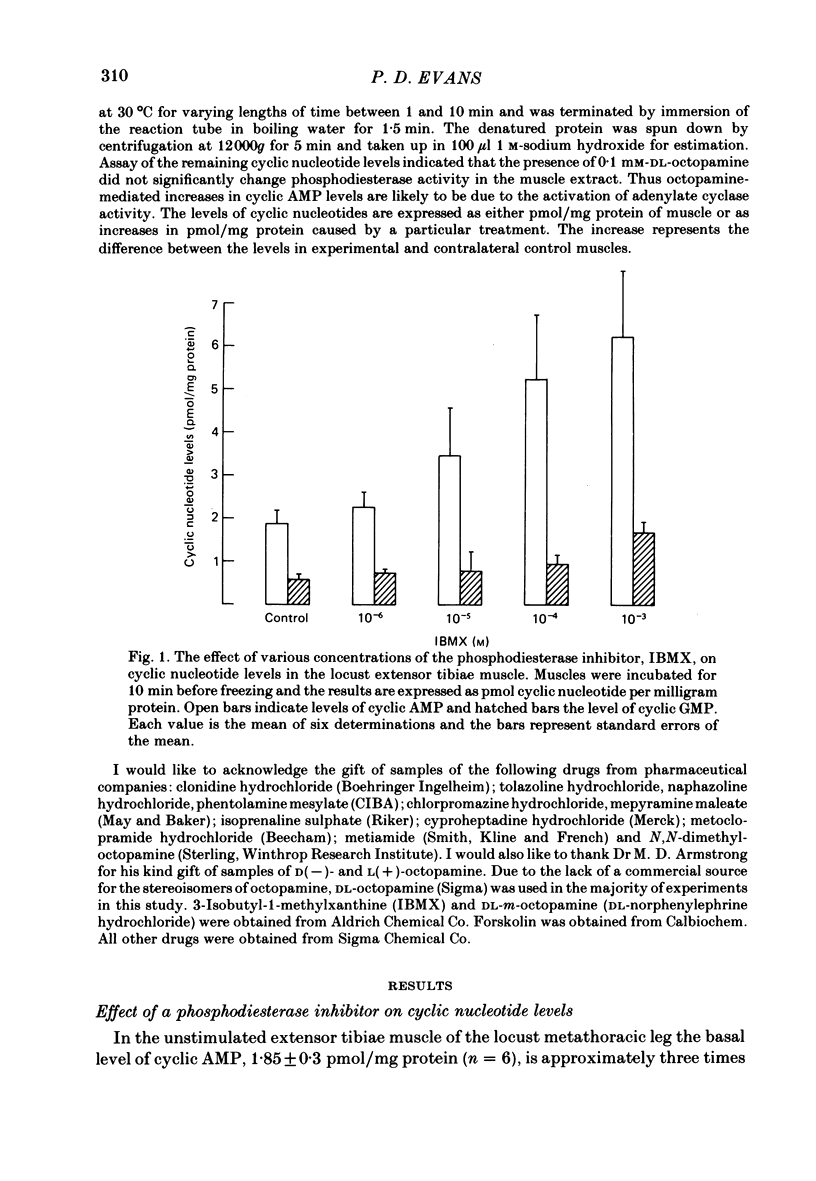
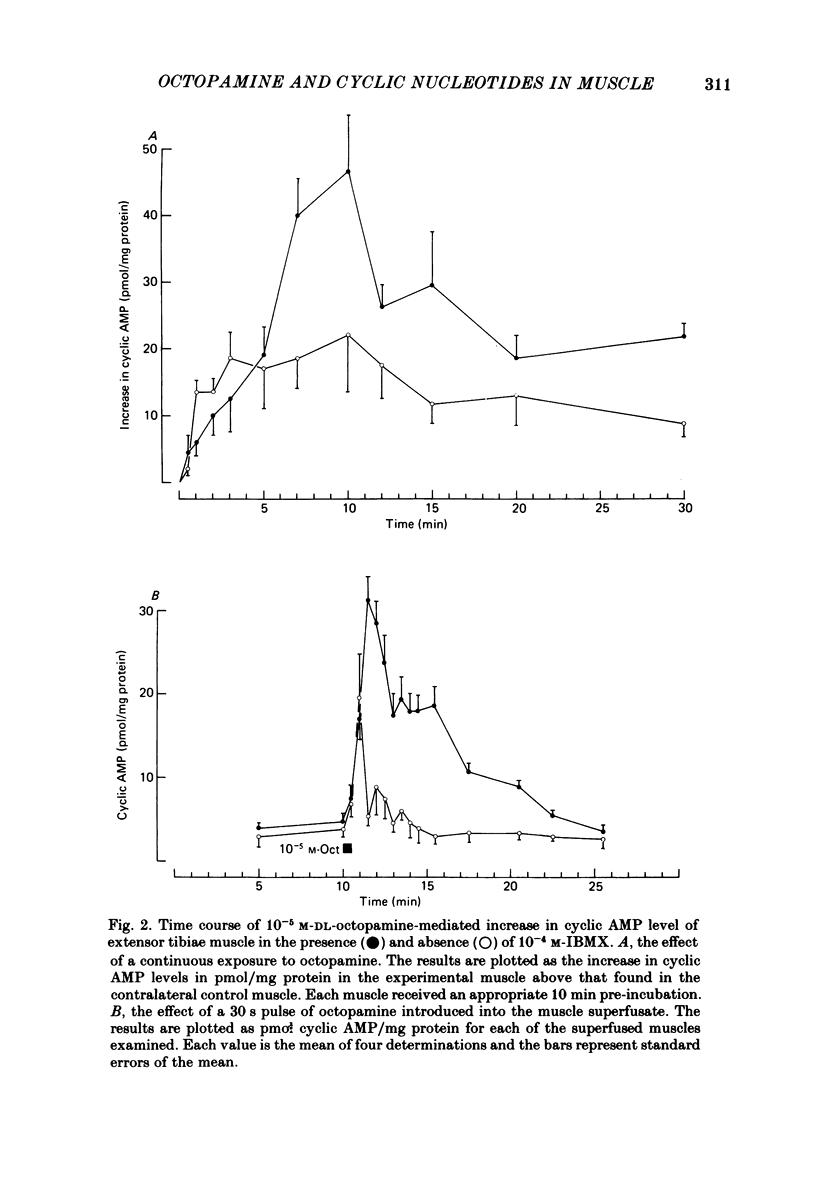
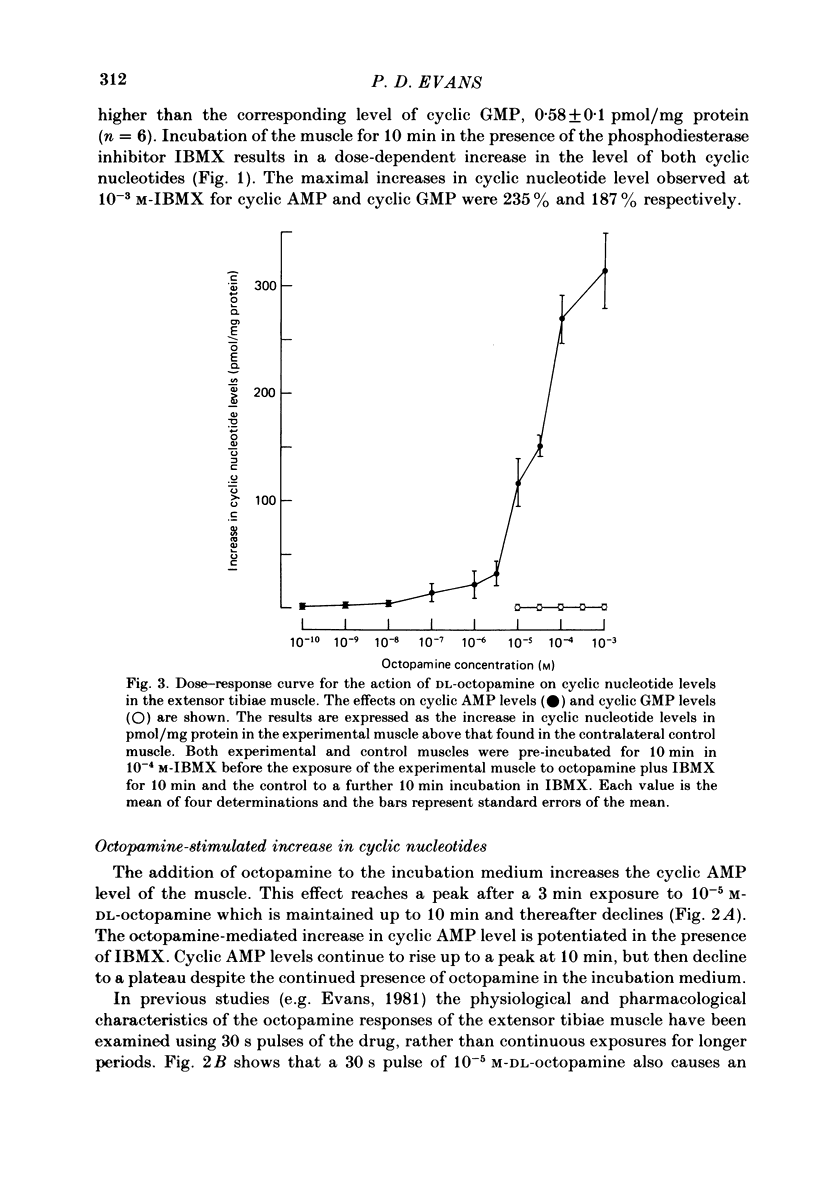
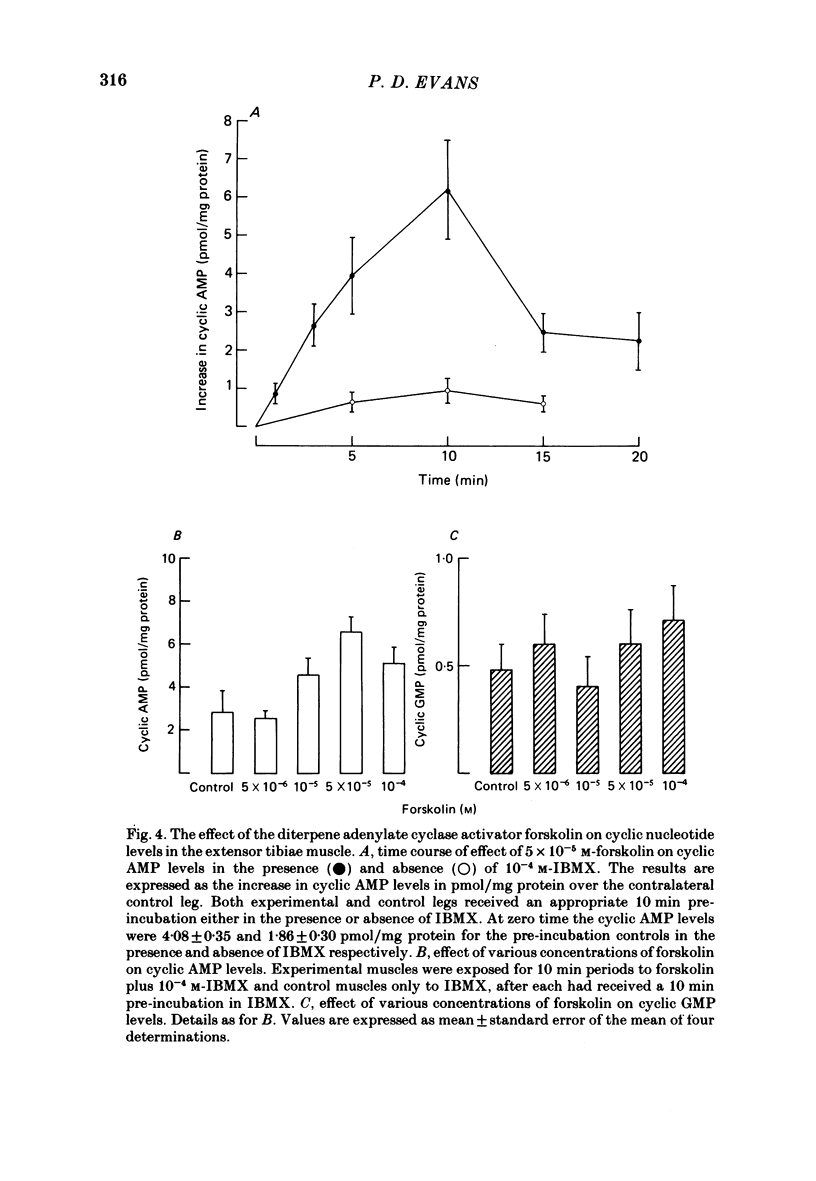
Octopamine increases the level of cyclic AMP in a dose-dependent way in the locust extensor tibiae neuromuscular preparation. The response peaks after a 10 min exposure and then declines to a plateau. The effect of octopamine is potentiated in the presence of the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX). The levels of cyclic GMP in the muscle were not affected by octopamine. The response is stereospecific for the naturally occurring D(-) isomer of octopamine and is also specific for monophenolic biogenic amines. Studies with a range of synthetic agonists and antagonists reveal that the receptors mediating the response are of the OCTOPAMINE2 class. Forskolin, a diterpene activator of adenylate cyclase activity, increases cyclic AMP but not cyclic GMP levels in the extensor muscle. The response has a prolonged time course and is again potentiated by IBMX. Stimulation of the octopaminergic neurone to the extensor muscle increases the levels of cyclic AMP but not those of cyclic GMP. The response is blocked by phentolamine, an alpha-adrenergic blocking agent that also blocks the effects of octopamine in this preparation. The results are discussed in terms of the parallels between the biochemical and physiological effects of octopamine on this muscle and in terms of the mode of action of the octopamine receptors present.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aston R. J. The role of adenosine 3':5'-cyclic monophosphate in relation to the diuretic hormone of Rhodnius prolixus. J Insect Physiol. 1975 Nov;21(11):1873–1877. doi: 10.1016/0022-1910(75)90256-5. [DOI] [PubMed] [Google Scholar]
