Abstract
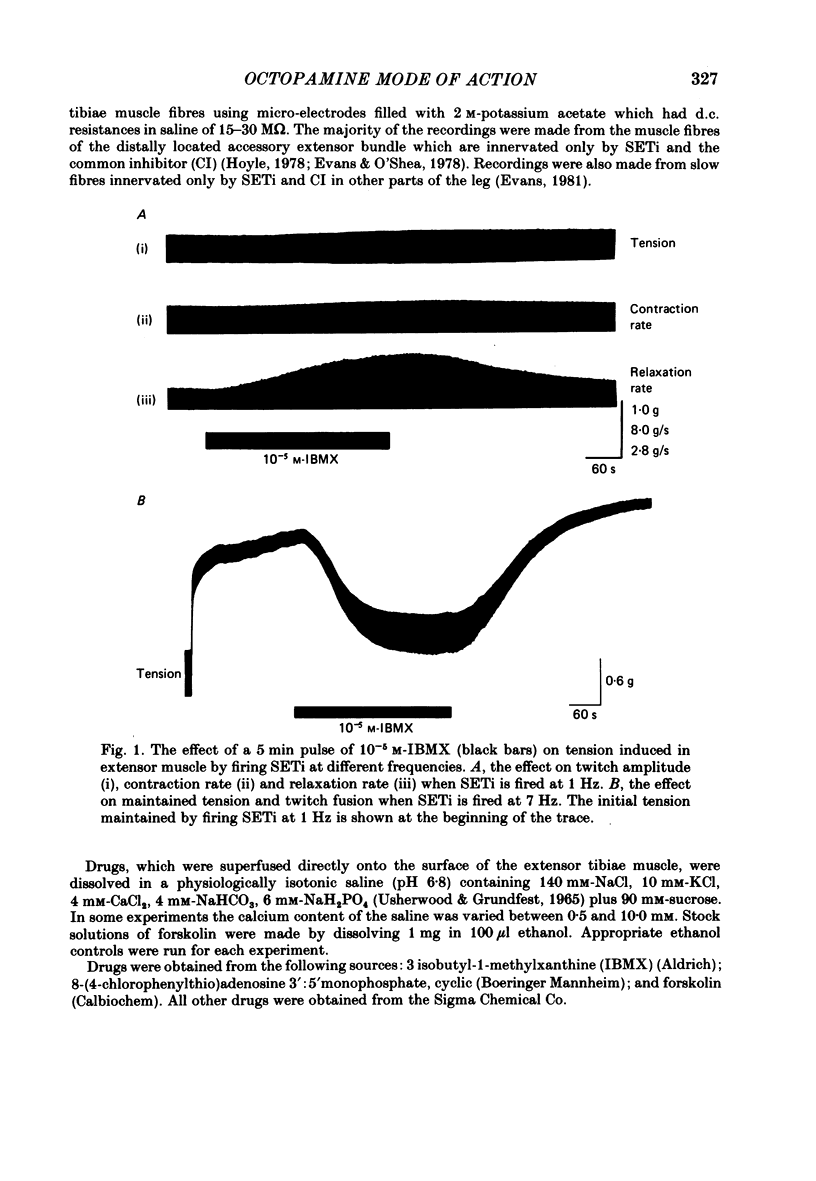
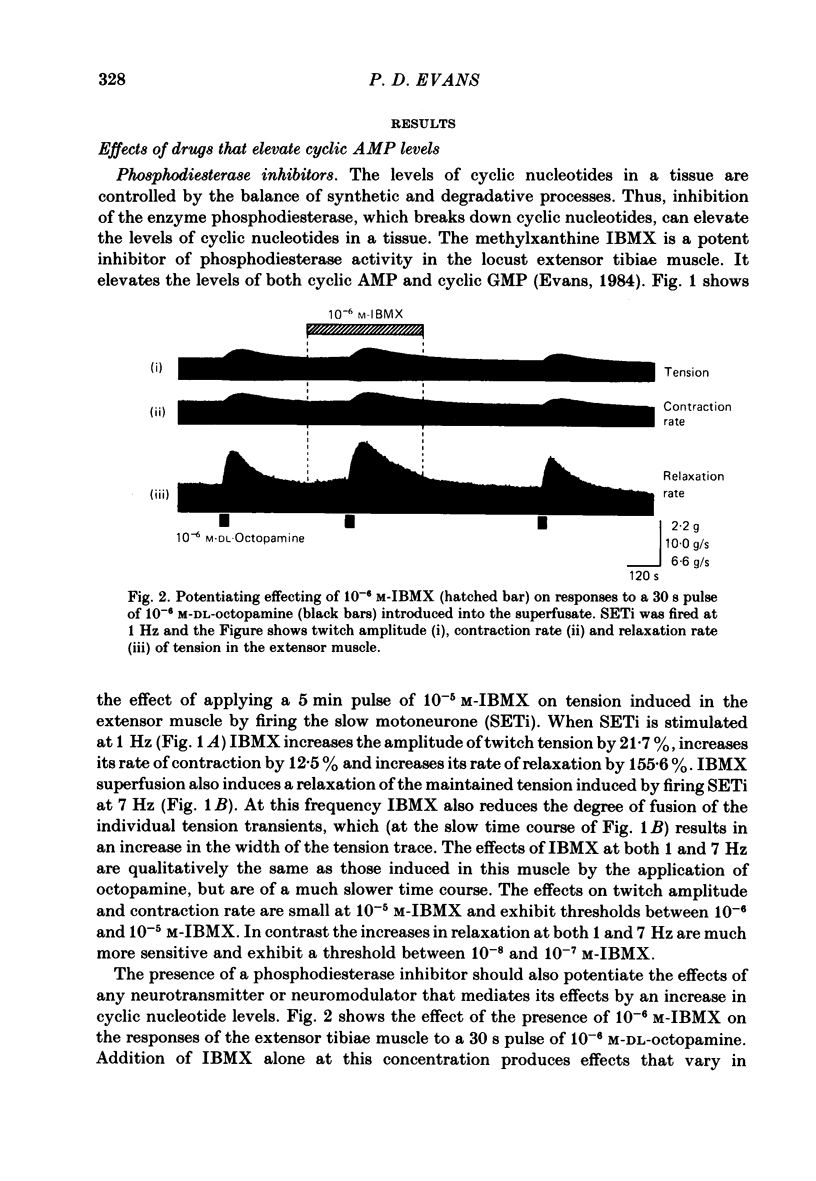
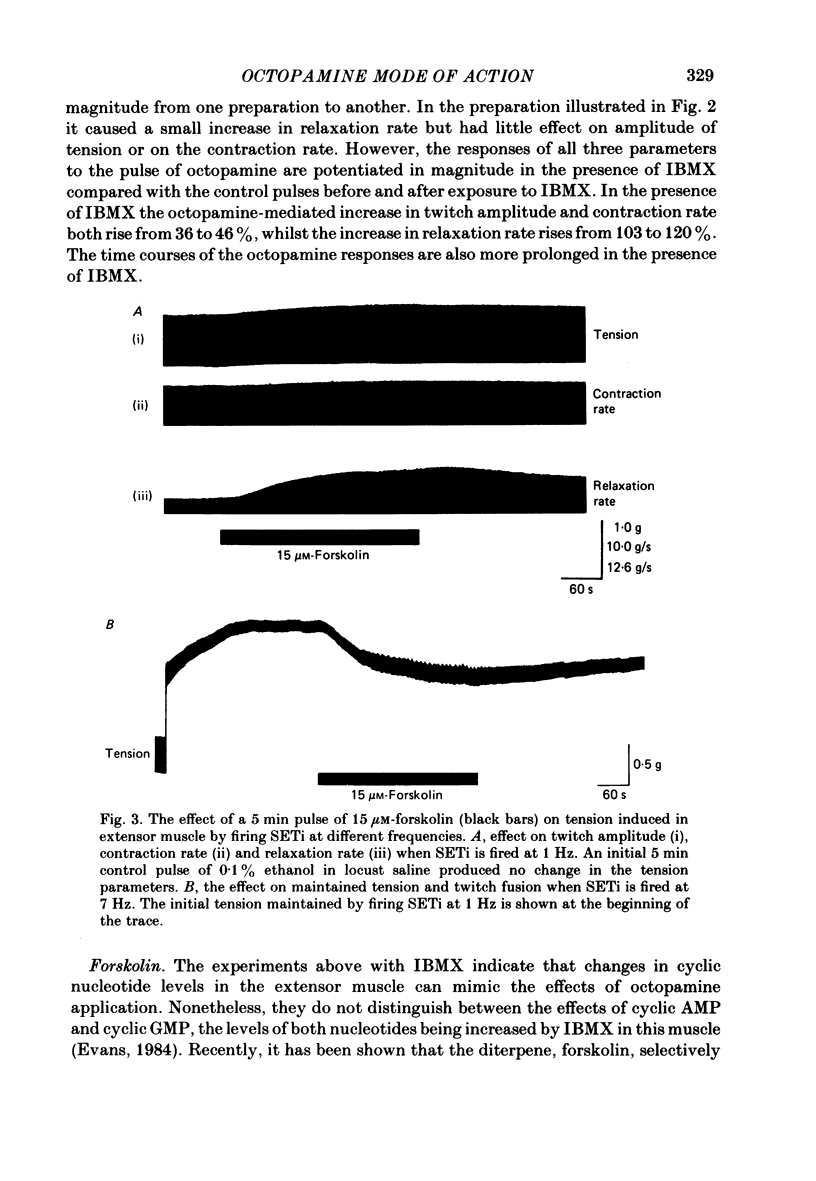
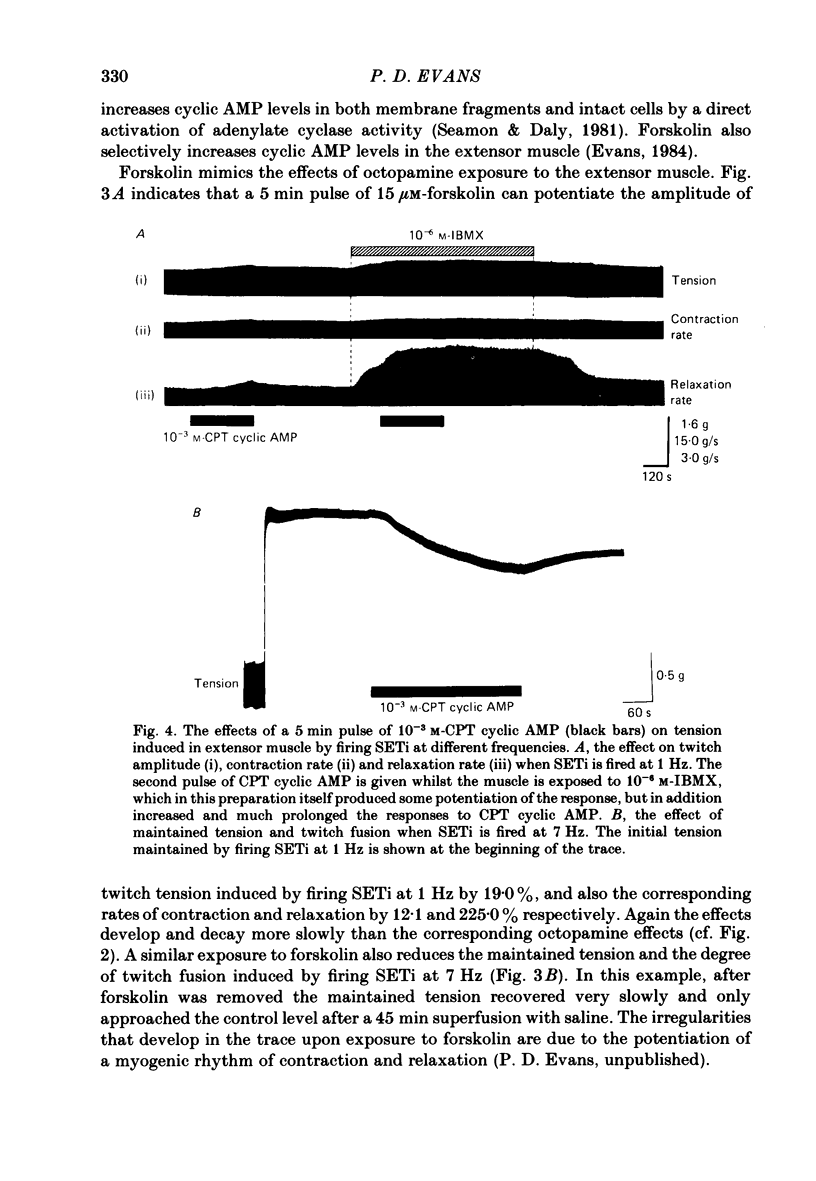
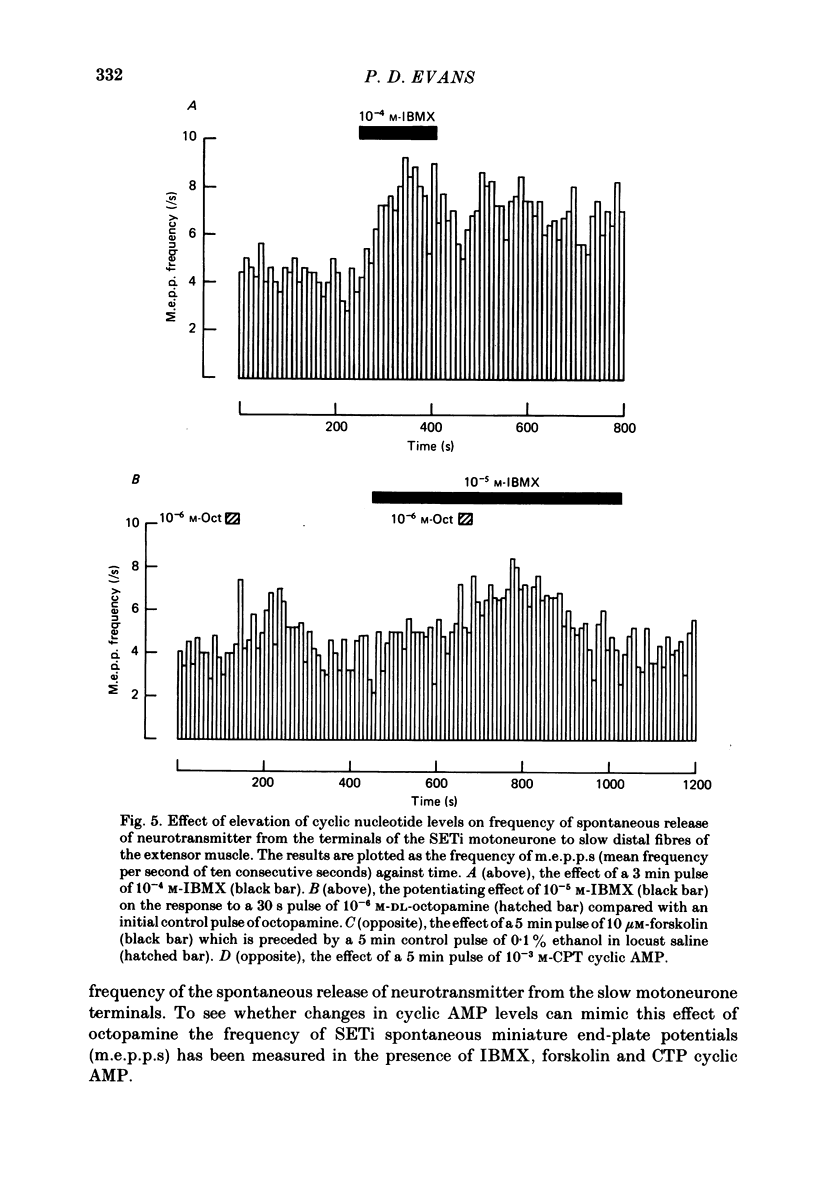
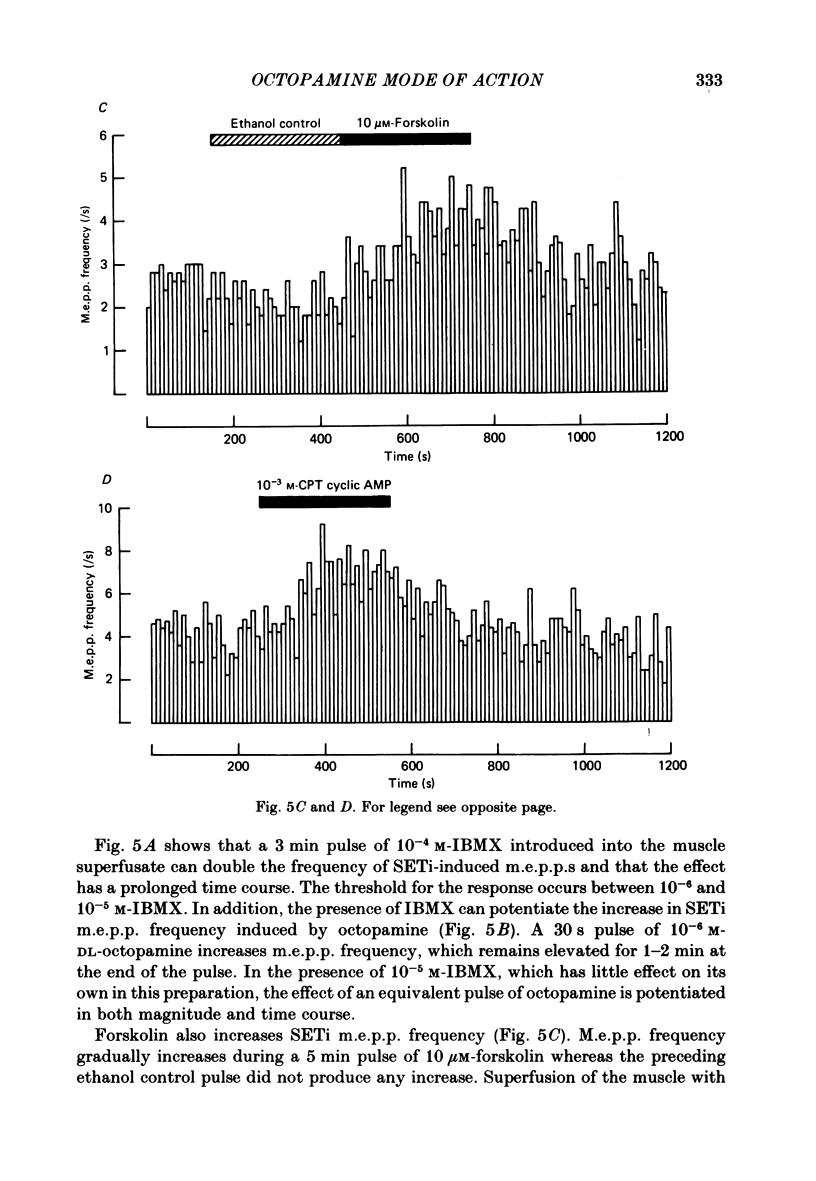
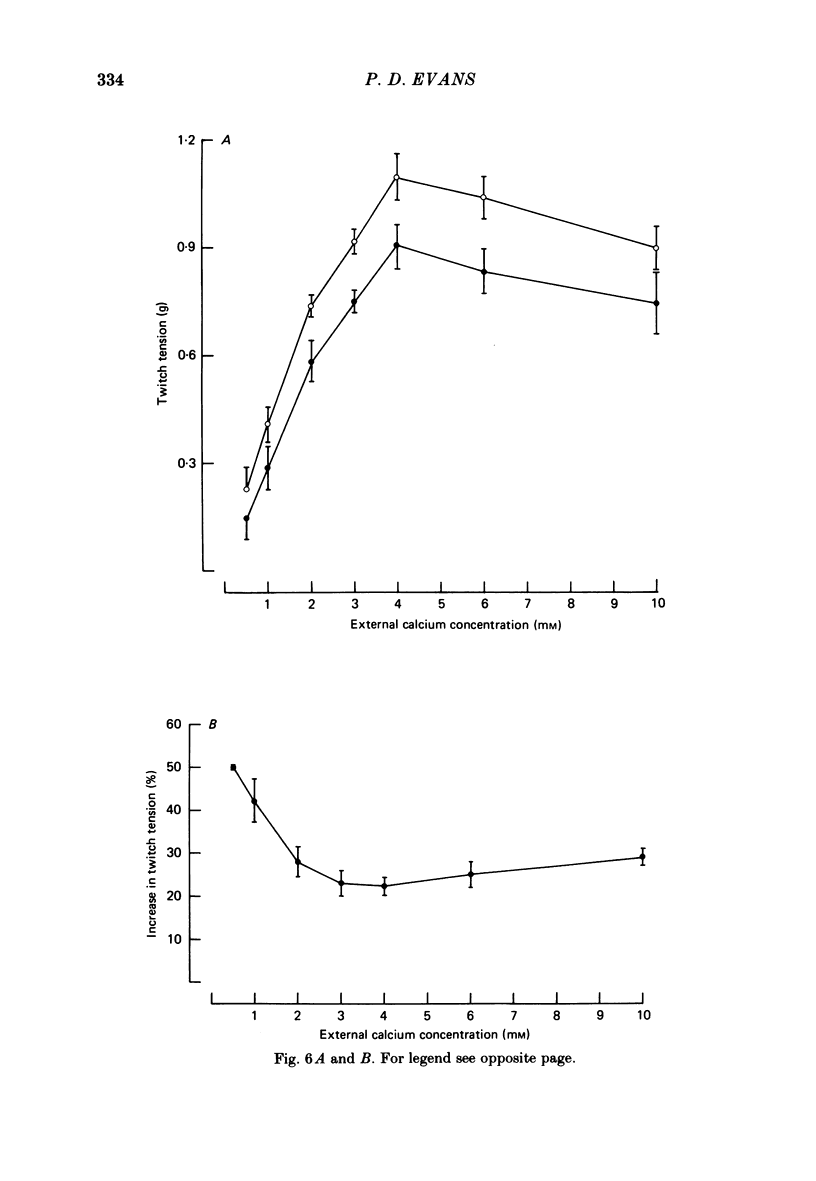
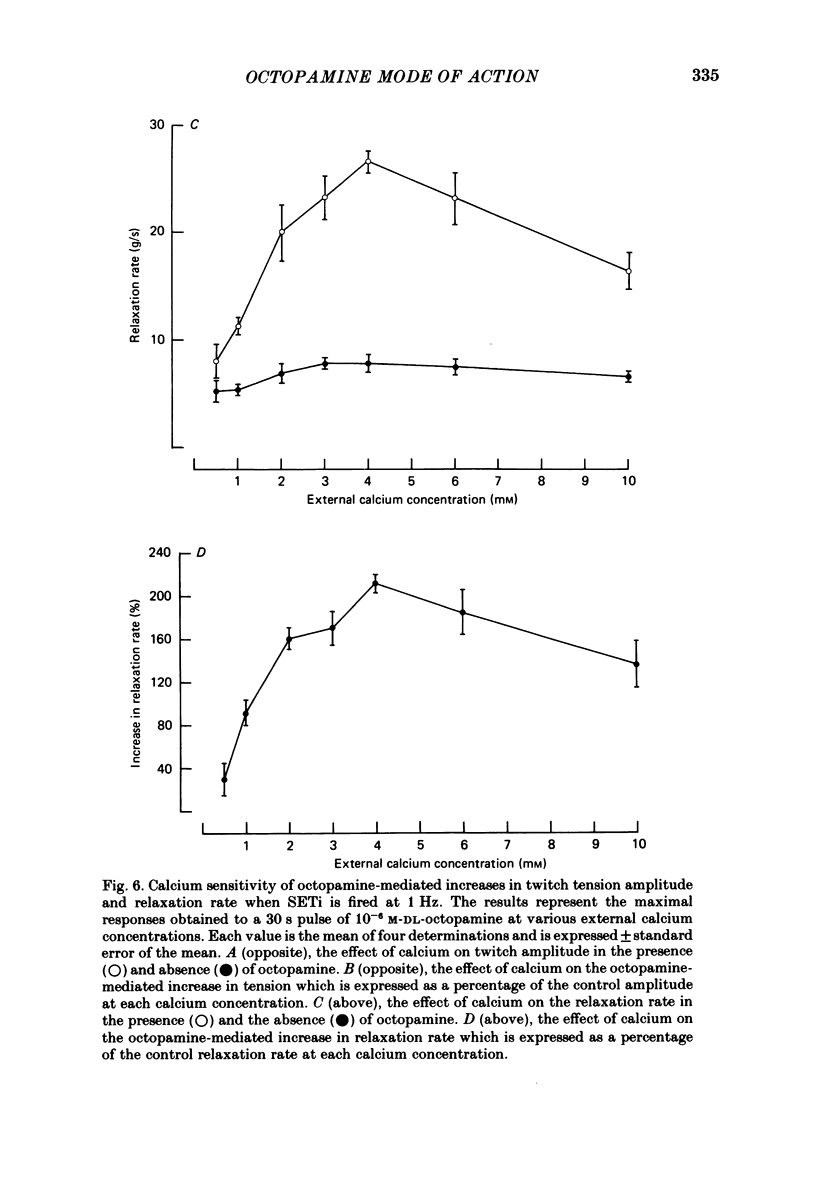
The role of cyclic AMP and calcium in the mediation of the effects of octopamine has been investigated in the extensor tibiae muscle of the hind leg of the locust. Elevation of cyclic AMP levels in the preparation by means of the phosphodiesterase inhibitor, 3-isobutyl-1-methylxanthine (IBMX), by means of the diterpene adenylate cyclase activator, forskolin, and by means of cyclic nucleotide analogues mimics the post-synaptic effects of octopamine