Abstract
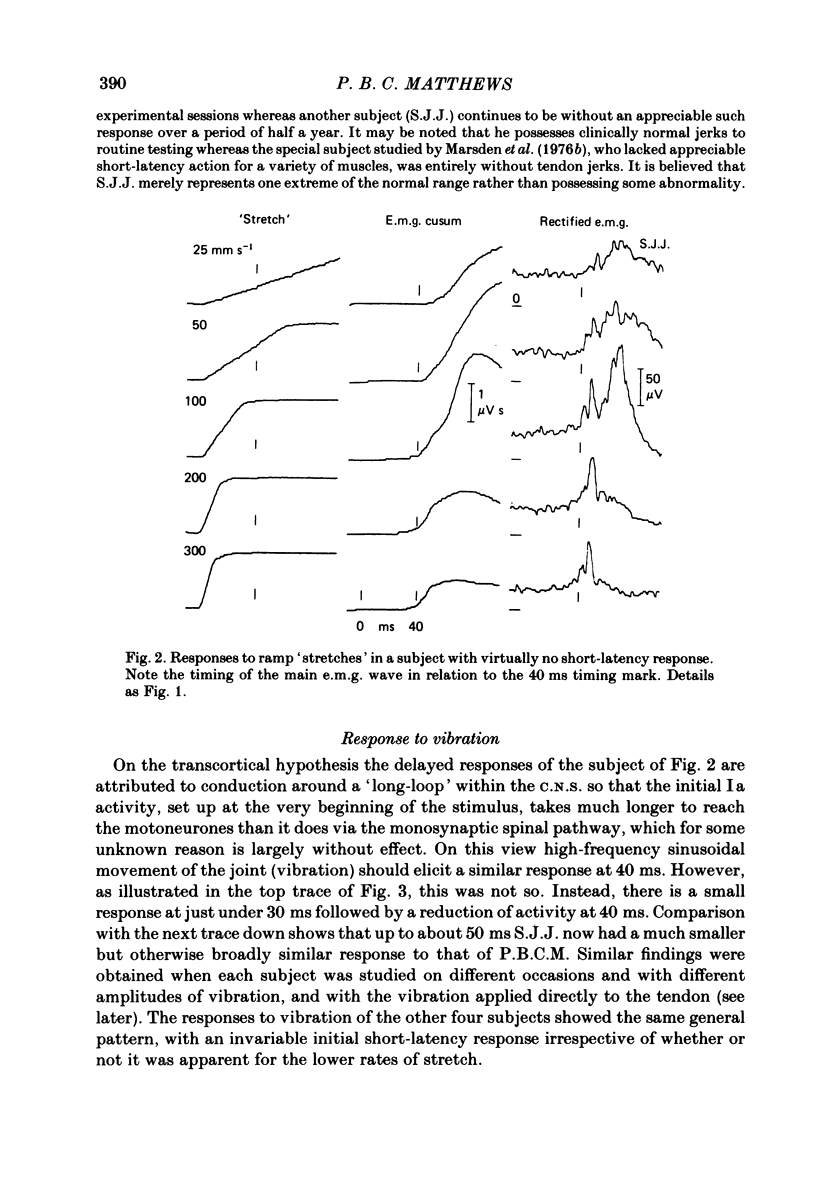
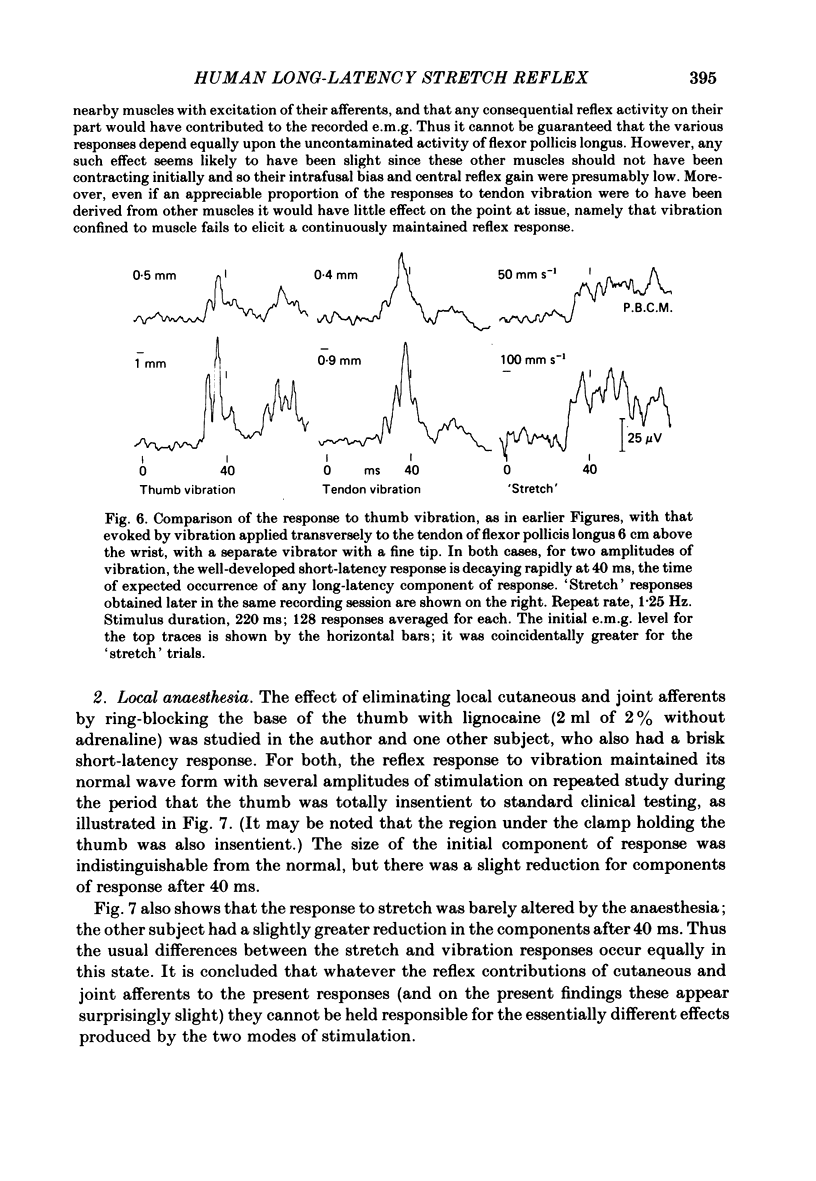
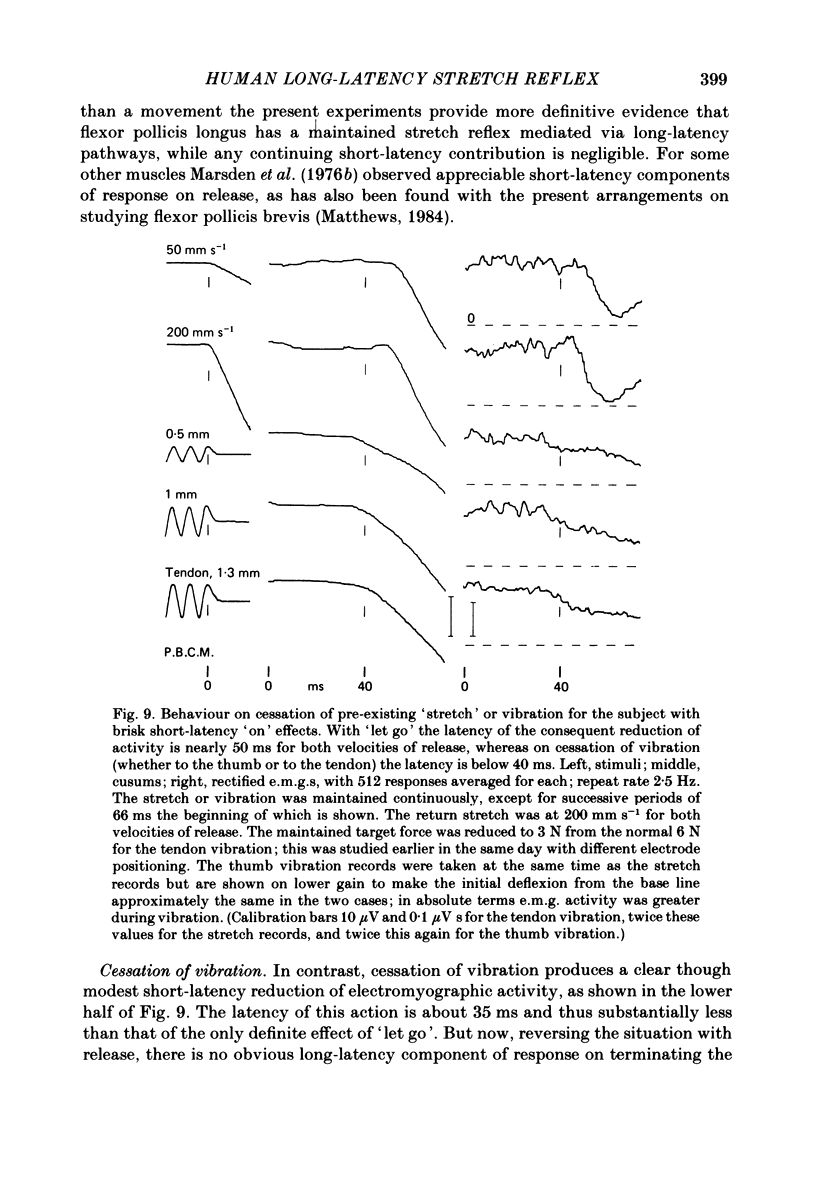
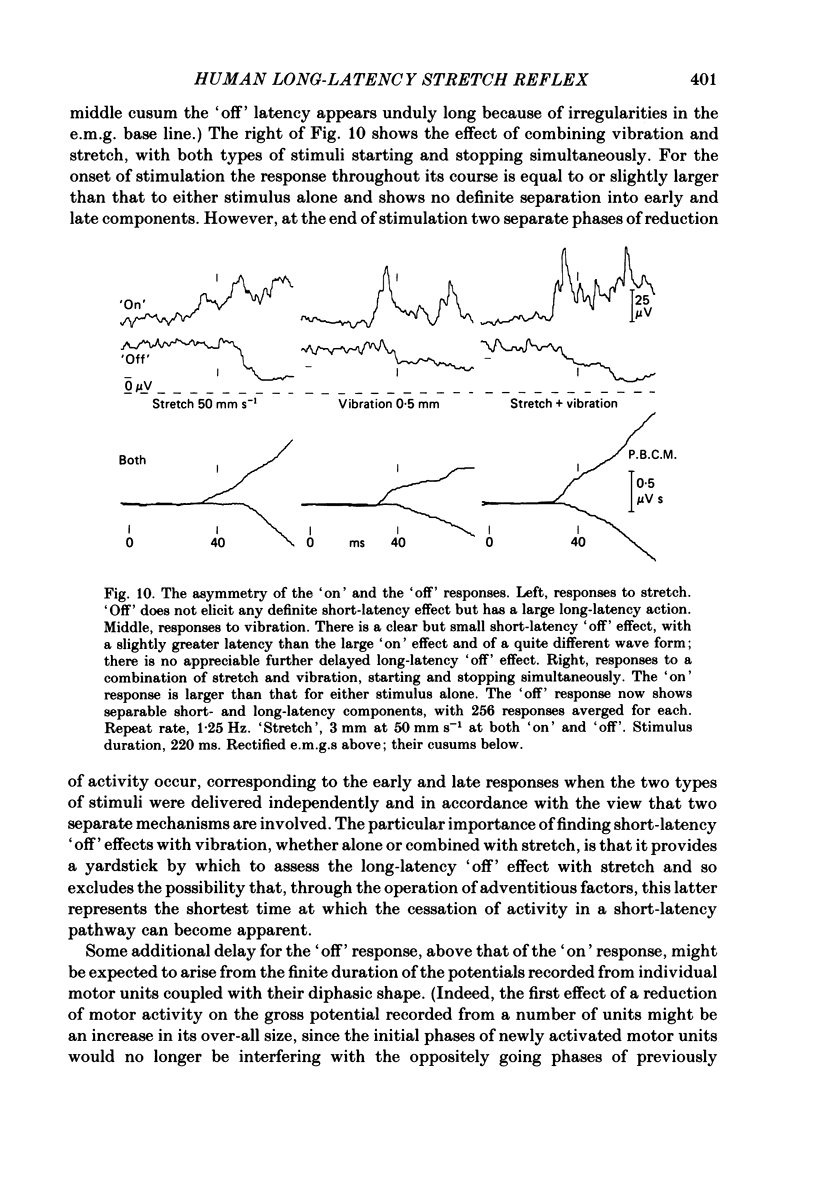
The electromyographic activity of flexor pollicis longus has been recorded in normal human subjects on moving the tip of the thumb with the proximal phalanx clamped. Ramp and hold displacements (stretches) were compared with high-frequency sinusoidal movement (vibration). The subject exerted a constant flexor force between stimuli and made no voluntary response to them. On stretching the muscle by forcibly extending the thumb at various constant velocities the usual combination of short-latency (ca. 25-30 ms) and long-latency (ca. 40 ms) components of response were observed. The short-latency response progressively predominated as the velocity was increased (60-900 deg s-1, 9 deg joint displacement). One subject still showed only a long-latency response with the fastest stretch, arguing that it is a distinct reflex entity. On commencing vibration (143 Hz, 3 deg movement peak-to-peak) a short-latency response was regularly obtained, but any long-latency response was always small in relation to that elicited by stretch. This was equally so when the short-latency responses to the two types of stimulation were matched by using appropriate parameters of stimulation. The time course of the vibration response did not change appreciably with change of amplitude of vibration, so that its temporal profile was always quite different from that of the stretch response. The observed differences are in accordance with the hypothesis that the spindle group II afferents produce the long-latency excitation, with the time lost peripherally in afferent conduction rather than centrally. In relation to the strength of their Ia excitatory actions, stretch is known to excite secondary afferents more powerfully