Abstract
Topical steroid withdrawal (TSW) is a skin condition characterized by red burning, itchy, painful skin lesions, often accompanied by peeling, and cracking. Patients experience sleep disturbances due to intense itching, significantly impacting their quality of life. A majority of affected individuals develop secondary bacterial infection, marked by heavy colonization of Staphylococcus aureus (S. aureus) and alterations in the skin microbiome. TSW is described as a rebound effect following discontinuation of prolonged use of mid-to-high-potency topical corticosteroids. There exist no definitive diagnostic criteria for this entity, and it is often misdiagnosed as a flare-up of an underlying condition or a contact allergy. Despite numerous personal reports and experiences shared on online platforms, studies on TSW remain scarce in scientific literature. Recognizing and effectively managing this condition is critical for healthcare providers seeking to develop comprehensive management plans. These plans typically include supportive therapy for both physical and psychological symptoms, as well as the gradual tapering of corticosteroid use before complete discontinuation. This review aims to consolidate the existing knowledge on TSW, providing a comprehensive resource for its identification, management, and treatment. By enhancing understanding of TSW, this review seeks to support healthcare providers in implementing optimal management strategies and improving patient outcomes.
Keywords: topical steroid withdrawal, topical steroid addiction, corticosteroids, atopic dermatitis, treatment and management
1. Introduction
Atopic dermatitis (AD), a prevalent inflammatory skin disorder, affects an estimated 17% of children and 7% of adults in the United States (1). Redness, swelling, cracking, crusting, scaling due to intense itching and pruritus are key hallmarks of AD, Topical corticosteroids (TCS, or TS) have been the mainstays in managing AD for more than 40 years and are endorsed as the first-line anti-inflammatory treatment for eczema and other atopic conditions in international guidelines (1, 2). Despite their demonstrated safety and efficacy in both short-term daily use and long-term intermittent application, concerns have surfaced regarding the cumulative effects of prolonged TCS use, specifically regarding topical steroid addiction (TSA) and withdrawal (TSW) (3), with numerous websites and patient blogs warning against these potential risks (4).
TSA and TSW are adverse outcomes associated with inappropriate or prolonged TCS use. Even though TCS has been used for AD for more than 40-years, and despite the term “steroid addiction” being introduced in 1979 (5), TSA and TSW have only recently gained attention on online platforms. TSA tends to precede TSW (6) and is defined as physical dependence on TCS with prolonged use (5). Patients with TSA typically present with increasing resistance and may require more potent steroids or frequent applications to prevent flares (7, 8). Furthermore, TSA is also characterized by the exacerbation of dermatological conditions following TCS withdrawal compared to pre- application (9, 10). This cycle of increasing dependency on TSA is often referred to as “steroid addiction syndrome” (4, 8). On the other hand, TSW, topical steroid withdrawal syndrome (TSWS), or “red skin syndromes” specifically refers to manifestations occurring after TCS cessation (2, 11) and usually results from long-term application of moderate-to-high potency TCS on sensitive areas such as the face, genitals, and intertriginous regions. In severe cases, the skin lesion extends to the entire body. It is often depicted as severe cutaneous rebound inflammation and new distressing symptoms upon TCS discontinuation (3, 12, 13). Various terms have been used to describe TSA/TSW, including, but not limited to, “red skin syndromes” (2, 11), “red face” (4), and “red scrotum” (4). The face is the most affected area in TSA/TSW; however, other areas with high percutaneous penetration, such as the genitals and intertriginous regions, are more likely to be affected as well (3, 4, 10, 12).
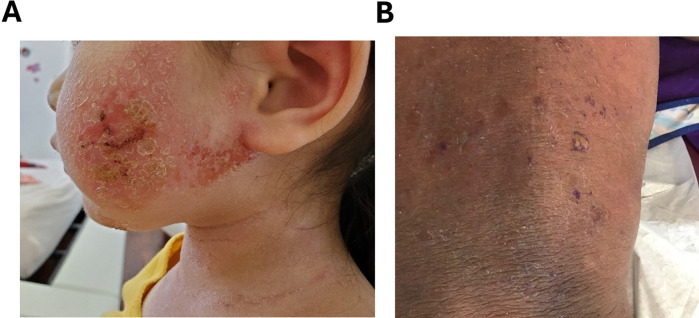
Two primary morphological subtypes of TSW have been identified: erythematoedematous (47.9%) (Figure 1A) and papulopustular (52.1%) (Figure 1B) (4). The erythematoedematous subtype is prevalent among patients with chronic eczematous conditions such as AD and seborrheic dermatitis. It is characterized by erythema, scaling, edema, and burning sensations. Conversely, the papulopustular subtype is more common in patients using TCS for the treatment of cosmetic, pigmentary, or acneiform conditions and frequently occurs in those who develop steroid rosacea, although its presence is not required for diagnosis. As the name suggests, the papulopustular subtype typically presents with erythema, papules, and pustules and is associated with less frequent occurrences of swelling, edema, burning and stinging (4). While clinical overlaps between the two variants exist, the erythematoedematous subtype is distinguished by more pronounced burning, stinging sensations, and edema. Specifically, burning sensations and edema are present in 94.6% and 43.3% of erythematoedematous cases, respectively, compared to 35.4% and 2.9% of papulopustular cases (4). The terms “topical steroid induced facial rosaceiform dermatitis” (15) and “topical corticosteroid-induced rosacea-like dermatitis” (16) have been used to describe patients experiencing erythema and telangiectasias with prolonged TCS use. These conditions are believed to correspond with the erythematoedematous subtype of TSW (13).
Figure 1.
(A) Erythematoedematous subtype of steroid withdrawal characterized by severe oozing, crust, redness and excoriation. (B) Papulopustular subtype of steroid withdrawal with erythema, papules and pustules. Permission obtained to reuse figure from (14).
Despite TSA/TSW being recognized in 1979, and patients reporting it on online platforms, research on these conditions has been limited. Furthermore, an absence of consensus in diagnostic criteria contributes to the incomplete understanding of these conditions within the dermatological community (2, 3). Therefore, a more comprehensive understanding of TSA/TSW among medical professionals is required to mitigate the burden of these conditions. Accordingly, this review summarizes key information regarding TSA/TSW.
2. Epidemiology and risk factors
Determining the prevalence of TSA/TSW is challenging due to the lack of clear diagnostic criteria and significant variation in literature reports. For example, a Japanese study conducted in 2000 found that 12% of patients with AD using TCS were experiencing TSA (9). On the other hand, a multinational study involving 2,160 subjects with eczema (aged 18 years or older or caregivers of children with eczema) reported that 79% of adults and 43% of caregivers observed symptoms consistent with TSW in themselves and in their children, respectively (3).
