Abstract
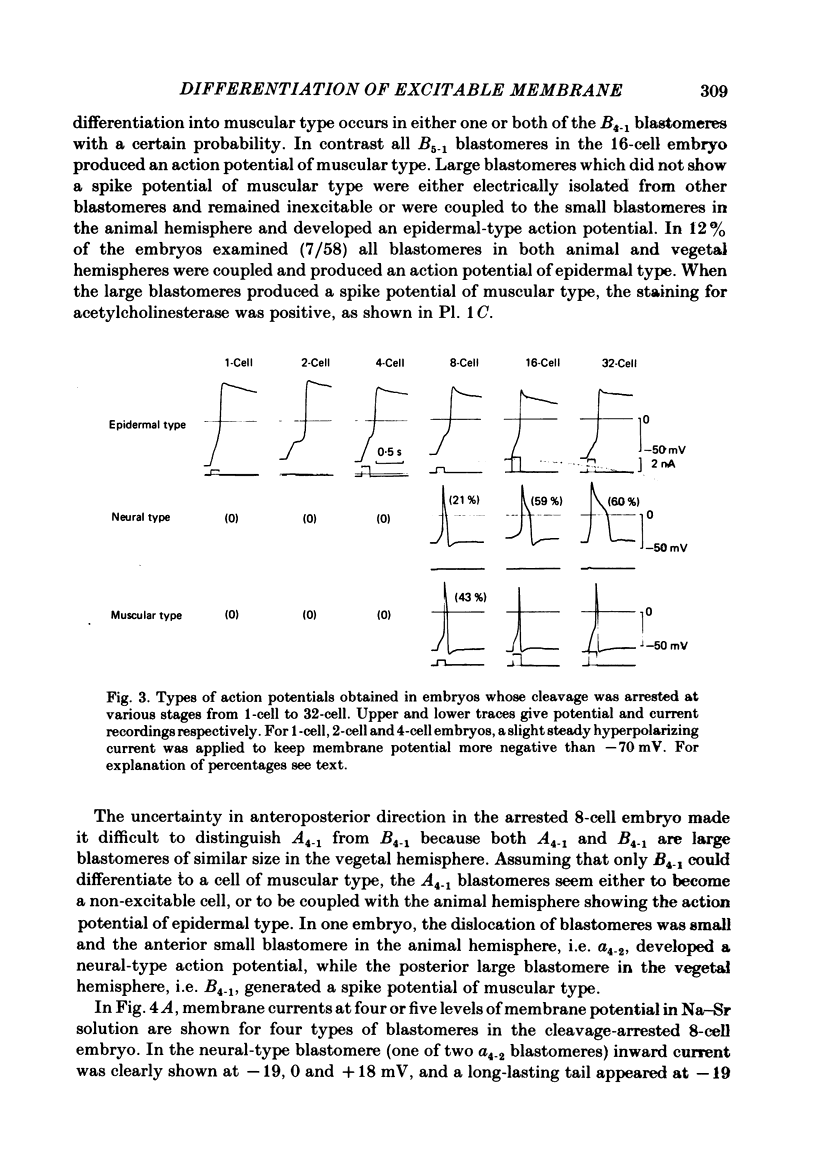
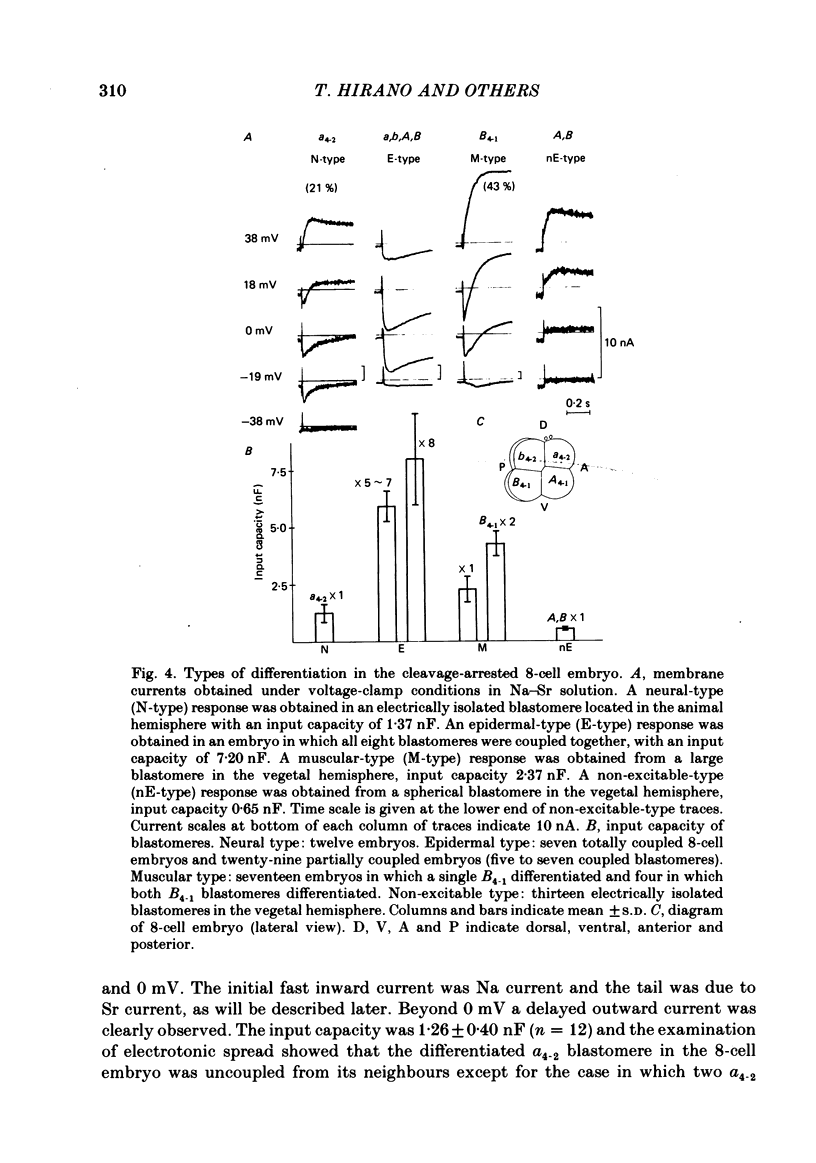
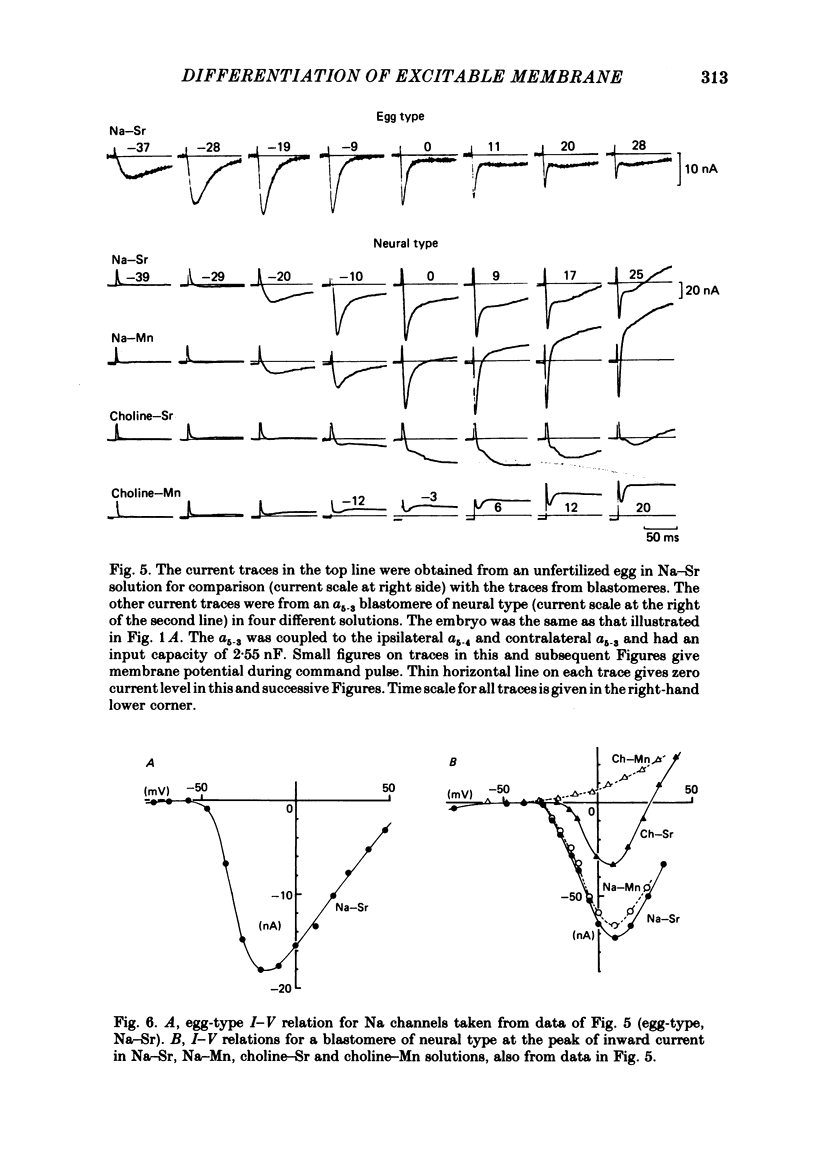
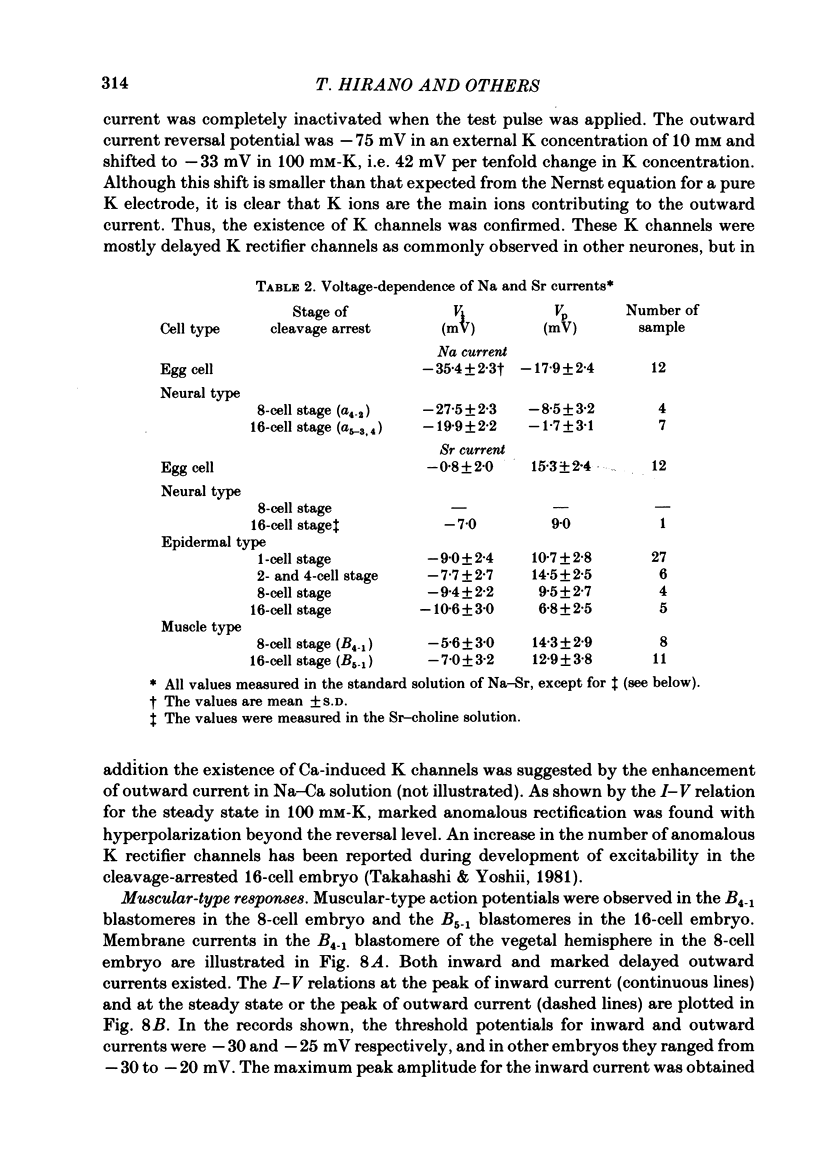
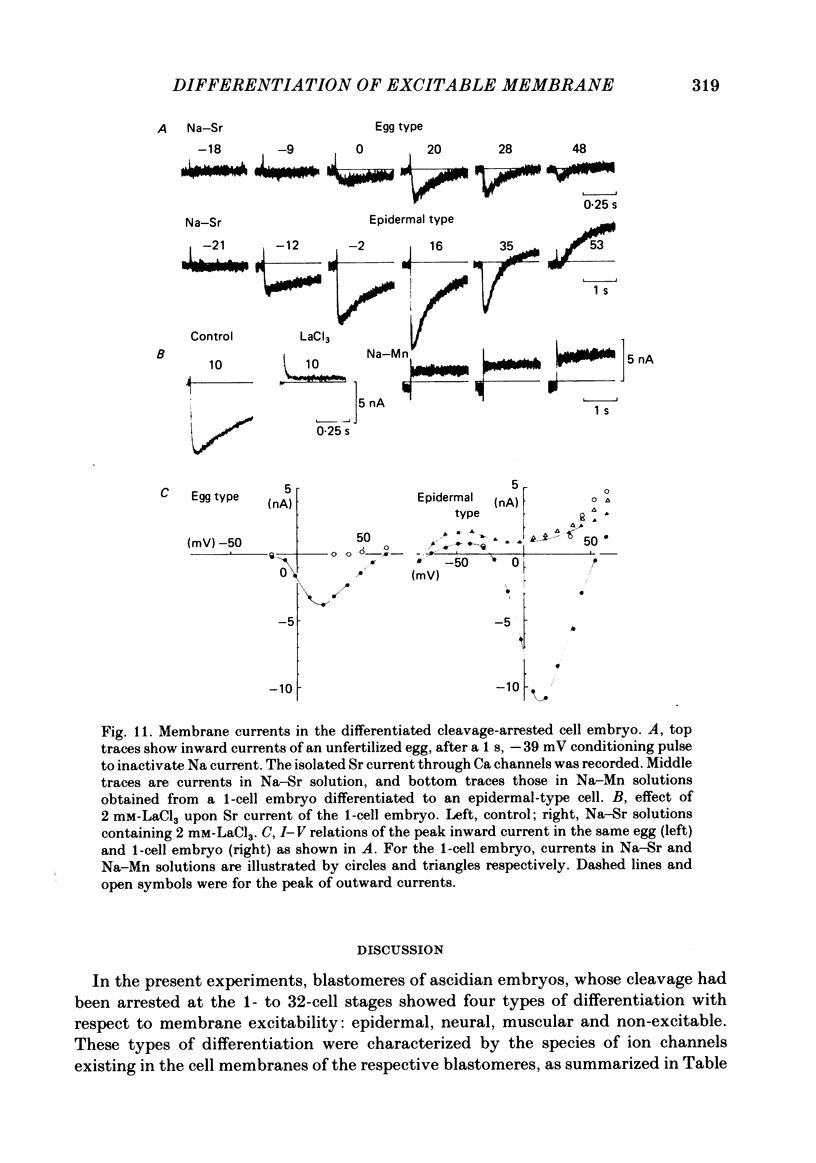
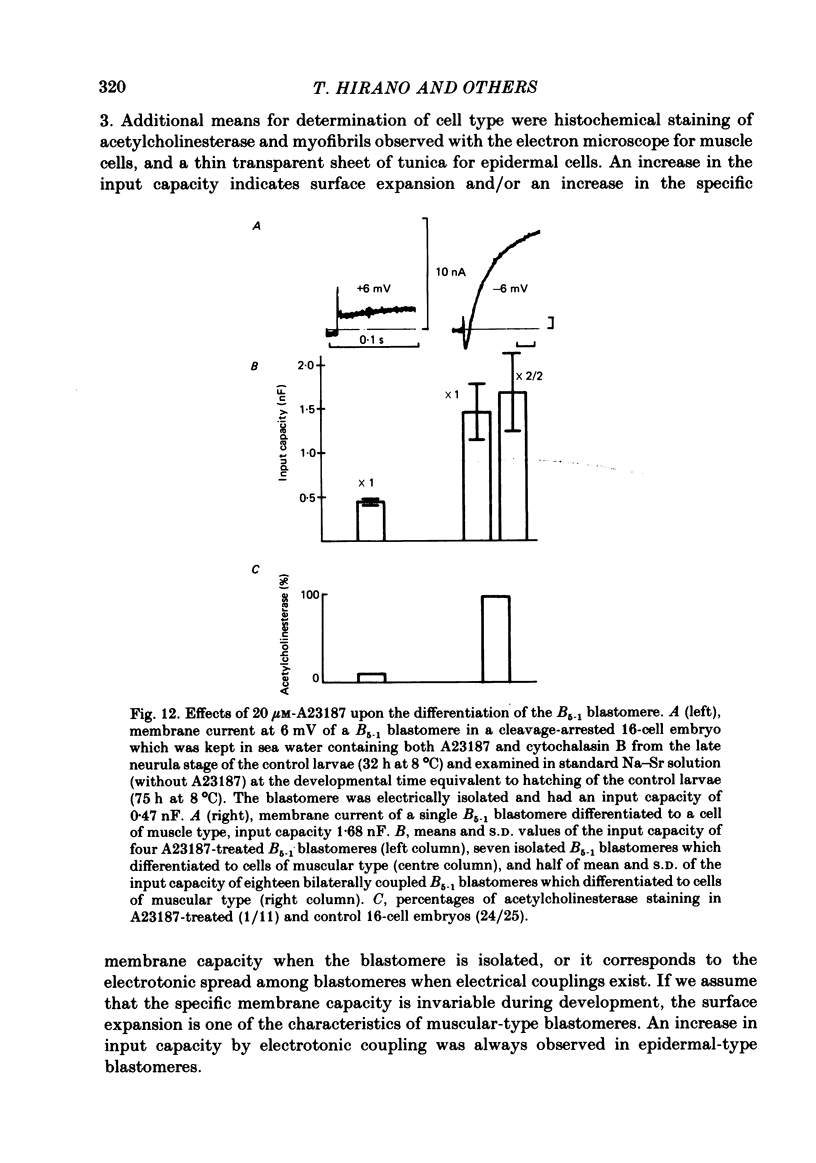
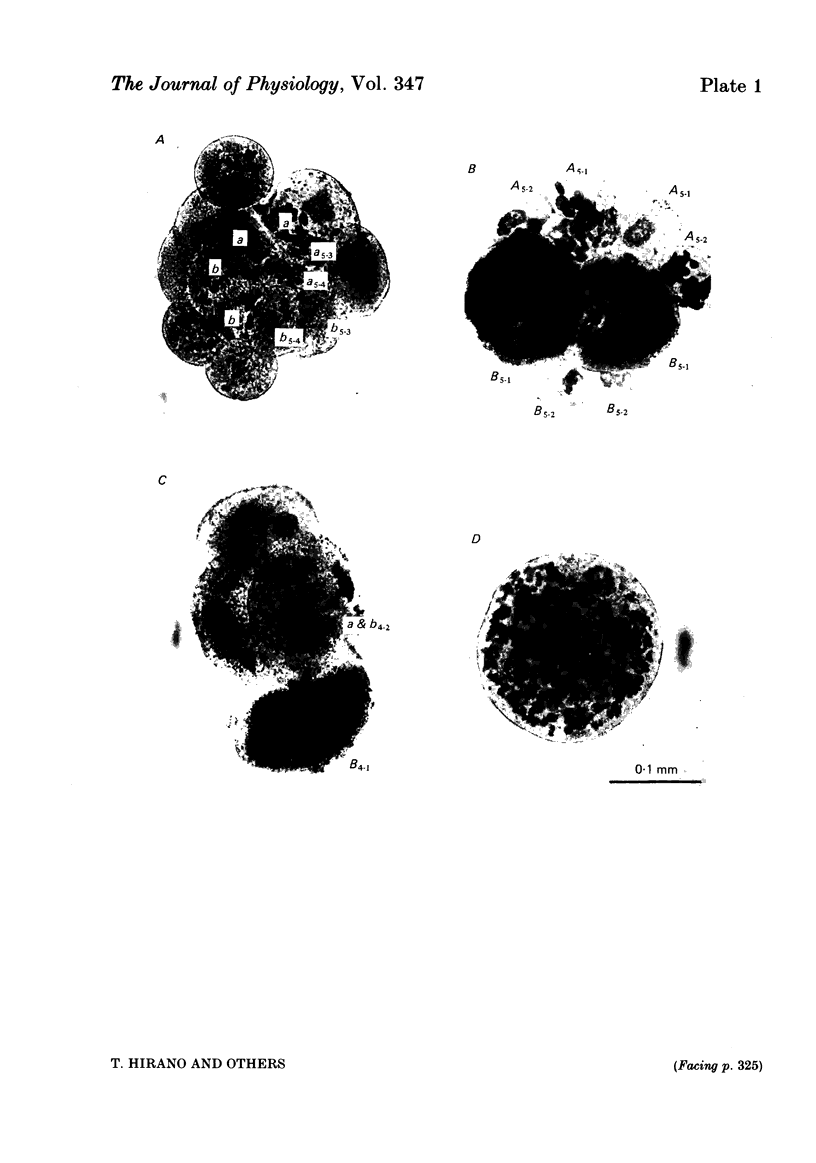
Cleavage of the embryo of Halocynthia roretzi was arrested with cytochalasin B at 1- to 32-cell stages and the embryo was cultured in sea water containing cytochalsin B until a developmental time equivalent to the hatching of the control larva. Membrane properties of the blastomeres were studied with constant-current and voltage-clamp techniques. Four types of membrane response - neural, epidermal, muscular and non-excitable - were identified on the basis of the