- BOWMAN W. C., ZAIMIS E. The effects of adrenaline, noradrenaline and isoprenaline on skeletal muscle contractions in the cat. J Physiol. 1958 Nov 10;144(1):92–107. doi: 10.1113/jphysiol.1958.sp006088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battelle B. A., Kravitz E. A. Targets of octopamine action in the lobster: cyclic nucleotide changes and physiological effects in hemolymph, heart and exoskeletal muscle. J Pharmacol Exp Ther. 1978 May;205(2):438–448. [PubMed] [Google Scholar]
- Beam K. G., Greengard P. Cyclic nucleotides, protein phosphorylation and synaptic function. Cold Spring Harb Symp Quant Biol. 1976;40:157–168. doi: 10.1101/sqb.1976.040.01.017. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Rogers N. L., Crofford O. B., Hardman J. G., Sutherland E. W., Newman E. V. Effects of xanthine derivatives on lipolysis and on adenosine 3',5'-monophosphate phosphodiesterase activity. Mol Pharmacol. 1970 Nov;6(6):597–603. [PubMed] [Google Scholar]
- Bowman W. C., Nott M. W. Actions of sympathomimetic amines and their antagonists on skeletal muscle. Pharmacol Rev. 1969 Mar;21(1):27–72. [PubMed] [Google Scholar]
- Bowman W. C., Nott M. W. Effects of catecholamines, cyclic nucleotides and phosphodiesterase inhibitors on contractions of skeletal muscles in anaesthetized cats. Clin Exp Pharmacol Physiol. 1974 Jul-Aug;1(4):309–323. doi: 10.1111/j.1440-1681.1974.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Ekins R. P., Albano J. D. Saturation assay for cyclic AMP using endogenous binding protein. Adv Cyclic Nucleotide Res. 1972;2:25–40. [PubMed] [Google Scholar]
- Clayberger C. A., Goodman D. B., Rasmussen H. Regulation of cyclic AMP metabolism in the rat erythrocyte during chronic beta-adrenergic stimulation. Evidence for calmodulin-mediated alteration of membrane-bound phosphodiesterase activity. J Membr Biol. 1981 Feb 28;58(3):191–201. doi: 10.1007/BF01870905. [DOI] [PubMed] [Google Scholar]
