Abstract
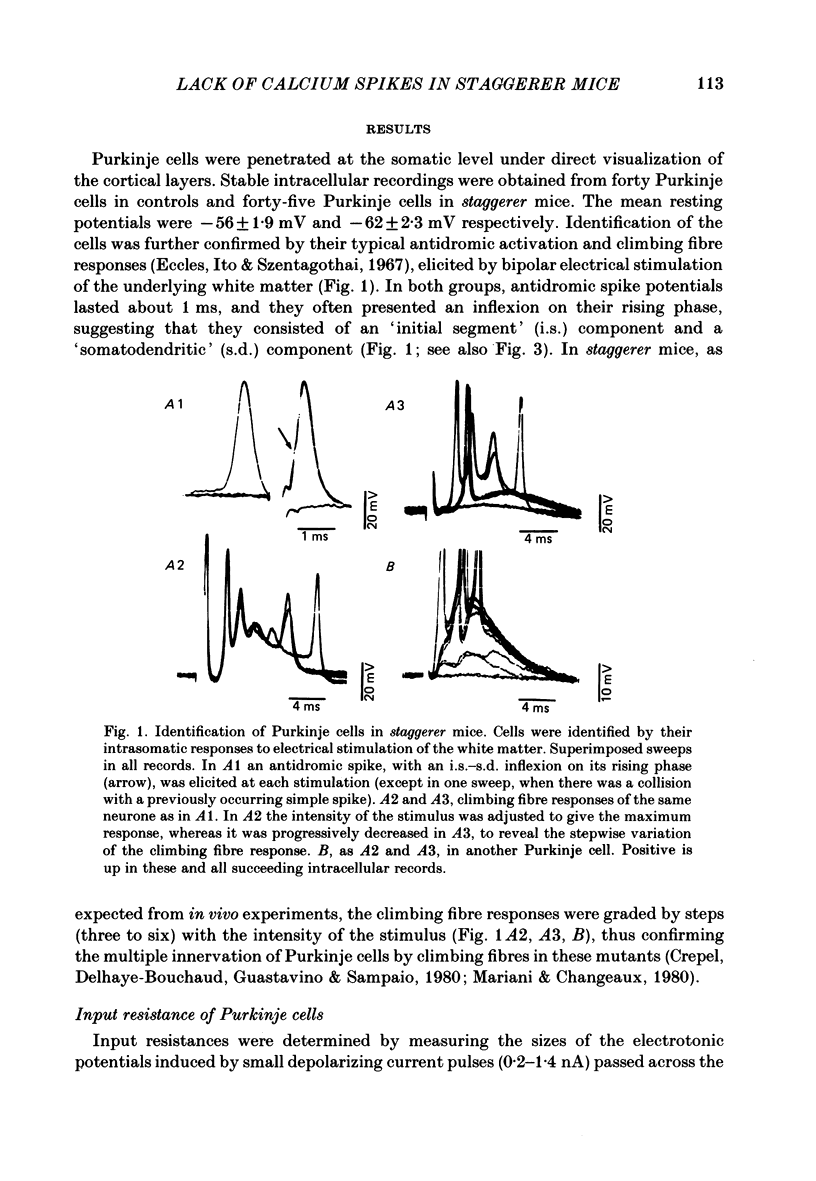
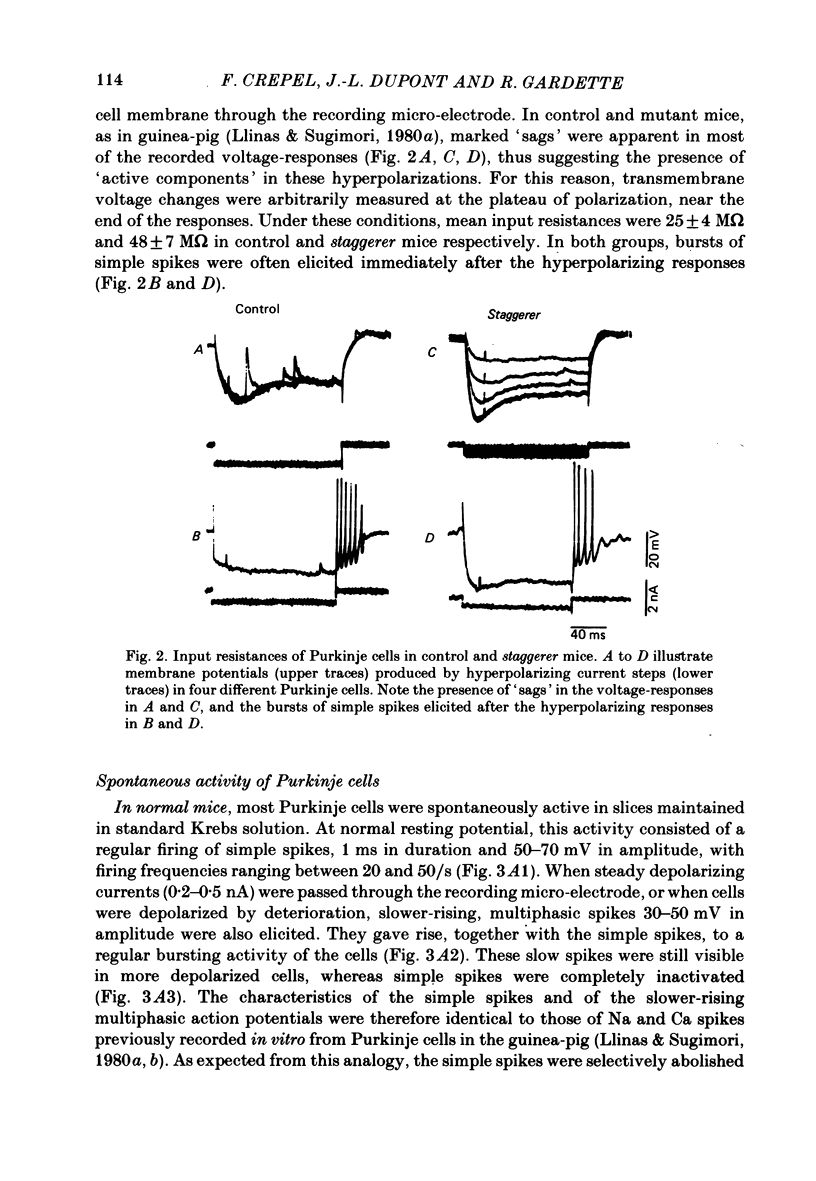
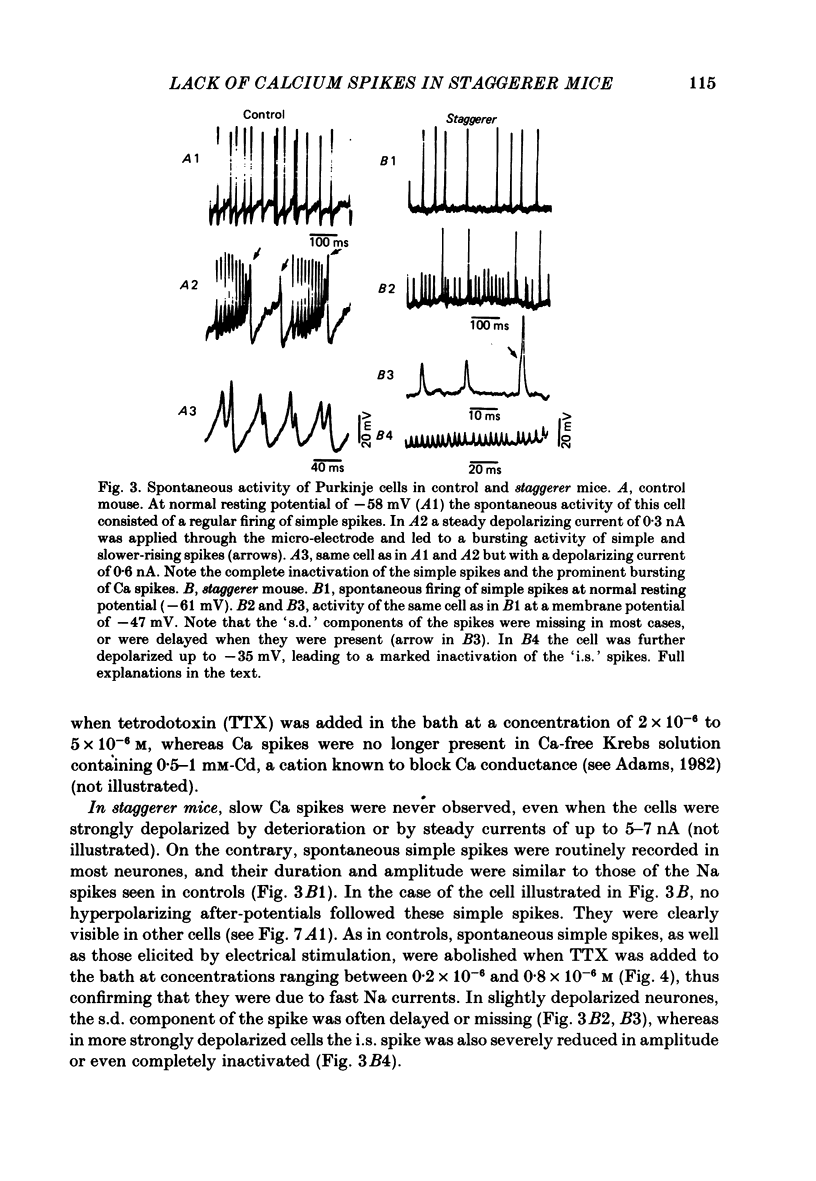
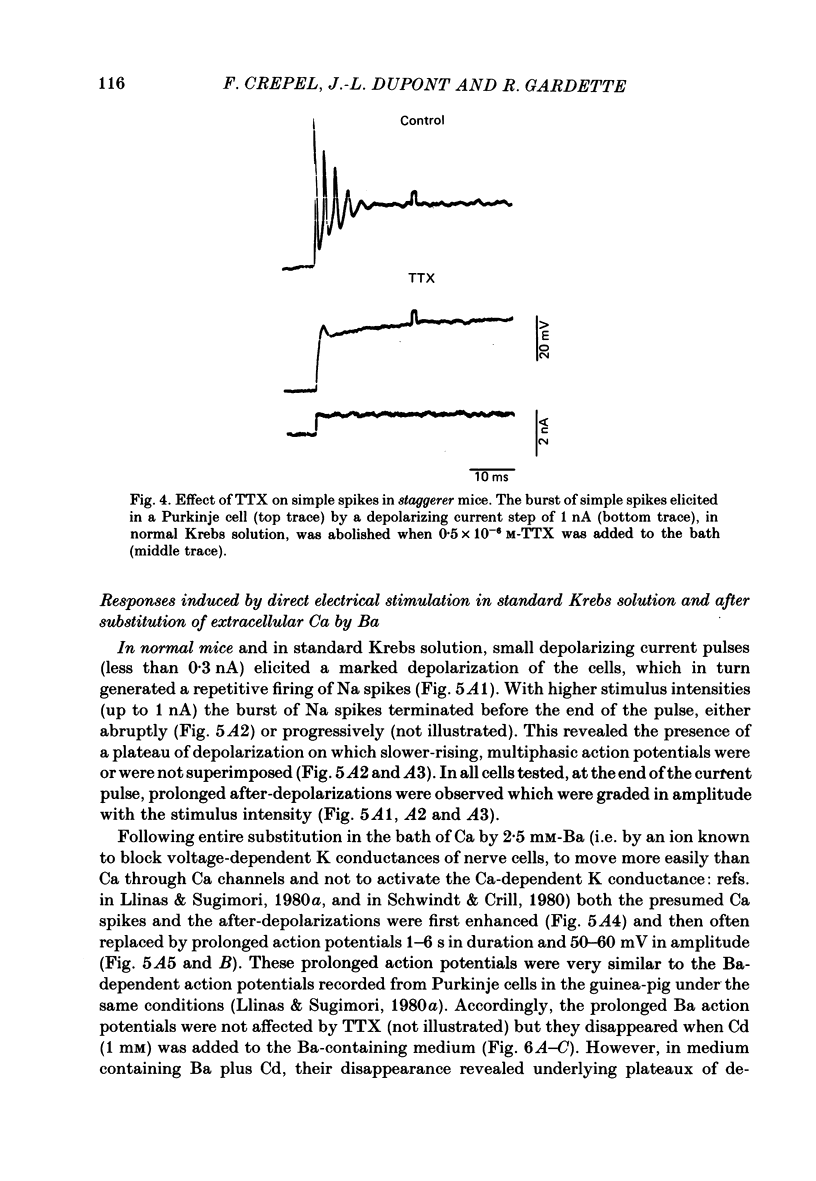
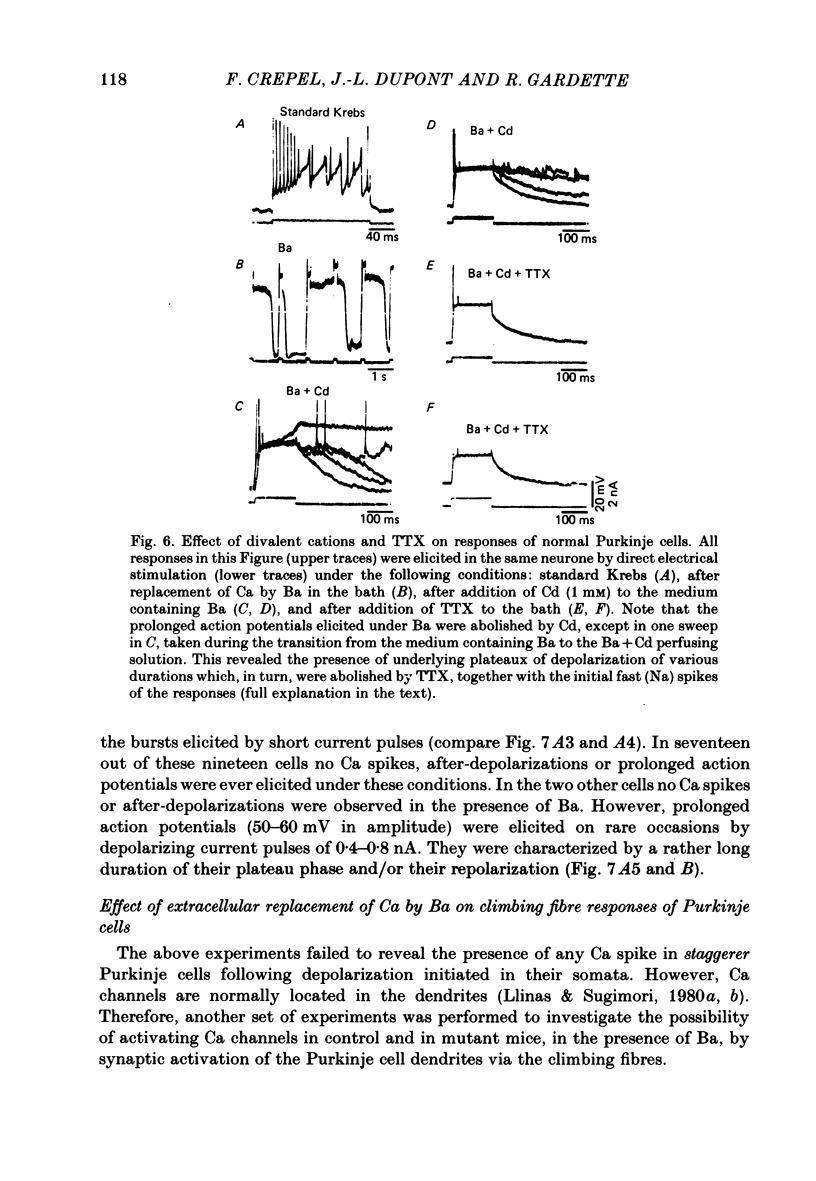
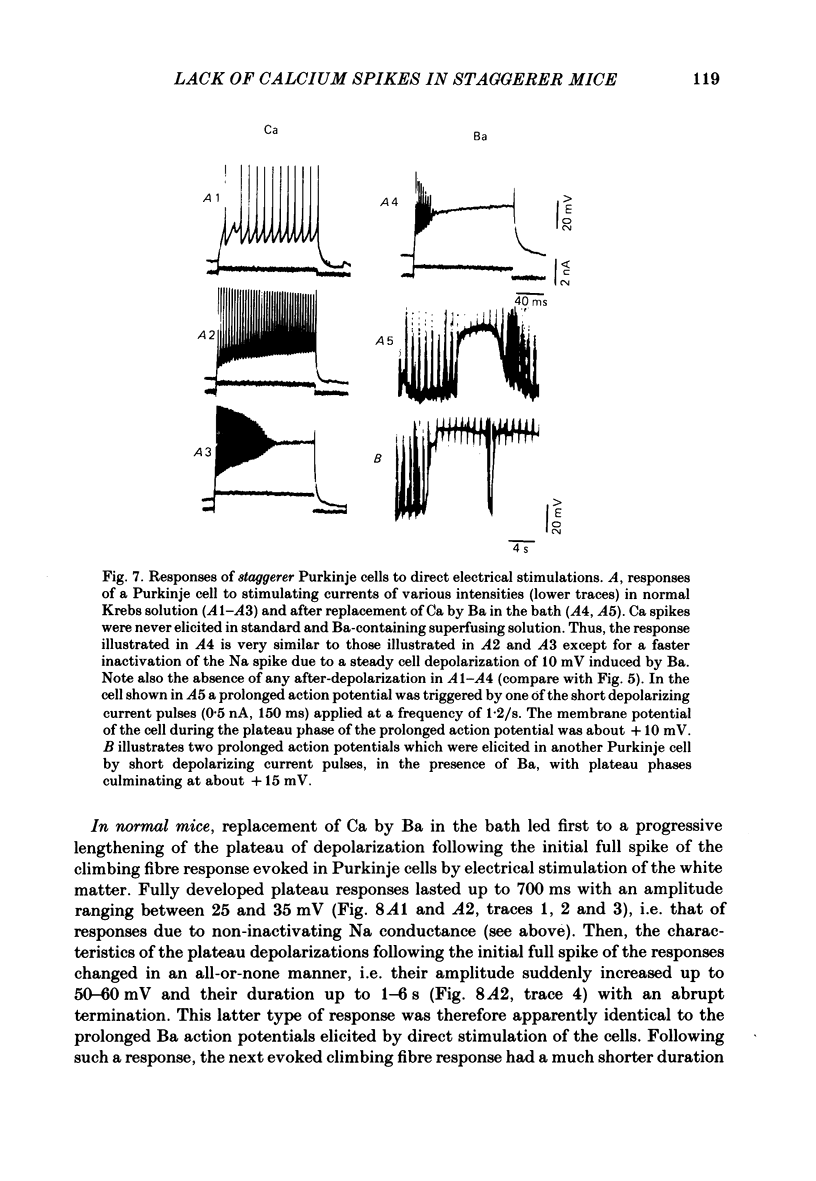
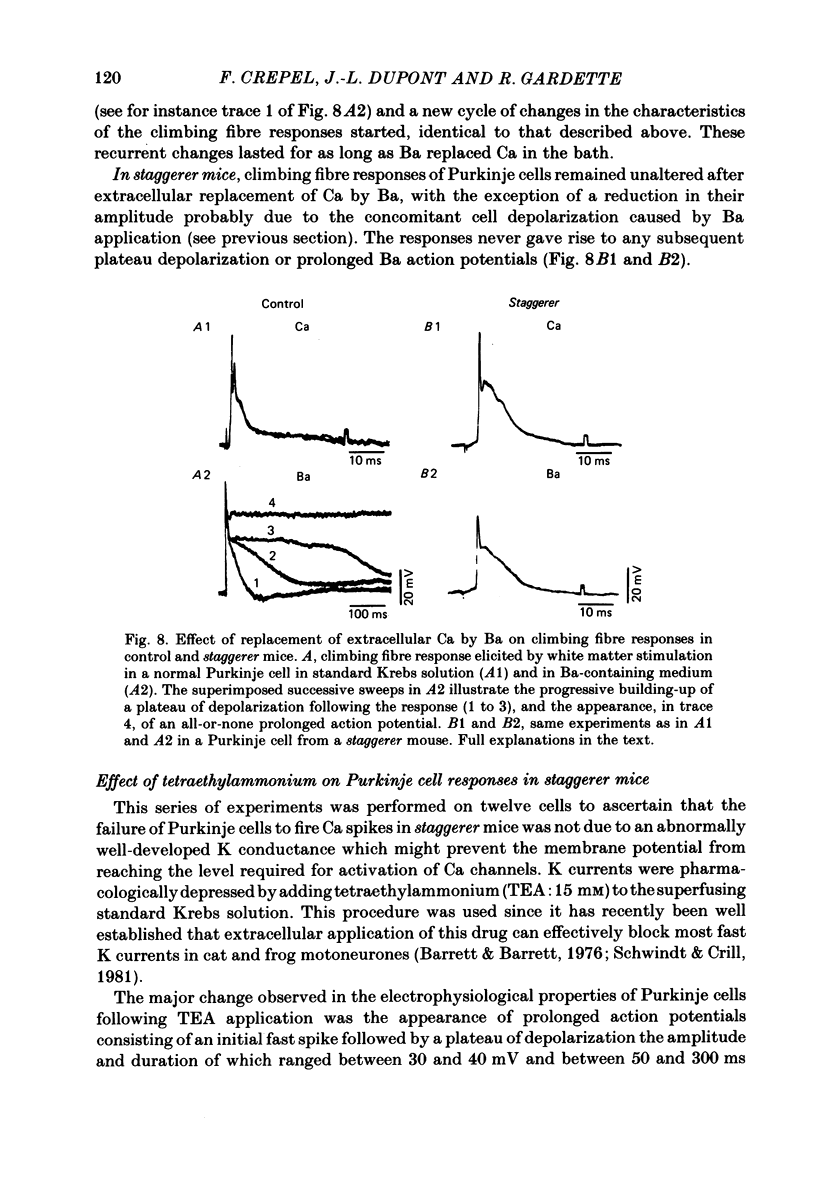
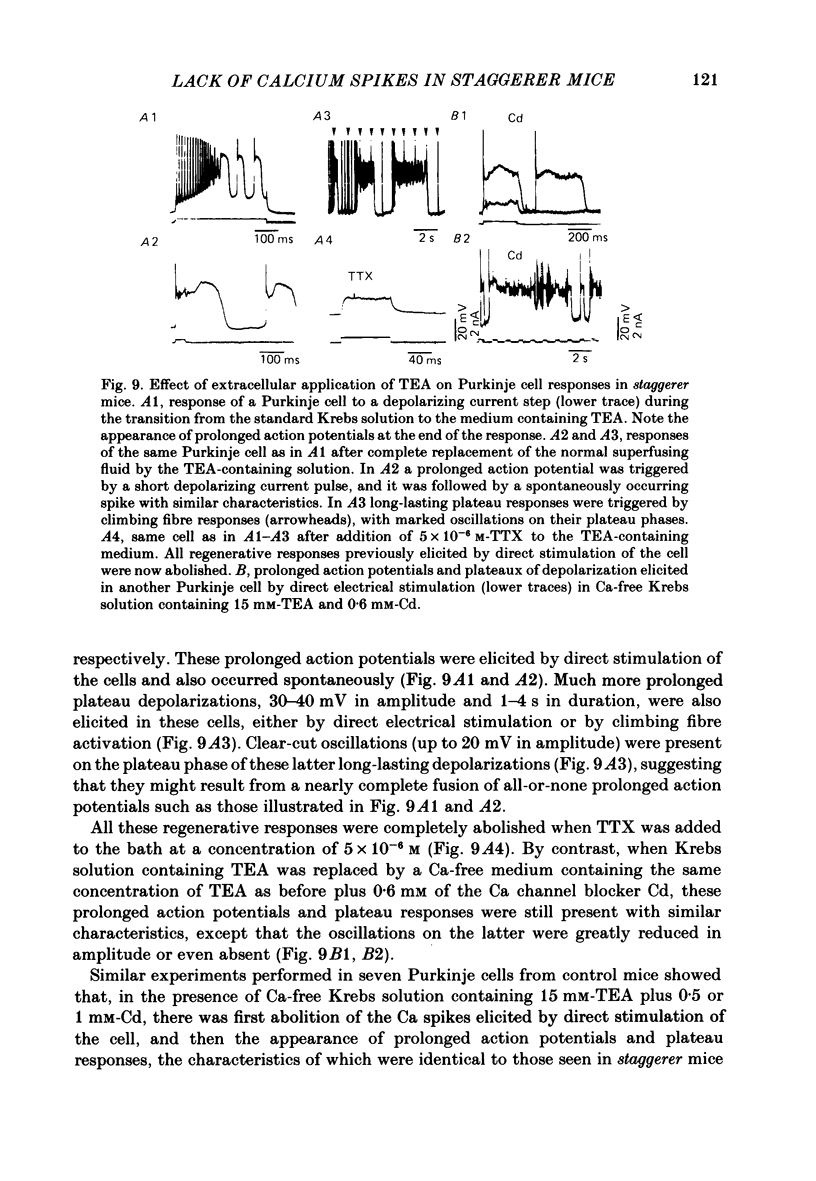
The bioelectrical properties of Purkinje cells (intrasomatic recordings) were studied in sagittal cerebellar slices of both adult staggerer and control mice. Mean input resistances of Purkinje cells were 25 +/- 4 M omega and 48 +/- 7 M omega in normal and staggerer mice respectively. In both groups, time-dependent inward rectifications were apparent in the hyperpolarizing voltage-responses. In normal mice, tetrodotoxin (TTX)-sensitive simple spikes and slower-rising multiphasic spikes, abolished when Ca was replaced by Cd in the bath, spontaneously occurred in Purkinje cells. These Na- and Ca-dependent