than does vibration. The findings are not readily accommodated on the hypothesis that the long-latency response is a transcortical reflex elicited by the initial Ia input, since vibration should then also have had a powerful long-latency action. Similar responses to vibration were obtained when it was applied percutaneously to the tendon of flexor pollicis longus 6 cm above the wrist. Also, those elicited by thumb vibration persisted largely unchanged when the thumb was anaesthetized. This confirms that they were dependent upon the excitation of receptors in flexor pollicis longus, presumably the Ia afferents, rather than upon cutaneous or joint receptors in the thumb. The stretch responses also depended upon muscle receptors, since they too survived anaesthesia.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bawa P., McKenzie D. C. Contribution of joint and cutaneous afferents to longer-latency reflexes in man. Brain Res. 1981 Apr 27;211(1):185–189. doi: 10.1016/0006-8993(81)90081-0. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Engberg I., Matthews P. B. The relative sensitivity to vibration of muscle receptors of the cat. J Physiol. 1967 Oct;192(3):773–800. doi: 10.1113/jphysiol.1967.sp008330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. I., Rack P. M., Ross H. F. Forces generated at the thumb interphalangeal joint during imposed sinusoidal movements. J Physiol. 1982 Nov;332:69–85. doi: 10.1113/jphysiol.1982.sp014401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller N. P., Garnett R., Stephens J. A. The reflex responses of single motor units in human hand muscles following muscle afferent stimulation. J Physiol. 1980 Jun;303:337–349. doi: 10.1113/jphysiol.1980.sp013289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Hagbarth K. E., Löfstedt L., Wallin B. G. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976 Oct;261(3):695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C. W., Jones G. M., Catchlove R. F. The 'late' electromyographic response to limb displacement in man. II. Sensory origin. Electroencephalogr Clin Neurophysiol. 1979 Feb;46(2):182–188. doi: 10.1016/0013-4694(79)90067-1. [DOI] [PubMed] [Google Scholar]
- Chan C. W., Jones G. M., Kearney R. E., Watt D. G. The 'late' electromyographic response to limb displacement in man. I. Evidence for supraspinal contribution. Electroencephalogr Clin Neurophysiol. 1979 Feb;46(2):173–181. doi: 10.1016/0013-4694(79)90066-x. [DOI] [PubMed] [Google Scholar]
- Chofflon M., Lachat J. M., Rüegg D. G. A transcortical loop demonstrated by stimulation of low-threshold muscle afferents in the awake monkey. J Physiol. 1982 Feb;323:393–402. doi: 10.1113/jphysiol.1982.sp014079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago P. E., Houk J. C., Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol. 1976 Sep;39(5):925–935. doi: 10.1152/jn.1976.39.5.925. [DOI] [PubMed] [Google Scholar]
- Eklund G., Hagbarth K. E., Hägglund J. V., Wallin E. U. Mechanical oscillations contributing to the segmentation of the reflex electromyogram response to stretching human muscles. J Physiol. 1982 May;326:65–77. doi: 10.1113/jphysiol.1982.sp014177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund G., Hagbarth K. E., Hägglund J. V., Wallin E. U. The 'late' reflex responses to muscle stretch: the 'resonance hypothesis' versus the 'long-loop hypothesis'. J Physiol. 1982 May;326:79–90. doi: 10.1113/jphysiol.1982.sp014178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund G., Hagbarth K. E., Torebjörk E. Exteroceptive vibration-induced finger flexion reflex in man. J Neurol Neurosurg Psychiatry. 1978 May;41(5):438–443. doi: 10.1136/jnnp.41.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. An application of cumulative sum technique (cusums) to neurophysiology [proceedings]. J Physiol. 1977 Feb;265(1):1P–2P. [PMC free article] [PubMed] [Google Scholar]