- Dao W. P., Walker R. J. Effect of cyproheptadine on the octopamine-induced responses in the mammalian central nervous system. Experientia. 1980 May 15;36(5):584–585. doi: 10.1007/BF01965816. [DOI] [PubMed] [Google Scholar]
- Evans P. D. Multiple receptor types for octopamine in the locust. J Physiol. 1981 Sep;318:99–122. doi: 10.1113/jphysiol.1981.sp013853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. D., O'Shea M. An octopaminergic neurone modulates neuromuscular transmission in the locust. Nature. 1977 Nov 17;270(5634):257–259. doi: 10.1038/270257a0. [DOI] [PubMed] [Google Scholar]
- Evans P. D., O'Shea M. The identification of an octopaminergic neurone and the modulation of a myogenic rhythm in the locust. J Exp Biol. 1978 Apr;73:235–260. doi: 10.1242/jeb.73.1.235. [DOI] [PubMed] [Google Scholar]
- Evans P. D., Siegler M. V. Octopamine mediated relaxation of maintained and catch tension in locust skeletal muscle. J Physiol. 1982 Mar;324:93–112. doi: 10.1113/jphysiol.1982.sp014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. D. The role of cyclic nucleotides and calcium in the mediation of the modulatory effects of octopamine on locust skeletal muscle. J Physiol. 1984 Mar;348:325–340. doi: 10.1113/jphysiol.1984.sp015113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain J. N., Berridge M. J. Relationship between hormonal activation of phosphatidylinositol hydrolysis, fluid secretion and calcium flux in the blowfly salivary gland. Biochem J. 1979 Jan 15;178(1):45–58. doi: 10.1042/bj1780045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flitney F. W., Singh J. Inotropic responses of the frog ventricle to adenosine triphosphate and related changes in endogenous cyclic nucleotides. J Physiol. 