spikes were also elicited by depolarizing current pulses. In the mutant, Ca spikes were never observed, even in strongly depolarized cells. On the contrary, TTX-sensitive simple spikes occurred spontaneously or were elicited by depolarizing current pulses. When Ca was replaced by Ba in the bath, the Ca spikes evoked in normal Purkinje cells by direct stimulation were first enhanced and then replaced by prolonged action potentials (1-6 s in duration) which were TTX-resistant and Cd-sensitive. These (Ba) action potentials were also triggered by climbing fibre activation of the cells. In staggerer mice, Ca spikes were never elicited by direct stimulation in Ba-containing medium, although in a few cells prolonged action potentials were occasionally elicited by depolarizing current pulses. However, this latter type of response was never evoked by climbing fibre activation of Purkinje cells. In the mutant, extracellular application of tetraethylammonium (TEA) generated prolonged action potentials, the plateaux of depolarization of which were less positive than those elicited by Ba in control mice. These plateaux were abolished by TTX and left unaffected by the substitution of Ca by Cd in the bath, suggesting that they were due to a non-inactivating Na conductance. On the whole, the present study strongly suggests that voltage-dependent Ca channels are missing in most staggerer Purkinje cells or at least that their characteristics and/or distribution are such that they cannot be activated. Na channels appear unaffected.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett E. F., Barret J. N. Separation of two voltage-sensitive potassium currents, and demonstration of a tetrodotoxin-resistant calcium current in frog motoneurones. J Physiol. 