While determining the prevalence of TSA/TSW is challenging, more is known about the associated risk factors. Increased TCS potency has been identified as a risk factor for TSA/TSW, with moderate- to high-potency TCS use being associated with an increased likelihood of disease (2–4, 13). Mid- or high-potency TCS use has been reported in 98.6% of patients with TSW (4), and in 73% of children with AD demonstrating symptoms of TSW (12). The vasoconstrictor assay categorizes TCS into seven potency groups, ranging from ultra-high potency (Group I) to low potency (Group VII) based on the extent of cutaneous vasoconstriction (17). For example, mometasone is a medium potency steroid (class IV–V) with strong topical efficacy but limited systemic absorption, making it more suitable for long term use. In contrast, clobetasol propionate is ultra-high potency (class I) with significantly higher penetration and ability to cause irritation, skin atrophy, striae and systemic side effects such as hypothalamic-pituitary-adrenal suppression, glaucoma, septic necrosis of the femoral head, hyperglycemia, hypertension (17). Given that a high proportion of patients with TSA/TSW have used mid- or high- potency TCS and more potent molecules are associated with greater dependence (18), caution must be practiced when prescribing these medications. Being mindful of TCS potency is particularly important in pediatric populations, who have a larger body surface area (BSA)-to-weight-ratio, promoting greater TCS absorption and increased systemic effects such as hypothalamic-pituitary-adrenal (HPA) axis suppression. Mid-potency TCS may be a safer alternative to high-potency TCS when treating TSW in these populations, as they do not affect the HPA axis when used “up to 3 times a day for 4 weeks or twice daily for 16 weeks” (19). Furthermore, the use of a topical steroid ladder, which classifies TCS based on potency, may be a useful tool in facilitating tapering strategies and minimizing adverse effects in both adult and pediatric patient population (20).
Similarly, dosage also influences the risk of TSW, with the use of multiple TCSs increasing the likelihood of its occurrence. A previous study reported that, among 1,702 participants with symptoms consistent with TSW, 82% experienced TSW symptoms when using two TCSs, compared to 64% with one TCS (3). Not only does TCS dosage matter, but total corticosteroid use also plays a role. History of oral corticosteroid use may indicate more severe clinical conditions and is associated with TCS overuse, contributing to a greater likelihood of TSW (21). Lastly, longer duration of TCS application is another critical factor; 86% of individuals using TCS for 6 or more years reported symptoms consistent with TSW, compared to 53% of individuals using TCS for less than 1 year (3). Generally, the duration of TCS usage is 6 months or more (13), with 85.2% of patients with TSW reporting use longer than 12 months (4).
Certain patient factors, such as gender, primary concern, and region of application, have also been identified to increase risk. Individuals with AD are particularly susceptible to TSW, with AD being the initial indication for TCS use in one-third of cases. Furthermore, adult women are the largest demographic, comprising 81% of cases, and nearly all patients with TSW (97%) having a history of applying TCS to the face (4). The reason for the predominance in women is not fully understood (13), but it is believed to result from TCS use related to cosmetic and pigmentary concerns of the face (22). In particular, the anti-melanogenic properties of TCS may be favorable in those seeking skin lightening, contributing to misuse and subsequent TSA/TSW (13, 22). Additionally, patient age, accessibility of TCS, and behavior can also be risk factors. Studies show that younger patients are associated with an increased sensitivity to TCS and are likely to develop TSA/TSW. Unlike adults, who typically develop TSW after 6 months of TCS use (13), children can develop TSW after just 2 months of use, with symptoms potentially persisting for over 12 months (12). Studies show that recovery rates and prognosis also differ between children and adults, with 44% of children recovering from TSW compared to 28% of adults, and 8% of adults experiencing symptoms for more than 5 years (3). Additionally, TCS misuse is more prevalent in developing countries due to unregulated and over-the-counter sales of TCS and self-treatment, often for cosmetic purposes or non-responsive dermatoses. Moreover, patient behaviors such as self-treatment, seeking prescriptions from practitioners other than dermatologists, reusing old prescriptions for recurrent or similar rashes, and sharing prescriptions with others are concerning and can increase one's susceptibility (22). Furthermore, clinical evidence suggests that young females, especially those with skin of color are predominantly affected by this condition owing their vulnerability to TCS misuse for cosmetic reasons, such as use of fairness creams (22).
Lastly, an often-overlooked risk factor for TSW is occupational exposure to steroids. Specifically, healthcare professionals treating eczema patients may be exposed to TCS when applying them to patients, which can lead to skin irritation or allergic reactions if proper handling procedures are not followed—particularly with repeated exposure to high-potency steroids. This exposure can be mitigated if appropriate safety measures are taken. Wearing protective gloves, maintaining proper hand hygiene, minimizing direct skin contact, and employing correct application techniques are essential strategies to reduce the likelihood of developing TSW due to occupational exposure. Furthermore, occupational contact allergy, often presented as chronic irritative hand dermatitis, is primarily caused by water exposure or humidity in gloves, and corticosteroid use in such cases may be problematic. In these cases, protection and allergen avoidance are of utmost importance and corticosteroids should be the last choice. Alternative treatments such as tacrolimus and delgocitinib could be considered for its management.
3. Pathophysiology
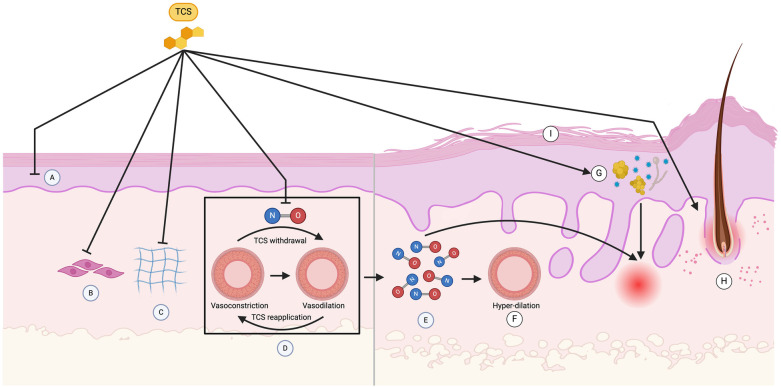
The pathophysiology of TSA/TSW is not fully understood. However, previous research and literature suggest that the progression of TSA/TSW is multifactorial. Some of the key contributing factors are outlined in Figure 2.
Figure 2.