shapes and ionic dependence of action potentials in the blastomeres of 8- to 32-cell embryos. Only the epidermal type of response was found in the blastomeres of 1- to 4-cell embryos. The blastomeres with responses of neural type had Na, Ca, delayed K rectifier, anomalous K rectifier and Ca-induced K channels. Those of epidermal type had Ca, anomalous K rectifier and Ca-induced K channels. Those of muscular type had Ca, delayed K rectifier, anomalous K rectifier and possibly Ca-induced K channels. Those of non-excitable type had almost none or small amounts of outward- and inward-going rectifier channels. The characteristic responses of neural type were found in small blastomeres in the animal hemisphere, which included some presumptive neural regions. The responses of muscular type were found in large blastomeres of the vegetal hemisphere, which included some presumptive regions for muscle. Those of epidermal type were found in the blastomeres of the animal hemisphere which did not differentiate into the neural type. Those of non-excitable type were found in some blastomeres of the vegetal hemisphere. Blasomeres of 1- to 32-cell cleavage-arrested embryos, which were presumed to possess more than one possible developmental fate, did not develop mosaic membrane properties but differentiated into one of the four types, with a probability dependent upon a gradient of ooplasmic segregation at the time of arrest.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hirano T., Takahashi K. Comparison of properties of calcium channels between the differentiated 1-cell embryo and the egg cell of ascidians. J Physiol. 1984 Feb;347:327–344. doi: 10.1113/jphysiol.1984.sp015068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kozuka M., Takahashi K. Changes in holding and ion-channel currents during activation of an ascidian egg under voltage clamp. J Physiol. 1982 Feb;323:267–286. doi: 10.1113/jphysiol.1982.sp014072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Takahashi K., Tsuda K. Electrical excitability in the egg cell membrane of the tunicate. J Physiol. 1974 Apr;238(1):37–54. doi: 10.1113/jphysiol.1974.sp010509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S., Takahashi K., Tsuda K. Calcium and sodium contributions to regenerative responses in the embryonic excitable cell membrane. Science. 