- Evarts E. V., Fromm C. Transcortical reflexes and servo control of movement. Can J Physiol Pharmacol. 1981 Jul;59(7):757–775. doi: 10.1139/y81-112. [DOI] [PubMed] [Google Scholar]
- Garnett R., Stephens J. A. The reflex responses of single motor units in human first dorsal interosseous muscle following cutaneous afferent stimulation. J Physiol. 1980 Jun;303:351–364. doi: 10.1113/jphysiol.1980.sp013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C., Shinoda Y. Spinal mechanisms of the functional stretch reflex. Exp Brain Res. 1978 May 12;32(1):55–68. doi: 10.1007/BF00237390. [DOI] [PubMed] [Google Scholar]
- Goodwin G. M., Hulliger M., Matthews P. B. The effects of fusimotor stimulation during small amplitude stretching on the frequency-response of the primary ending of the mammalian muscle spindle. J Physiol. 1975 Dec;253(1):175–206. doi: 10.1113/jphysiol.1975.sp011186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagbarth K. E., Hägglund J. V., Wallin E. U., Young R. R. Grouped spindle and electromyographic responses to abrupt wrist extension movements in man. J Physiol. 1981 Mar;312:81–96. doi: 10.1113/jphysiol.1981.sp013617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrie A., Lee R. G. Selective effects of vibration on human spinal and long-loop reflexes. Brain Res. 1978 Nov 24;157(2):369–375. doi: 10.1016/0006-8993(78)90044-6. [DOI] [PubMed] [Google Scholar]
- Hulliger M., Nordh E., Vallbo A. B. The absence of position response in spindle afferent units from human finger muscles during accurate position holding. J Physiol. 1982 Jan;322:167–179. doi: 10.1113/jphysiol.1982.sp014030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles J. F. Responses in human pretibial muscles to sudden stretch and to nerve stimulation. Exp Brain Res. 1977 Dec 19;30(4):451–470. doi: 10.1007/BF00237637. [DOI] [PubMed] [Google Scholar]