Pathophysiology of topical steroid addiction (TSA) and withdrawal (TSW). Exogenous application of topical corticosteroids (TCS) suppresses intrinsic cortisol production by keratinocytes (A) through negative feedback mechanisms involving the downregulation of enzymes like CYP11A1, CYP17A1, 3βHSD1, CYP21, and CYP11B1. Upon TCS withdrawal, the elimination of exogenous cortisol and reduced enzymatic activity leads to a temporary reduction in cortisol production, worsening inflammation in TSW. TCS also inhibits the proliferation and extracellular matrix protein synthesis of keratinocytes and fibroblasts (B), reducing steroidogenesis of epidermal lipids such as ceramides, cholesterol, and fatty acids, resulting in a thinned stratum corneum and increased transepidermal water loss (I). Skin atrophy arises from TCS-induced reduction in collagen synthesis (C), caused by decreased prolyl hydroxylase activity and collagen mRNA degradation. Chronic TCS use also inhibits endothelial nitric oxide (NO) production, leading to vasoconstriction, which reverses upon TCS withdrawal, causing excess NO release and subsequent vasodilation (D). The vasodilation is associated with adverse effects such as erythema, itching and burning. Reapplication of TCS is often sought to reverse these adverse effects, resulting in a cycle of vasoconstriction and vasodilation (D), termed the “trampoline effect” or “neon sign,” and excess NO accumulation (E), leading to hyperdilation of blood vessels beyond their pre-steroid state (F). This excess NO and prolonged hyperdilation are thought to contribute significantly to the erythema characteristic of TSA/TSW. Prolonged TCS use induces immunosuppression as well, making the skin more susceptible to the overgrowth of microorganisms. When TCS are withdrawn, these microorganisms can act as superantigens, inducing cytokine release and subsequent inflammation (G). Papulopustular and acneiform lesions, characteristic of the papulopustular subtype of TSW, are thought to result from TCS degradation of the follicular epithelium and subsequent extrusion of follicular contents (H). These interconnected processes illustrate the impact of TCS on keratinocyte and fibroblast function, endothelial response, immune function, and follicular epithelium and skin barrier integrity, ultimately driving the pathological changes observed in TSA and TSW (Created with BioRender).
3.1. Dysregulation of endogenous cortisol production by keratinocytes
Former studies have revealed that keratinocytes possess all the necessary mRNA and enzymes for the metabolic conversion of cholesterol to cortisol, suggesting the presence of a non-adrenal steroidal system in human skin (23, 24). During TSA, exogenous application of TCS may thus suppress the intrinsic production of cortisol by keratinocytes (Figure 2A) through negative feedback mechanisms. This could involve the downregulation of enzymes involved in cortisol synthesis in keratinocytes such as CYP11A1, CYP17A1, 3bHSD1, CYP21, and CYP11B1 (24). It is likely that TCS withdrawal results in elimination of exogenous cortisol, and the suppressed enzymatic activity of keratinocytes may take a while to return to baseline levels, resulting in a period of decreased cortisol production. Given that cortisol produced from keratinocytes helps to modulate inflammatory reactions, the temporary reduction in cortisol may be responsible for the worsening inflammation seen in TSW.
3.2. Skin atrophy
TCS suppress proliferation and the extracellular matrix (ECM) protein synthesis of keratinocytes (Figure 2A) and fibroblasts (Figure 2B), which can be viewed histopathologically. This inhibition results in reduced steroidogenesis of epidermal lipids such as ceramides, cholesterol, and fatty acids. As a result, the stratum corneum is thinned (Figure 2I), and greater transepidermal water loss (TEWL) transpires. The resultant skin has reduced skin barrier function, tensile strength, and elasticity (25), which could correspond to the formation of “elephant wrinkles” seen in TSA/TSW.
Similarly, skin atrophy may also occur from reduced collagen synthesis (Figure 2C). TCS indirectly inhibits collagen synthesis by reducing prolyl hydroxylase activity or promoting collagen mRNA degradation (25). They also directly inhibit synthesis by reducing procollagen gene expression through glucocorticoid response elements that negatively regulate Smad proteins, which are necessary for type I collagen transcription (25). Additionally, they may promote epidermal atrophy by overexpression of tissue proteases and keratinocyte proteins (12). For instance, TCS increases thymic stromal lymphopoietin activity, which shifts the T-helper (Th) lymphocyte ratio from a balanced Th1/Th2 population to Th2 predominance, like that seen in AD (12).
3.3. Role of nitric oxide
Nitric oxide (NO), an endothelium-derived relaxing factor (EDRF), released by vascular endothelial cells, functions as a potent vasodilator (26). Chronic TCS use inhibits endothelial NO, causing persistent vasoconstriction. Following TCS removal, accumulated endothelial NO is released, resulting in vasodilation and TSW symptoms such as erythema, itching, and burning sensation. Reapplying TCS to alleviate these unwanted symptoms results in vasoconstriction. The dependency on TCS results in alternating cycles of vasoconstriction and vasodilation, known as the “trampoline effect” or “neon sign,” (Figure 2D) and leads to excess NO accumulation (Figure 2E) (22) and hyperdilation of blood vessels beyond their pre- steroid diameter (Figure 2F) (22, 27). In summary, excess NO results in prolonged hyperdilation of vessels in TSA/TSW and is thought to contribute to the cardinal sign of erythema.
While NO is believed to contribute to the pathophysiology of TSA/TSW, its effects are difficult to discern and are inconclusive. Previous studies have demonstrated higher serum NO levels in inflammatory skin diseases like AD (10, 28), making it challenging to identify if elevated NO is due to TSA/TSW or the underlying condition. Furthermore, NO acts as a signaling molecule with both pro-inflammatory and anti-inflammatory effects depending on the context of its production. It is anti-inflammatory under normal physiological conditions (29) and participates in various biological functions including barrier homeostasis, wound healing, and antimicrobial defense (30). On the other hand, it is also pro- inflammatory and can participate in cutaneous inflammation in pathological conditions (29, 30). Topical NO-releasing products have also been shown to improve AD symptoms in humans and murine models (30). The dual nature of NO's effects in inflammation complicates the understanding of its role in TSA/TSW. However, despite its contradictory effects, NO levels have been found to be elevated in patients with TSA compared to cured patients and patients with eczema, indicating that serum NO may be useful in identifying addicted individuals (31). Enhancing our understanding of NO and its role in the pathology of TSA/TSW, as well as other inflammatory conditions like atopic dermatitis, could lead to the development of more effective treatments.
3.4. Topical corticosteroid-induced immunosuppression
Prolonged TCS use induces immunosuppression, making the skin more susceptible to the overgrowth of microorganisms. This may initially be helpful to patients with AD and other skin conditions, as a skin microbiome lacking biodiversity has been linked to many skin problems (32). However, when TCS are discontinued or withdrawn, these flourishing microorganisms can act as superantigens. As a result, the immune system which is no longer being suppressed induces cytokine release in response to the microbes and subsequent inflammation (Figure 2G) (18, 22, 32).
3.5. Direct effects on follicular epithelium
Papulopustular and acneiform lesions, characteristic of the papulopustular subtype, are thought to result from TCS degradation of the follicular epithelium and subsequent extrusion of follicular contents (Figure 2H) (22).
3.6. Glucocorticoid receptor expression
TCS acts by binding to intracellular glucocorticoid receptors (GR), of which two splicing variants have been identified, the GRa and GRb isoforms (10). Patients with a poor response following TCS treatment were shown to have upregulated GRb expression, while patients with a good response showed no changes in expression (10). Having higher GRb expression is thought to correlate to TCS insensitivity (10). Patients exhibiting TCS insensitivity may consequently use higher doses of TCS for extended periods, thereby elevating their risk of developing TSA/TSW.