1980 Jul;304:21–42. doi: 10.1113/jphysiol.1980.sp013307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Serratos H., Hill L., Valle-Aguilera R. Effects of catecholamines and cyclic amp on excitation--contraction coupling in isolated skeletal muscle fibres of the frog. J Physiol. 1981 Jun;315:267–282. doi: 10.1113/jphysiol.1981.sp013747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Possible role for cyclic nucleotides and phosphorylated membrane proteins in postsynaptic actions of neurotransmitters. Nature. 1976 Mar 11;260(5547):101–108. doi: 10.1038/260101a0. [DOI] [PubMed] [Google Scholar]
- Harmar A. J., Horn A. S. Octopamine-sensitive adenylate cyclase in cockroach brain: effects of agonists, antagonists, and guanylyl nucleotides. Mol Pharmacol. 1977 May;13(3):512–520. [PubMed] [Google Scholar]
- Hicks T. P., McLennan H. Actions of octopamine upon dorsal horn neurones of the spinal cord. Brain Res. 1978 Nov 24;157(2):402–406. doi: 10.1016/0006-8993(78)90050-1. [DOI] [PubMed] [Google Scholar]
- Horn J. P., McAfee D. A. Modulation of cyclic nucleotide levels in peripheral nerve without effect on resting or compound action potentials. J Physiol. 1977 Aug;269(3):753–766. doi: 10.1113/jphysiol.1977.sp011927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle G. Evidence that insect dorsal unpaired medican (DUM) neurons are octopaminergic. J Exp Zool. 1975 Sep;193(3):425–431. doi: 10.1002/jez.1401930321. [DOI] [PubMed] [Google Scholar]
- Janis R. A., Diamond J. Relationship between cyclic nucleotide levels and drug-induced relaxation of smooth muscle. J Pharmacol Exp Ther. 1979 Dec;211(3):480–484. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morad M., Sanders C., Weiss J. The inotropic actions of adrenaline on frog ventricular muscle: relaxing versus potentiating effects. J Physiol. 