- Jaeger R. J., Gottlieb G. L., Agarwal G. C., Tahmoush A. J. Afferent contributions to stretch-evoked myoelectric responses. J Neurophysiol. 1982 Aug;48(2):403–418. doi: 10.1152/jn.1982.48.2.403. [DOI] [PubMed] [Google Scholar]
- Jenner J. R., Stephens J. A. Cutaneous reflex responses and their central nervous pathways studied in man. J Physiol. 1982 Dec;333:405–419. doi: 10.1113/jphysiol.1982.sp014461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz F. A., Tatton W. G., Tasker R. R. Electromyographic response to displacement of different forelimb joints in the squirrel monkey. J Neurosci. 1983 Apr;3(4):783–794. doi: 10.1523/JNEUROSCI.03-04-00783.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz F. A., Tatton W. G., Tasker R. R. The effect of cortical lesions on the electromyographic response to joint displacement in the squirrel monkey forelimb. J Neurosci. 1983 Apr;3(4):795–805. doi: 10.1523/JNEUROSCI.03-04-00795.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B., Rothwell J. C., Traub M. M. Reliability and efficacy of the long-latency stretch reflex in the human thumb. J Physiol. 1981 Jul;316:47–60. doi: 10.1113/jphysiol.1981.sp013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in human voluntary movement. Nature. 1972 Jul 21;238(5360):140–143. doi: 10.1038/238140a0. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Servo action in the human thumb. J Physiol. 1976 May;257(1):1–44. doi: 10.1113/jphysiol.1976.sp011354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. Stretch reflex and servo action in a variety of human muscles. J Physiol. 1976 Jul;259(2):531–560. doi: 10.1113/jphysiol.1976.sp011481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Merton P. A., Morton H. B. The sensory mechanism of servo action in human muscle. J Physiol. 