3.7. Cytokines
TCS withdrawal has been associated with upregulation of IL1-α TNF-α, inhibitor of nuclear factor kappa-B kinase subunits alpha and beta (IKK1, IKK2) and nuclear factor kappa-B (NF-kB) in the epidermis. These cytokines gradually diminish after 1 week (33).
4. Clinical features of TSA/TSW
4.1. Signs and symptoms
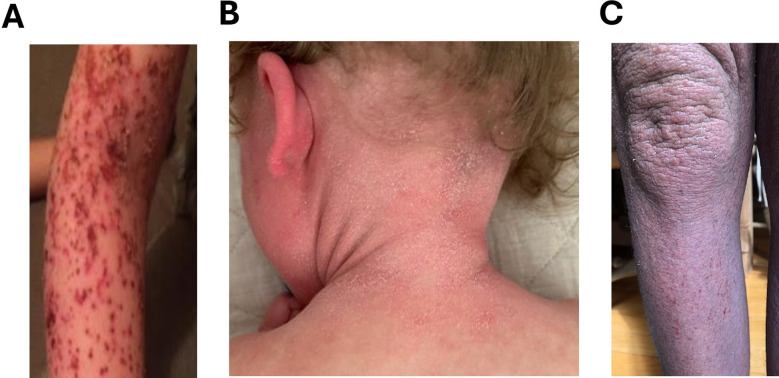
When patients with TSA discontinue TCS, they may experience widespread erythema (Figure 3A), the most common sign of TSW. The erythema can extend beyond original eczematous areas to previously untreated sites (22) and be accompanied by other signs associated with TSW such as the headlight sign (Figure 3B), red sleeve sign, and “elephant wrinkles” (Figure 3C). Patients undergoing TSW commonly exhibit symptoms such as intense burning sensations, pruritus, and edema, amongst others (4). Clinical signs and symptoms of TSW described in literature are summarized in Table 1.
Figure 3.
Signs and symptoms of TSW. (A) Widespread redness in the arm. (B) Serious redness in the ear. (C) Elephant wrinkles. Permission obtained to reuse figure from (14).
Table 1.
Clinical signs and symptoms.
| Major dermatological signs | Features |
|---|---|
| Erythema (cardinal sign) (3, 4, 9, 11, 13) | |
| Headlight sign (2, 4, 13) |
|
| Red sleeve (3) | |
| “Elephant wrinkles” (2, 3, 13) | |
| Other dermatological signs and symptoms | Features |
| Burning or stinging skin (3, 4, 9, 11) |
|
| Skin peeling, flaking, or exfoliation (3, 4, 9, 11) |
|
| Pruritis (3, 4, 9, 11) |
|
| New skin hypersensitivity or dysesthesias (to various materials or environmental conditions) (3, 4, 13) | |
| Papulopustules (4) | |
| Hyperhidrosis or itchy wheals |
|
| Alopecia (3) |
|
| Skin oozing or weeping (3, 4, 9, 11) |
|
| Changes to skin of the genitalia (11) |
|
| Skin atrophy (2, 22) |
|
| Telangiectasia (2, 3, 8, 13, 22) |
|
| Non-dermatological signs and symptoms | Features |
|
|
| Swelling/Edema (3, 4, 9, 11) |
|
| Nerve pain (3) |
|
| Lymphadenopathy (3) |
|
| Appetite changes (3) |
|
| Psychological changes | Features |
| Intense emotional fluctuations (3), depression (3, 9) anxiety (3, 11) |
|
| Suicidality (3) |
|
| Insomnia, sleep disturbances, or altered circadian rhythm (3, 11) |
|
| Fatigue (3) |
|
| Pediatric signs and symptoms | Features |
| Subjective growth delays (failure to meet milestones, decreased weight gain) (12) |
|
In patients with TSW, correlating clinical signs and symptoms with a detailed history of TCS use is essential. Key elements for identifying TSW include the specific TCS used, their potency, dosage, frequency, and duration of application. However, it is important to note that limited health literacy can hinder patients' ability to recall these details, complicating diagnosis. A 2012 review found that up to 48% of patients lack functional health literacy (34), which can make it challenging to attain accurate histories.
4.2. Histological features
The histological features of TSA/TSW are non-specific, rendering diagnosis by histology alone improbable. In a former study, biopsies from 4 patients with TSW demonstrated spongiosis and parakeratosis; however, these histological slides were excluded from the study (21). Nevertheless, research on TSA/TSW has identified some common histological findings between the two subtypes of TSW. Both subtypes have demonstrated dilated vessels in the dermis and collagen degeneration (4).
Additionally, histological analysis of the erythematoedematous subtype revealed a thinned epidermis, spongiosis, a thin or absent granular layer, sparse perivascular infiltrate, and prominent sebaceous glands surrounded by inflammatory cells. On the other hand, the papulopustular subtype, akin to rosacea, displayed a perifollicular or granulomatous infiltrate containing neutrophils and lymphocytes (4).
5. Differentiating TSW/TSA from other related conditions
5.1. Topical steroid damaged/dependent face
In recent years, instances of improper TCS use on the face have risen in India, prompting dermatologist Koushik Lahiri to introduce the term “topical steroid damaged/dependent face (TSDF)” in March 2008. Lahiri defined TSDF as “the semi-permanent or permanent damage to the skin of the face precipitated by the irrational, indiscriminate, unsupervised, or prolonged use of TCS resulting in a plethora of cutaneous signs and symptoms and psychological dependence on the drug” (35). Although TSDF shares characteristics with TSA, such as dependency on TCS and exacerbation of cutaneous symptoms upon withdrawal (22), TSDF is specifically localized to the face, whereas TSA encompasses a broader dependency affecting various body regions.
5.2. Tachyphylaxis
Additionally, the terms tachyphylaxis and steroid phobia are often incorrectly associated with TSA/TSW. Tachyphylaxis is an acute condition characterized by reduced medication efficacy after successive dosing. It can be distinguished from TSA/TSW, as tachyphylaxis occurs before TCS withdrawal, while TSA/TSW exhibit cutaneous eruptions after withdrawal (9). Moreover, steroid phobia, defined as the fear or reluctance to use TCS, is associated with TSA/TSW. Awareness of TSA/TSW may exacerbate steroid phobia. Conversely, steroid phobia may lead to improper TCS usage that could perpetuate TSA/TSW. It is important to note that TSA is often attributed to TCS misuse or overuse, while steroid phobia results in TCS underuse (2).