application at different frequencies of neuronal stimulation. These conditions also mimic the presynaptic effects of octopamine on spontaneous release of transmitter from the slow motoneurone. The effects of octopamine on the preparation are calcium sensitive, with the maximal sensitivity occurring between 0.5 and 4.0 mM-external calcium. The results are discussed in terms of the role of cyclic AMP and calcium in the mediation of the effects of octopamine.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AIDLEY D. J. THE EFFECT OF CALCIUM IONS ON POTASSIUM CONTRACTURE IN A LOCUST LEG MUSCLE. J Physiol. 1965 Mar;177:94–102. doi: 10.1113/jphysiol.1965.sp007577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWMAN W. C., ZAIMIS E. The effects of adrenaline, noradrenaline and isoprenaline on skeletal muscle contractions in the cat. J Physiol. 1958 Nov 10;144(1):92–107. doi: 10.1113/jphysiol.1958.sp006088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Greengard P. Cyclic nucleotides, protein phosphorylation and synaptic function. Cold Spring Harb Symp Quant Biol. 1976;40:157–168. doi: 10.1101/sqb.1976.040.01.017. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Bowman W. C., Nott M. W. Effects of catecholamines, cyclic nucleotides and phosphodiesterase inhibitors on contractions of skeletal muscles in anaesthetized cats. Clin Exp Pharmacol Physiol. 1974 Jul-Aug;1(4):309–323. doi: 10.1111/j.1440-1681.1974.tb00553.x. [DOI] [PubMed] [Google Scholar]
- Enyeart J. Cyclic AMP, 5-HT, and the modulation of transmitter release at the crayfish neuromuscular junction. J Neurobiol. 1981 Sep;12(5):505–513. doi: 10.1002/neu.480120509. [DOI] [PubMed] [Google Scholar]