1981 Feb;311:585–604. doi: 10.1113/jphysiol.1981.sp013606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson J. A., Greengard P. Octopamine-sensitive adenylate cyclse: evidence for a biological role of octopamine in nervous tissue. Science. 1973 Apr 20;180(4083):308–310. doi: 10.1126/science.180.4083.308. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A., Hunnicutt E. J. N-demethylchlordimeform: a potent partial agonist of octopamine-sensitive adenylate cyclase. Mol Pharmacol. 1981 Jul;20(1):68–75. [PubMed] [Google Scholar]
- Nathanson J. A., Hunnicutt E. J. Neural control of light emission in Photuris larvae: identification of octopamine-sensitive adenylate cyclase (1). J Exp Zool. 1979 May;208(2):255–262. doi: 10.1002/jez.1402080213. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A. Octopamine receptors, adenosine 3',5'-monophosphate, and neural control of firefly flashing. Science. 1979 Jan 5;203(4375):65–68. doi: 10.1126/science.214856. [DOI] [PubMed] [Google Scholar]
- Orchard I., Carlisle J. A., Loughton B. G., Gole J. W., Downer R. G. In vitro studies on the effects of octopamine on locust fat body. Gen Comp Endocrinol. 1982 Sep;48(1):7–13. doi: 10.1016/0016-6480(82)90031-4. [DOI] [PubMed] [Google Scholar]
- Prince W. T., Berridge M. J., Rasmussen H. Role of calcium and adenosine-3':5'-cyclic monophosphate in controlling fly salivary gland secretion. Proc Natl Acad Sci U S A. 1972 Mar;69(3):553–557. doi: 10.1073/pnas.69.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Localization of beta adrenergic receptors, and effects of noradrenaline and cyclic nucleotides on action potentials, ionic currents and tension in mammalian cardiac muscle. J Physiol. 