5.3. Psoriasis
Psoriasis flares can be triggered by the abrupt cessation of systemic or topical corticosteroids (36). Withdrawal of these medications may lead to rebound psoriasis, often presenting with symptoms that are more severe than the initial presentation (37). These drug-associated flares of psoriasis should not be misinterpreted as TSW. Drug- induced psoriasis is similar to conventional psoriasis, but is associated with an eosinophilic infiltrate in the dermis and a lichenoid pattern on histology (36, 38).
6. Phases of TCS withdrawal
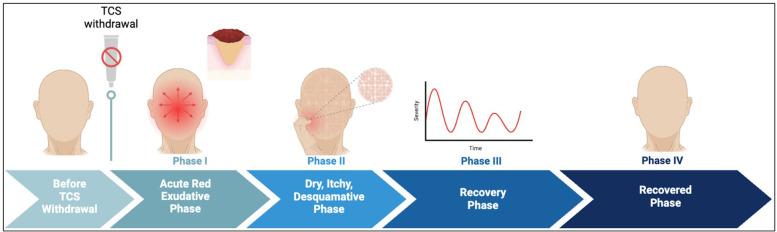
Before discontinuing TCS, patients' skin may appear normal or well-controlled by TCS, although some begin to experience symptoms such as increased pruritis or diminished TCS efficacy. In certain cases, prurigo-like eruptions or intractable nodules with severe itching emerge, often signaling addiction (9). As symptoms worsen, patients may choose to discontinue TCS for various personal reasons, such as steroid phobia, or diminished benefits despite prolonged or increased usage (2, 12, 39). TCS discontinuation is particularly relevant for patients with recalcitrant eczema, where withdrawal is a critical aspect of management. Four phases of TCS withdrawal have been proposed from previous studies (10). The sequence of events in each phase is described and illustrated in Table 2 and Figure 4 respectively.
Table 2.
Phases of TSW.
| Phases | Features |
|---|---|
| Phase I: Acute Red Exudative Phase (4, 11) |
|
| Phase II: Dry, Itchy, Desquamative Phase (Acute Phase) (11) | |
| Phase III: Recovery Phase (11) | |
| Phase IV: Recovered Phase (11) |
|
Figure 4.
Phases of TCS withdrawal. Before TCS withdrawal patients’ skin appears normal. Phase I is characterized by acute red skin which spreads gradually. It lasts for a few days to weeks. Phase II is marked by dry, itchy, thickened skin. Some patients show signs of depression due to lack of treatment and continuous worsening of skin lesion. Phase III is the recovery phase where patients show gradual improvement of skin lesion with occasional aggravated skin flares. Phase IV is the recovered phase where patients skin normalizes and regains its original appearance. This phase may take weeks to years.
7. Diagnosing TSA/TSW
The non-specific features of TSA/TSW can propagate misdiagnosis and mistreatment. Several conditions can be considered in the case of TSA/TSW, such as rosacea, cutaneous T-cell lymphoma, and psoriasis (13). However, the 3 main differential diagnoses include eczematous/AD flares, allergic contact dermatitis (ACD), and infection (2).
Differentiating TSW from a flare-up of the underlying disease may be challenging but is of utmost importance. Misdiagnosing a withdrawal episode as an AD flare can lead to inappropriate treatment, often escalating TCS use. Conversely, AD flares can be misinterpreted as TSW. Both scenarios result in insufficient treatment, worsened patient outcomes, and unnecessary morbidity (6). No consensus criteria for distinguishing TSW from eczematous flares have been established. Nonetheless, previous studies suggest 3 essential diagnostic criteria for favoring a diagnosis of TSW over worsening AD:
- 1.
-
2.
Erythema- confluent redness within days to weeks of TCS withdrawal (4, 13, 21)
-
3.
History of prolonged, frequent TCS use on the affected region (typically the face or genitals) (4, 13, 21)
Additionally, to rule out ACD to an active steroid molecule or its vehicular excipients, patch testing may be indicated (4, 12). Localization of a rash can help identify the offending allergen or point clinicians toward suspecting ACD, such as supraumbilical dermatitis from ACD to nickel in a belt buckle (1). Despite challenges, like lack of clear skin for testing in atopic individuals (13), and potential false positives due to vasodilation (10), patch testing can determine reactions to the active steroid, its vehicle, or other environmental allergens. Given a high clinical suspicion of TSW, patch testing should be performed with the awareness of potential delayed positive reactions (13), as ACD is a type IV delayed hypersensitivity reaction. When indicated, patch testing should be administered, as mistaking ACD for TSW could prevent patients from receiving necessary anti-inflammatory therapy (4).
8. Treatment
Despite the lack of general guidelines for treating TSA/TSW, several treatment approaches have shown potential value for both physicians and patients. The most widely recommended intervention is TCS discontinuation, which is advised in nearly all cases of TSA/TSW. In addition, some physicians may prescribe immune-modulating agents such as oral steroids, monoclonal antibodies, non-steroidal anti-inflammatory drugs (NSAIDs), among others. In addition, use of multi-component TCM therapy, complementary integrative medicine, black tea, coal tar and tannins are widely recognized in the literature. A summary of all these immune-modulating therapies, along with other recommended treatments, is provided in Table 3 and Figure 5.
Table 3.
Treatment of TSA/TSW.
| Treatment | Details | Adverse effects |
|---|---|---|
| TCS discontinuation |
|
|
| TCS discontinuation with oral steroid supplementation |
|
|
| NSAIDs |
|
|
| Antibiotic treatment: doxycycline, tetracycline, and erythromycin |
|
|
| Supportive therapy (antihistamines, ice/cool compresses, etc.) |
|
|
| Dupilumab (Dupixent®) |
|
|
| Complementary integrative medicine |
|
|
| Black tea formulation |
|
|
| Coal tar |
|
|
| Tannins |
|
|
| Basic skin care |
|
Figure 5.
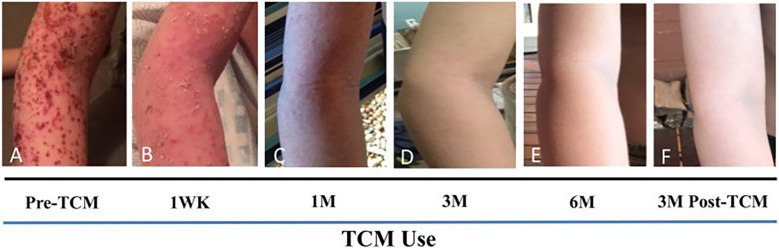
TCM therapy showed marked improved skin lesion. (A) 12 days after the last course of prednisone, with MSSA Staphylococcus aureus infection and poor response to steroid injection. (B) Improvement of skin lesions by 1 Week of TCM use including internal tea, external herbal bath and creams. (C) 1 month of TCM use, (D) 3 months of TCM use, (E) By 6 months, skin remained to be well controlled. No steroids or antibiotics were used during the course of TCM therapy. (F) Skin reveals no apparent recurrence 3 months after TCM discontinuation. Figure permitted to use from (45).