- Evans P. D. A modulatory octopaminergic neurone increases cyclic nucleotide levels in locust skeletal muscle. J Physiol. 1984 Mar;348:307–324. doi: 10.1113/jphysiol.1984.sp015112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. D. Multiple receptor types for octopamine in the locust. J Physiol. 1981 Sep;318:99–122. doi: 10.1113/jphysiol.1981.sp013853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. D., O'Shea M. An octopaminergic neurone modulates neuromuscular transmission in the locust. Nature. 1977 Nov 17;270(5634):257–259. doi: 10.1038/270257a0. [DOI] [PubMed] [Google Scholar]
- Evans P. D., O'Shea M. The identification of an octopaminergic neurone and the modulation of a myogenic rhythm in the locust. J Exp Biol. 1978 Apr;73:235–260. doi: 10.1242/jeb.73.1.235. [DOI] [PubMed] [Google Scholar]
- Evans P. D., Siegler M. V. Octopamine mediated relaxation of maintained and catch tension in locust skeletal muscle. J Physiol. 1982 Mar;324:93–112. doi: 10.1113/jphysiol.1982.sp014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Serratos H., Hill L., Valle-Aguilera R. Effects of catecholamines and cyclic amp on excitation--contraction coupling in isolated skeletal muscle fibres of the frog. J Physiol. 1981 Jun;315:267–282. doi: 10.1113/jphysiol.1981.sp013747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Possible role for cyclic nucleotides and phosphorylated membrane proteins in postsynaptic actions of neurotransmitters. Nature. 1976 Mar 11;260(5547):101–108. doi: 10.1038/260101a0. [DOI] [PubMed] [Google Scholar]