1976 Mar;255(3):737–774. doi: 10.1113/jphysiol.1976.sp011306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Delhaye-Bouchaud N., Guastavino J. M., Sampaio I. Multiple innervation of cerebellar Purkinje cells by climbing fibres in staggerer mutant mouse. Nature. 1980 Jan 31;283(5746):483–484. doi: 10.1038/283483a0. [DOI] [PubMed] [Google Scholar]
- Crepel F., Dhanjal S. S. Cholinergic mechanisms and neurotransmission in the cerebellum of the rat. An in vitro study. Brain Res. 1982 Jul 22;244(1):59–68. doi: 10.1016/0006-8993(82)90904-0. [DOI] [PubMed] [Google Scholar]
- Crepel F., Dhanjal S. S., Garthwaite J. Morphological and electrophysiological characteristics of rat cerebellar slices maintained in vitro. J Physiol. 1981 Jul;316:127–138. doi: 10.1113/jphysiol.1981.sp013777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Dhanjal S. S., Sears T. A. Effect of glutamate, aspartate and related derivatives on cerebellar purkinje cell dendrites in the rat: an in vitro study. J Physiol. 1982 Aug;329:297–317. doi: 10.1113/jphysiol.1982.sp014304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Mariani J. Anatomical, physiological and biochemical studies of the cerebellum from mutant mice. I. Electrophysiological analysis of cerebellar cortical neurons in the staggerer mouse. Brain Res. 1975 Nov 7;98(1):135–147. doi: 10.1016/0006-8993(75)90514-4. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Gerschenfeld H. M., Quandt F. N. Calcium spikes in toad rods. J Physiol. 1980 Jun;303:495–513. doi: 10.1113/jphysiol.1980.sp013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449–472. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan Y. N., Jan L. Y., Dennis M. J. Two mutations of synaptic transmission in Drosophila. Proc R Soc Lond B Biol Sci. 1977 Jul 28;198(1130):87–108. doi: 10.1098/rspb.1977.0087. [DOI] [PubMed] [Google Scholar]
- Kung C., Chang S. Y., Satow Y., Houten J. V., Hansma H. Genetic dissection of behavior in paramecium. Science. 1975 May 30;188(4191):898–904. [PubMed] [Google Scholar]