1974 Oct;242(2):429–451. doi: 10.1113/jphysiol.1974.sp010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N. L., Cox P. M., Greengard P. Glutamate regulates adenylate cyclase and guanylate cyclase activities in an isolated membrane preparation from insect muscle. Nature. 1982 Mar 25;296(5855):354–356. doi: 10.1038/296354b0. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Schultz K. D., Böhme E., Kreye V. A., Schultz G. Relaxation of hormonally stimulated smooth muscular tissues by the 8-bromo derivative of cyclic GMP. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jan;306(1):1–9. doi: 10.1007/BF00515586. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Tran V. T., Chang R. S., Snyder S. H. Histamine H1 receptors identified in mammalian brain membranes with [3H]mepyramine. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6290–6294. doi: 10.1073/pnas.75.12.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USHERWOOD P. N., GRUNDFEST H. PERIPHERAL INHIBITION IN SKELETAL MUSCLE OF INSECTS. J Neurophysiol. 1965 May;28:497–518. doi: 10.1152/jn.1965.28.3.497. [DOI] [PubMed] [Google Scholar]
- Uzzan A., Dudai Y. Aminergic receptors in Drosophila melanogaster: responsiveness of adenylate cyclase to putative neurotransmitters. J Neurochem. 1982 Jun;38(6):1542–1550. doi: 10.1111/j.1471-4159.1982.tb06631.x. [DOI] [PubMed] [Google Scholar]
- Weiss K. R., Mandelbaum D. E., Schonberg M., Kupfermann I. Modulation of buccal muscle contractility by serotonergic metacerebral cells in Aplysia: evidence for a role of cyclic adenosine monophosphate. J Neurophysiol. 1979 May;42(3):791–803. doi: 10.1152/jn.1979.42.3.791. [DOI] [PubMed] [Google Scholar]
- Wolff J., Londos C., Cooper D. M. Adenosine receptors and the regulation of adenylate cyclase. Adv Cyclic Nucleotide Res. 1981;14:199–214. [PubMed] [Google Scholar]