9. Prevention
To prevent TSA/TSW, it is recommended that continuous TCS use be limited to 2–4 weeks to prevent long-term histological effects. Periods of interruption where TCS use is discontinued may allow the skin to recover from the previous TCS cycle to permit continuation of treatment (9). Additionally, another method of preventing TSW is by dampening moderate or severe AD before disease escalation. In such cases, administering a short-term systemic corticosteroid as a rescue therapy, followed by site-specific TCS, and tapering of all steroids may be appropriate (Figure 6). In doing so, the severity of AD should dampen with time, reducing the need for corticosteroids and limiting adverse effects like TSA/TSW (6).
Figure 6.
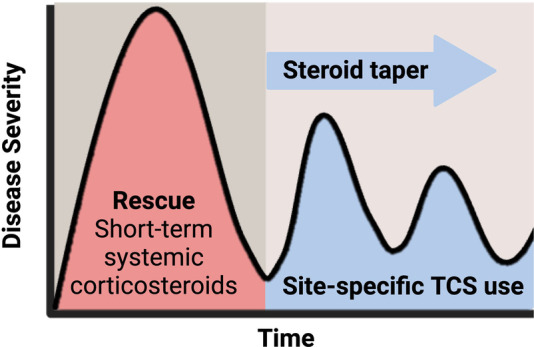
Damping moderate to severe AD with corticosteroid. Topical corticosteroid use should be limited to 2–4 weeks for appropriate response. Tapering steroid dose, followed by site-specific corticosteroids may help dampen AD severity by reducing the need for corticosteroids and limiting adverse effects like TSW.
10. Prognosis and psychological impact
Active TSA/TSW symptoms can persist even for 20 months after discontinuing the offending agent (12). One survey found that 26% of patients with TSA/TSW who had stopped TCS for over 5 years still experienced symptoms. Not only can TSA/TSW be long-lasting, but it also has a sizeable psychological impact. Concern over TSW was high among adults with AD and caregivers of children with AD, with 74% of adults and 49% of caregivers reporting concern about TSW. Alarmingly, 47% of adults with symptoms consistent with TSW reported having suicidal thoughts (3). Given that TSW can persist for months to years, and is associated with high levels of concern, suicidal ideation, and significant psychological toll, frequent check-ins are crucial for evaluating and maintaining patients' emotional well-being (2). Despite increasing patient concern over TCS use and TSA/TSW, many patients find that they are met with a dismissive, ignorant or unempathetic response from their doctors. The response has resulted in patient mistrust of the medical profession, with one study reporting that 9 of 26 participants withdrew from typical dermatologic care. The resultant lack of validation for patient concerns has led some to discontinue TCS without guidance from providers and has led many to seek alternative care such as TCM (39).
11. Discussion and conclusion
Estimates of the prevalence of TSA/TSW vary widely, with studies suggesting that 12%–79% of patients with atopic dermatitis (AD) may experience TSW (3, 9). Therefore, it is necessary to calm down the underlying Th2 mediated immune response in early stages of AD development. Additionally, 47% of patients with symptoms consistent with TSW experience suicidal ideations. Despite the large potential burden, studying TSA/TSW presents several limitations, such as the unclear temporal relationship between TCS use and symptom onset (4), a predominance of female survey respondents (3), reliance on self-reported symptoms, and the absence of standardized diagnostic criteria. Given the substantial physical and psychological impact of TSA/TSW, continued research is necessary to establish consensus diagnostic criteria and effective treatment protocols. TSA/TSW share similarities with other dermatologic conditions; however, cardinal signs have been identified. Determination of consensus diagnostic criteria may thus be achieved by combination of these clinical signs with histological findings and detailed history of TCS use. Additionally, inflammatory markers involved in pathophysiology, such as NO, may enable quantitative differentiation from other conditions.
While diagnostic criteria are needed, proposals of such criteria cannot occur unless physicians, particularly dermatologists, recognize the legitimacy of TSA/TSW. Despite numerous patients sharing their experiences with chronic TCS use and withdrawal on online platforms, TSA/TSW remains a contentious topic within the dermatologic community. Therefore, emphasis should be given on educational training, patient-clinician symposiums and appropriate presentations on management of TSA/TSW are essential to ensure effective care. Well-informed providers will not only enhance patient trust and treatment adherence but also help mitigate steroid phobia and prevent therapeutic failures associated with improper TCS use. Enhanced provider education will lead to greater recognition of these conditions, drive the establishment of standardized diagnostic criteria, and ultimately improve clinical outcomes for patients.
Several corticosteroid-sparing strategies have been explored, however, there is an urgent need for continued research to develop new therapeutic regimens that effectively manage TSA/TSW with minimal side effects. Besides, use of natural active compounds based therapeutic strategies for steroid-dependent or steroid withdrawal associated with severe eczema along with early introduction of TCM in young adults with eczema is well discussed previously (14). A case series summary shows remarkable improvement of skin lesion, reduced itch sensation, and sleep improvement following initiation of TCM therapy protocol. Furthermore, patients undergoing substantial topical steroids, light therapy, and biological treatment showed no improvement in quality of life, however introduction of TCM therapy led to significantly improved skin integrity within 3 months. This evidence suggests TCM therapy has great potential in managing TSA/TSW. This should be further studies in controlled clinical studies of patients with severe refractory eczema and TSW to better achieve expected milestones. Additionally, advancing our understanding and treatment of TSA/TSW is crucial to restoring patient trust and ensuring compassionate care for those afflicted by these conditions.
Funding Statement
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the The Lie-Artati Family Fund (ORA LOG NO.:012756-101) and Study of Integrative Medicine (ORA LOG NO.: 012874-101) to X-ML at New York Medical College. AM and XML received salary support from the two funds.