- Landis D. M., Sidman R. L. Electron microscopic analysis of postnatal histogenesis in the cerebellar cortex of staggerer mutant mice. J Comp Neurol. 1978 Jun 15;179(4):831–863. doi: 10.1002/cne.901790408. [DOI] [PubMed] [Google Scholar]
- Llinás R., Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2520–2523. doi: 10.1073/pnas.73.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Calcium conductances in Purkinje cell dendrites: their role in development and integration. Prog Brain Res. 1979;51:323–334. doi: 10.1016/S0079-6123(08)61312-6. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani J., Changeux J. P. Multiple innervation of Purkinje cells by climbing fibers in the cerebellum of the adult staggerer mutant mouse. J Neurobiol. 1980;11(1):41–50. doi: 10.1002/neu.480110106. [DOI] [PubMed] [Google Scholar]
- Mariani J., Crepel F., Mikoshiba K., Changeux J. P., Sotelo C. Anatomical, physiological and biochemical studies of the cerebellum from Reeler mutant mouse. Philos Trans R Soc Lond B Biol Sci. 1977 Nov 2;281(978):1–28. doi: 10.1098/rstb.1977.0121. [DOI] [PubMed] [Google Scholar]
- Salkoff L., Wyman R. Genetic modification of potassium channels in Drosophila Shaker mutants. Nature. 1981 Sep 17;293(5829):228–230. doi: 10.1038/293228a0. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Slawsky M. Probable calcium spikes in hippocampal neurons. Brain Res. 1977 Oct 21;135(1):157–161. doi: 10.1016/0006-8993(77)91060-5. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Crill W. E. Differential effects of TEA and cations on outward ionic currents of cat motoneurons. J Neurophysiol. 1981 Jul;46(1):1–16. doi: 10.1152/jn.1981.46.1.1. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Crill W. E. Effects of barium on cat spinal motoneurons studied by voltage clamp. J Neurophysiol. 1980 Oct;44(4):827–846. doi: 10.1152/jn.1980.44.4.827. [DOI] [PubMed] [Google Scholar]
- Sotelo C., Changeux J. P. Transsynaptic degeneration 'en cascade' in the cerebellar cortex of staggerer mutant mice. Brain Res. 1974 Mar 8;67(3):519–526. doi: 10.1016/0006-8993(74)90499-5. [DOI] [PubMed] [Google Scholar]
- Trenkner E. Postnatal cerebellar cells of staggerer mutant mice express immature components on their surface. Nature. 1979 Feb 15;277(5697):566–567. doi: 10.1038/277566a0. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A., Basbaum A. I. Intradendritic recordings from hippocampal neurons. Proc Natl Acad Sci U S A. 1979 Feb;76(2):986–990. doi: 10.1073/pnas.76.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. F., Ganetzky B. Genetic alteration of nerve membrane excitability in temperature-sensitive paralytic mutants of Drosophila melanogaster. Nature. 1980 Aug 21;286(5775):814–816. doi: 10.1038/286814a0. [DOI] [PubMed] [Google Scholar]