1977 Feb;265(2):521–535. doi: 10.1113/jphysiol.1977.sp011728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Rothwell J. C., Traub M. M. Effect of thumb anaesthesia on weight perception, muscle activity and the stretch reflex in man. J Physiol. 1979 Sep;294:303–315. doi: 10.1113/jphysiol.1979.sp012931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Bawa P., Matthews H. R. Synchronisation of motor firing by vibration during stretch evoked responses of the human wrist flexors. Exp Brain Res. 1982;45(1-2):313–316. doi: 10.1007/BF00235792. [DOI] [PubMed] [Google Scholar]
- Matthews P. B. Evidence that the secondary as well as the primary endings of the muscle spindles may be responsible for the tonic stretch reflex of the decerebrate cat. J Physiol. 1969 Oct;204(2):365–393. doi: 10.1113/jphysiol.1969.sp008918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. B., Watson J. D. Action of vibration on the response of cat muscle spindle Ia afferents to low frequency sinusoidal stretching. J Physiol. 1981 Aug;317:365–381. doi: 10.1113/jphysiol.1981.sp013830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noth J., Friedemann H. H., Podoll K., Lange H. W. Absence of long latency reflexes to imposed finger displacements in patients with Huntington's disease. Neurosci Lett. 1983 Jan 31;35(1):97–100. doi: 10.1016/0304-3940(83)90533-5. [DOI] [PubMed] [Google Scholar]
- Rymer W. Z., Houk J. C., Crago P. E. Mechanisms of the clasp-knife reflex studied in an animal model. Exp Brain Res. 1979 Sep;37(1):93–113. doi: 10.1007/BF01474257. [DOI] [PubMed] [Google Scholar]
- Wiesendanger M., Miles T. S. Ascending pathway of low-threshold muscle afferents to the cerebral cortex and its possible role in motor control. Physiol Rev. 1982 Oct;62(4 Pt 1):1234–1270. doi: 10.1152/physrev.1982.62.4.1234. [DOI] [PubMed] [Google Scholar]