Author contributions
AM: Conceptualization, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. AS: Writing – original draft, Writing – review & editing. MS: Writing – original draft, Writing – review & editing. MK: Formal analysis, Supervision, Writing – original draft, Writing – review & editing. RT: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. JG: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. BS: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. X-ML: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Conflict of interest
X-ML receives grants to her institution from the National Institutes of Health, New York State Department of Health/ Empire Clinical Research Investigator Program (ECRIP), the Lie- Artati Family Fund, Study of Integrative Medicine, and Fidelity Charitable DAS Fund; received consultancy fees from FARE, Johnson & Johnson Pharmaceutical Research & Development, L.L.C, Bayer Global Health LLC; received royalties from UpToDate; shares US patent US7820175B2, US10500169B, US10406191B2, US10028985B2, US11351157B2; takes compensation from her practice at the Center for Integrative Health and Acupuncture PC; US Times Technology Inc. is managed by her related party; is a member of General Nutraceutical Technology LLC. AM received partial salary from the Lei-Artati Family fund at New York Medical College.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Butala S, Paller AS. Optimizing topical management of atopic dermatitis. Ann Allergy Asthma Immunol. (2022) 128(5):488–504. 10.1016/j.anai.2022.03.004 [DOI] [PubMed] [Google Scholar]
- 2.Sheary B. Topical corticosteroid addiction and withdrawal - an overview for GPs. Aust Fam Physician. (2016) 45(6):386–8. [PubMed] [Google Scholar]
- 3.Barta K, Fonacier LS, Hart M, Lio P, Tullos K, Sheary B, et al. Corticosteroid exposure and cumulative effects in patients with eczema: results from a patient survey. Ann Allergy Asthma Immunol. (2023) 130(1):93–9.e10. 10.1016/j.anai.2022.09.031 [DOI] [PubMed] [Google Scholar]
- 4.Hajar T, Leshem YA, Hanifin JM, Nedorost ST, Lio PA, Paller AS, et al. A systematic review of topical corticosteroid withdrawal (“steroid addiction”) in patients with atopic dermatitis and other dermatoses. J Am Acad Dermatol. (2015) 72(3):541–9.e2. 10.1016/j.jaad.2014.11.024 [DOI] [PubMed] [Google Scholar]
- 5.Kligman AM, Frosch PJ. Steroid addiction. Int J Dermatol. (1979) 18(1):23–31. 10.1111/j.1365-4362.1979.tb01905.x [DOI] [PubMed] [Google Scholar]
- 6.Lio P, Chandan N. Topical steroid withdrawal in atopic dermatitis. Pract Dermatol. (2019). Available at: https://practicaldermatology.com/topics/atopic-dermatitis/topical-steroid-withdrawal-in-atopic-dermatitis/23079/ (Accessed December 15, 2024). [Google Scholar]
- 7.Smith MC, Nedorost S, Tackett B. Facing up to withdrawal from topical steroids. Nursing. (2007) 37(9):60–1. 10.1097/01.NURSE.0000287732.08659.83 [DOI] [PubMed] [Google Scholar]
- 8.TSW Assist. Topical steroid withdrawal - everything we know so far: TSW assist 2023. Available at: https://tswassist.com/topical-steroid-withdrawal/ (Accessed December 14, 2024).
- 9.Fukaya M, Sato K, Sato M, Kimata H, Fujisawa S, Dozono H, et al. Topical steroid addiction in atopic dermatitis. Drug Healthc Patient Saf. (2014) 6:131–8. 10.2147/DHPS.S69201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan SY, Chandran NS, Choi EC. Steroid phobia: is there a basis? A review of topical steroid safety, addiction and withdrawal. Clin Drug Investig. (2021) 41(10):835–42. 10.1007/s40261-021-01072-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapaport MJ, Rapaport V. The red skin syndromes: corticosteroid addiction and withdrawal. Expert Rev Dermatol. (2006) 1(4):547–61. 10.1586/17469872.1.4.547 [DOI] [Google Scholar]
- 12.Juhász MLW, Curley RA, Rasmussen A, Malakouti M, Silverberg N, Jacob SE. Systematic review of the topical steroid addiction and topical steroid withdrawal phenomenon in children diagnosed with atopic dermatitis and treated with topical corticosteroids. J Dermatol Nurses Assoc. (2017) 9(5):233–40. 10.1097/JDN.0000000000000331 [DOI] [Google Scholar]
- 13.Hwang J, Lio PA. Topical corticosteroid withdrawal (“steroid addiction”): an update of a systematic review. J Dermatolog Treat. (2022) 33(3):1293–8. 10.1080/09546634.2021.1882659 [DOI] [PubMed] [Google Scholar]
- 14.Li X-M. Treating Eczema With Traditional Chinese Medicine, 1st ed. New Jersey, Singapore: World Scientific Publishing; (2022). [Google Scholar]
- 15.Tatu AL. Topical steroid induced facial rosaceiform dermatitis. Acta Endocrinol. (2016) 12(2):232–3. 10.4183/aeb.2016.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B, Pan F, Zhu K. Bilateral Morganella Morganii keratitis in a patient with facial topical corticosteroid-induced rosacea-like dermatitis: a case report. BMC Ophthalmol. (2017) 17(1):106. 10.1186/s12886-017-0504-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician. (2009) 79(2):135–40. [PubMed] [Google Scholar]
- 18.Ghosh A, Sengupta S, Coondoo A, Jana AK. Topical corticosteroid addiction and phobia. Indian J Dermatol. (2014) 59(5):465–8. 10.4103/0019-5154.139876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spergel JM, Leung DYM. Topical steroid withdrawal syndrome: should we worry? Ann Allergy Asthma Immunol. (2023) 130(1):8. 10.1016/j.anai.2022.09.005 [DOI] [PubMed] [Google Scholar]
- 20.Vergara J. Topical steroid ladder potency strength chart. Available at: https://tswassist.com/topical-steroid-ladder-potency-strength-chart/ (Accessed December 14, 2024).
- 21.Sheary B. Steroid withdrawal effects following long-term topical corticosteroid use. Dermatitis. (2018) 29(4):213–8. 10.1097/DER.0000000000000387 [DOI] [PubMed] [Google Scholar]
- 22.Bhat YJ, Bashir S. Topical steroid damaged face in females with skin of colour. In: Sarkar R, Sinha S, editors. Skin Diseases in Females. Singapore: Springer Nature Singapore; (2022). p. 121–35. [Google Scholar]
- 23.Cirillo N, Prime SS. Keratinocytes synthesize and activate cortisol. J Cell Biochem. (2011) 112(6):1499–505. 10.1002/jcb.23081 [DOI] [PubMed] [Google Scholar]
- 24.Hannen RF, Michael AE, Jaulim A, Bhogal R, Burrin JM, Philpott MP. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem Biophys Res Commun. (2011) 404(1):62–7. 10.1016/j.bbrc.2010.11.059 [DOI] [PubMed] [Google Scholar]
- 25.Schoepe S, Schacke H, May E, Asadullah K. Glucocorticoid therapy-induced skin atrophy. Exp Dermatol. (2006) 15(6):406–20. 10.1111/j.0906-6705.2006.00435.x [DOI] [PubMed] [Google Scholar]