1972 Jun 30;176(4042):1441–1443. doi: 10.1126/science.176.4042.1441. [DOI] [PubMed] [Google Scholar]
- Ohmori H. Inactivation kinetics and steady-state current noise in the anomalous rectifier of tunicate egg cell membranes. J Physiol. 1978 Aug;281:77–99. doi: 10.1113/jphysiol.1978.sp012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H., Sasaki S. Development of neuromuscular transmission in a larval tunicate. J Physiol. 1977 Jul;269(2):221–254. doi: 10.1113/jphysiol.1977.sp011900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Membrane currents of the tunicate egg under the voltage-clamp condition. J Physiol. 1976 Jan;254(3):607–638. doi: 10.1113/jphysiol.1976.sp011249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Takahashi K., Yoshii M. Two components of the calcium current in the egg cell membrane of the tunicate. J Physiol. 1976 Feb;255(2):527–561. doi: 10.1113/jphysiol.1976.sp011294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yoshii M. Development of sodium, calcium and potassium channels in the cleavage-arrested embryo of an ascidian. J Physiol. 1981 Jun;315:515–529. doi: 10.1113/jphysiol.1981.sp013761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker J. R. Segregation during ascidian embryogenesis of egg cytoplasmic information for tissue-specific enzyme development. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2096–2100. doi: 10.1073/pnas.70.7.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]