- Hoyle G., Colquhoun W., Williams M. Fine structure of an octopaminergic neuron and its terminals. J Neurobiol. 1980;11(1):103–126. doi: 10.1002/neu.480110109. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Distributions of nerve and muscle fibre types in locust jumping muscle. J Exp Biol. 1978 Apr;73:205–233. doi: 10.1242/jeb.73.1.205. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Evidence that insect dorsal unpaired medican (DUM) neurons are octopaminergic. J Exp Zool. 1975 Sep;193(3):425–431. doi: 10.1002/jez.1401930321. [DOI] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M., Shapiro E., Kandel E. R. Synaptic plasticity and the modulation of the Ca2+ current. J Exp Biol. 1980 Dec;89:117–157. doi: 10.1242/jeb.89.1.117. [DOI] [PubMed] [Google Scholar]
- Kuba K. Effects of catecholamines on the neuromuscular junction in the rat diaphragm. J Physiol. 1970 Dec;211(3):551–570. doi: 10.1113/jphysiol.1970.sp009293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. P., Beck A. H., Simon L. N., Meyer R. B., Jr Induction of hepatic tyrosine aminotransferase in vivo by derivatives of cyclic adenosine 3':5'-monophosphate. J Biol Chem. 1975 Jan 25;250(2):426–431. [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- USHERWOOD P. N., GRUNDFEST H. PERIPHERAL INHIBITION IN SKELETAL MUSCLE OF INSECTS. J Neurophysiol. 1965 May;28:497–518. doi: 10.1152/jn.1965.28.3.497. [DOI] [PubMed] [Google Scholar]
- Walsh M. P., Guilleux J. C. Calcium and cyclic AMP-dependent regulation of myofibrillar calmodulin-dependent myosin light chain kinases from cardiac and skeletal muscles. Adv Cyclic Nucleotide Res. 1981;14:375–390. [PubMed] [Google Scholar]
- Washio H. The ionic requirements for the initiation of action potentials in insect muscle fibers. J Gen Physiol. 1972 Feb;59(2):121–134. doi: 10.1085/jgp.59.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller M. Evidence for the presynaptic location of adenylate cyclase and the cyclic AMP-stimulated protein kinase which is bound to synaptic membranes. Biochim Biophys Acta. 1977 Sep 19;469(3):350–354. doi: 10.1016/0005-2736(77)90171-7. [DOI] [PubMed] [Google Scholar]
- Wolff J., Londos C., Cooper D. M. Adenosine receptors and the regulation of adenylate cyclase. Adv Cyclic Nucleotide Res. 1981;14:199–214. [PubMed] [Google Scholar]