- 26.Ignarro LJ. Nitric oxide. In: Reference Module in Biomedical Sciences. Amsterdam: Elsevier; (2014). 10.1016/B978-0-12-801238-3.00245-2 [DOI] [Google Scholar]
- 27.Chen AY, Zirwas MJ. Steroid-induced rosacealike dermatitis: case report and review of the literature. Cutis. (2009) 83(4):198–204. [PubMed] [Google Scholar]
- 28.Akdeniz N, Aktaş A, Erdem T, Akyüz M, Özdemir Ş. Nitric oxide levels in atopic dermatitis. Pain Clin. (2004) 16(4):401–5. 10.1163/1568569042664521 [DOI] [Google Scholar]
- 29.Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. (2007) 15(6):252–9. 10.1007/s10787-007-0013-x [DOI] [PubMed] [Google Scholar]
- 30.Man MQ, Wakefield JS, Mauro TM, Elias PM. Regulatory role of nitric oxide in cutaneous inflammation. Inflammation. (2022) 45(3):949–64. 10.1007/s10753-021-01615-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rapaport MJ, Rapaport VH. Serum nitric oxide levels in “red” patients: separating corticosteroid-addicted patients from those with chronic eczema. Arch Dermatol. (2004) 140(8):1013–4. 10.1001/archderm.140.8.1013 [DOI] [PubMed] [Google Scholar]
- 32.Wallen-Russell C, Gijsberts-Veens A, Wallen-Russell S. Could modifying the skin microbiome, diet, and lifestyle help with the adverse skin effects after stopping long-term topical steroid use? Allergies. (2021) 2(1):1–15. 10.3390/allergies2010001 [DOI] [Google Scholar]
- 33.Lin T-K, Wei K-J, Wu C-H, Lai F-J, Lan C-CE, Chang C-H, et al. Barrier abnormalities and keratinocyte-derived cytokine cascade after cessation of long-term topical glucocorticosteroid on hairless mouse skin. Dermatologica Sinica. (2015) 33(2):103–11. 10.1016/j.dsi.2015.05.002 [DOI] [Google Scholar]
- 34.Andrus MR, Roth MT. Health literacy: a review. Pharmacotherapy. (2012) 22(3):282–302. 10.1592/phco.22.5.282.33191 [DOI] [PubMed] [Google Scholar]
- 35.Lahiri K, Coondoo A. Topical steroid damaged/dependent face (TSDF): an entity of cutaneous pharmacodependence. Indian J Dermatol. (2016) 61(3):265–72. 10.4103/0019-5154.182417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balak DM, Hajdarbegovic E. Drug-induced psoriasis: clinical perspectives. Psoriasis. (2017) 7:87–94. 10.2147/PTT.S126727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mrowietz U, Domm S. Systemic steroids in the treatment of psoriasis: what is fact, what is fiction? J Eur Acad Dermatol Venereol. (2013) 27(8):1022–5. 10.1111/j.1468-3083.2012.04656.x [DOI] [PubMed] [Google Scholar]
- 38.Justiniano H, Berlingeri-Ramos AC, Sánchez JL. Pattern analysis of drug-induced skin diseases. Am J Dermatopathol. (2008) 30(4):352–69. 10.1097/DAD.0b013e3181722ef4 [DOI] [PubMed] [Google Scholar]
- 39.Tan S, Phan P, Law JY, Choi E, Chandran NS. Qualitative analysis of topical corticosteroid concerns, topical steroid addiction and withdrawal in dermatological patients. BMJ Open. (2022) 12(3):e060867. 10.1136/bmjopen-2022-060867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung DYM. Targeting the skin in atopic dermatitis. Ann Allergy Asthma Immunol. (2022) 128(5):481–2. 10.1016/j.anai.2022.01.044 [DOI] [PubMed] [Google Scholar]
- 41.Arnold KA, Treister AD, Lio PA. Dupilumab in the management of topical corticosteroid withdrawal in atopic dermatitis: a retrospective case series. JAAD Case Rep. (2018) 4(9):860–2. 10.1016/j.jdcr.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ou Z, Chen C, Chen A, Yang Y, Zhou W. Adverse events of dupilumab in adults with moderate-to-severe atopic dermatitis: a meta-analysis. Int Immunopharmacol. (2018) 54:303–10. 10.1016/j.intimp.2017.11.031 [DOI] [PubMed] [Google Scholar]
- 43.Hasan I, Parsons L, Duran S, Zinn Z. Dupilumab therapy for atopic dermatitis is associated with increased risk of cutaneous T cell lymphoma: a retrospective cohort study. J Am Acad Dermatol. (2024) 91(2):255–8. 10.1016/j.jaad.2024.03.039 [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Wang ZZ, Geliebter J, Tiwari R, Li XM. Traditional Chinese medicine for food allergy and eczema. Ann Allergy Asthma Immunol. (2021) 126(6):639–54. 10.1016/j.anai.2020.12.002 [DOI] [PubMed] [Google Scholar]
- 45.Uzun S, Wang Z, McKnight TA, Ehrlich P, Thanik E, Nowak-Wegrzyn A, et al. Improvement of skin lesions in corticosteroid withdrawal-associated severe eczema by multicomponent traditional Chinese medicine therapy. Allergy Asthma Clin Immunol. (2021) 17(1):68. 10.1186/s13223-021-00555-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maskey AR, Kopulos D, Kwan M, Reyes N, Figueroa C, Mo X, et al. Berberine inhibits the inflammatory response induced by Staphylococcus aureus isolated from atopic eczema patients via the TNF-α/inflammation/RAGE pathways. Cells. (2024) 13(19):1639. 10.3390/cells13191639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kwan M, Maskey A, Musa I, Yang N, Li X-M. Investigation of berberine’s potential in attenuating Staphylococcus aureus-induced inflammatory responses. J Allergy Clin Immunol. (2024) 153(2):AB70. 10.1016/j.jaci.2023.11.240 [DOI] [Google Scholar]
- 48.Reyes N, Figueroa C, Kwan M, Tiwari R, Geliebter J, Li X-M. Effects of multi-component TCM therapy on skin microbiome of infants with severe eczema associated with topical steroid withdrawal. J Allergy Clin Immunol. (2024) 153(2):AB66. 10.1016/j.jaci.2023.11.228 [DOI] [Google Scholar]
- 49.Yu C, Zhang P, Lv ZT, Li JJ, Li HP, Wu CH, et al. Efficacy of acupuncture in itch: a systematic review and meta-analysis of clinical randomized controlled trials. Evid Based Complement Alternat Med. (2015) 2015:208690. 10.1155/2015/208690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Witte M, Krause L, Zillikens D, Shimanovich I. Black tea dressings - a rapidly effective treatment for facial dermatitis. J Dermatolog Treat. (2019) 30(8):785–9. 10.1080/09546634.2019.1573306 [DOI] [PubMed] [Google Scholar]
- 51.van den Bogaard EH, Bergboer JG, Vonk-Bergers M, van Vlijmen-Willems IM, Hato SV, van der Valk PG, et al. Coal tar induces AHR-dependent skin barrier repair in atopic dermatitis. J Clin Invest. (2013) 123(2):917–27. 10.1172/JCI65642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaslavsky D, Tulenkova Е, Monakhov K, Kholodilova N, Kondratieva YS, Tamrazova O, et al. Eczema: tactics of choice external therapy. Vestn Dermatol Venerol. (2018) 94(3):56–66. 10.25208/0042-4609-2018-94-3-56-66 [DOI] [Google Scholar]
- 53.Ng JP, Liew HM, Ang SB. Use of emollients in atopic dermatitis. J Eur Acad Dermatol Venereol. (2015) 29(5):854–7. 10.1111/jdv.12864 [DOI] [PubMed] [Google Scholar]