Abstract
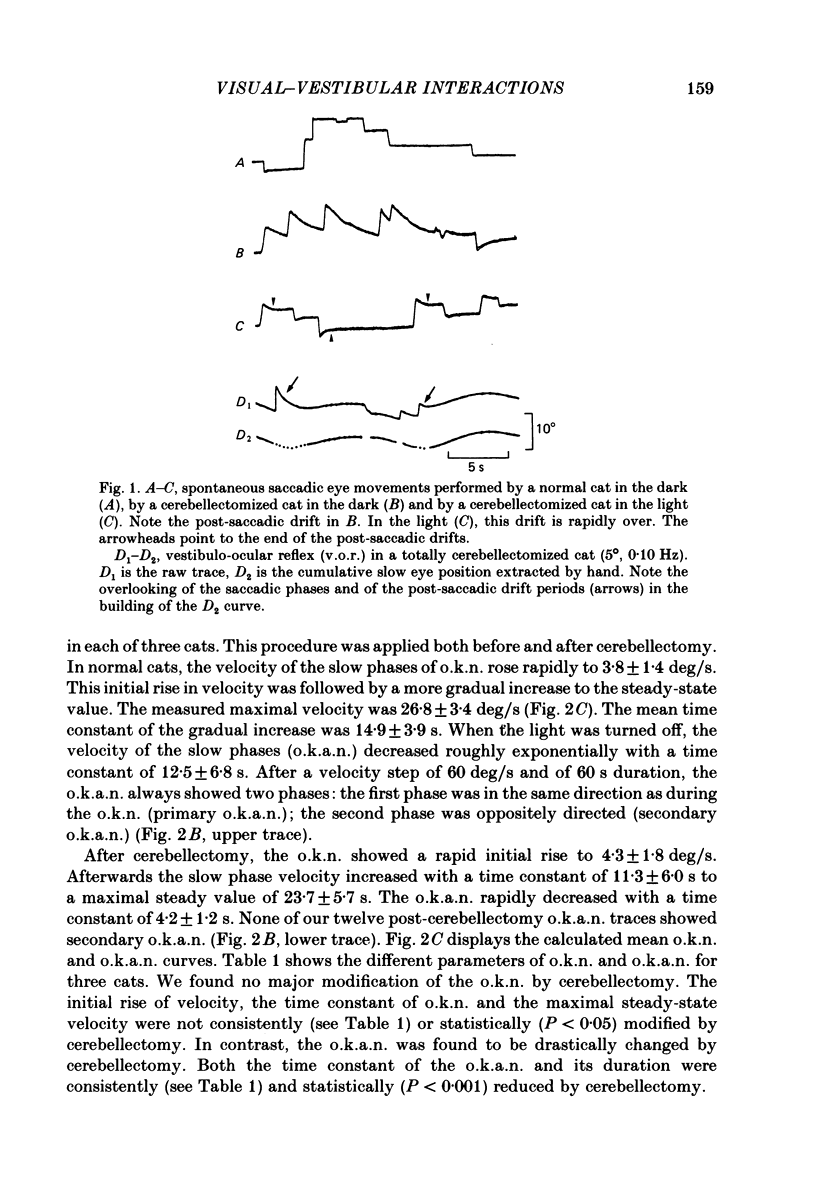
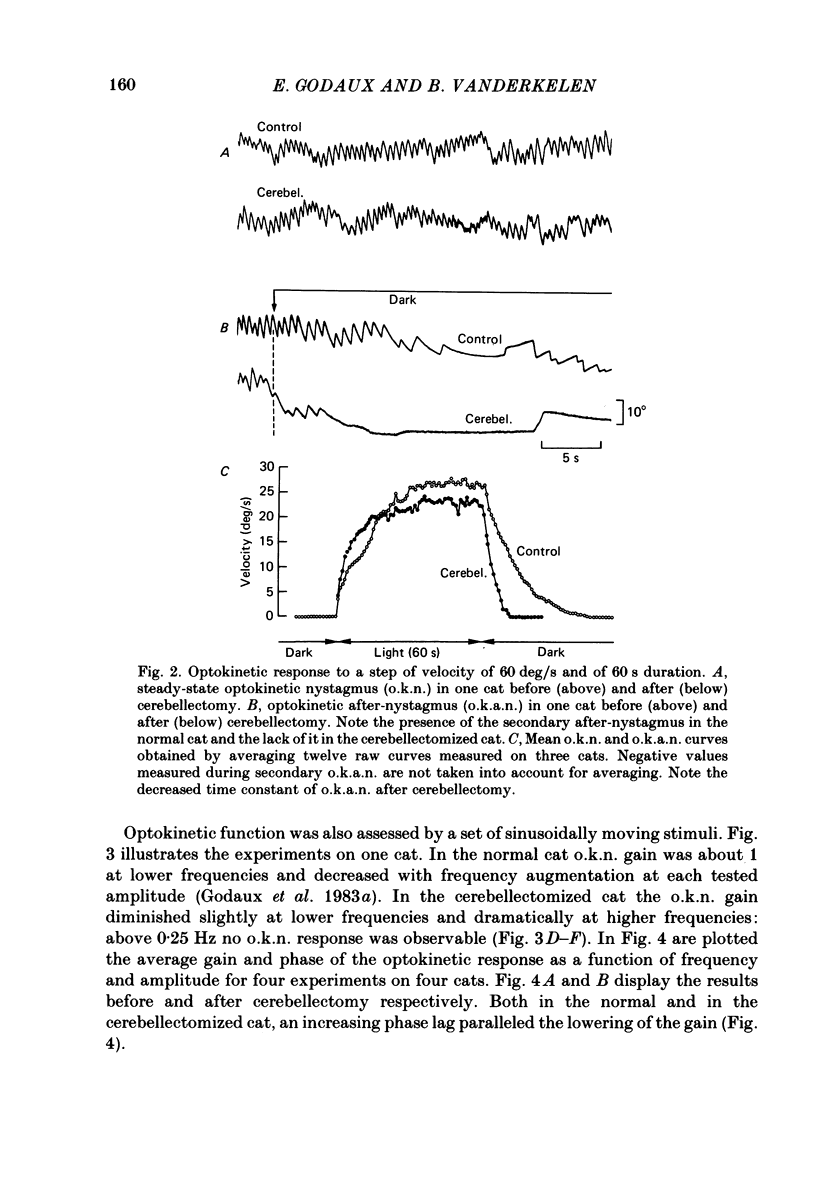
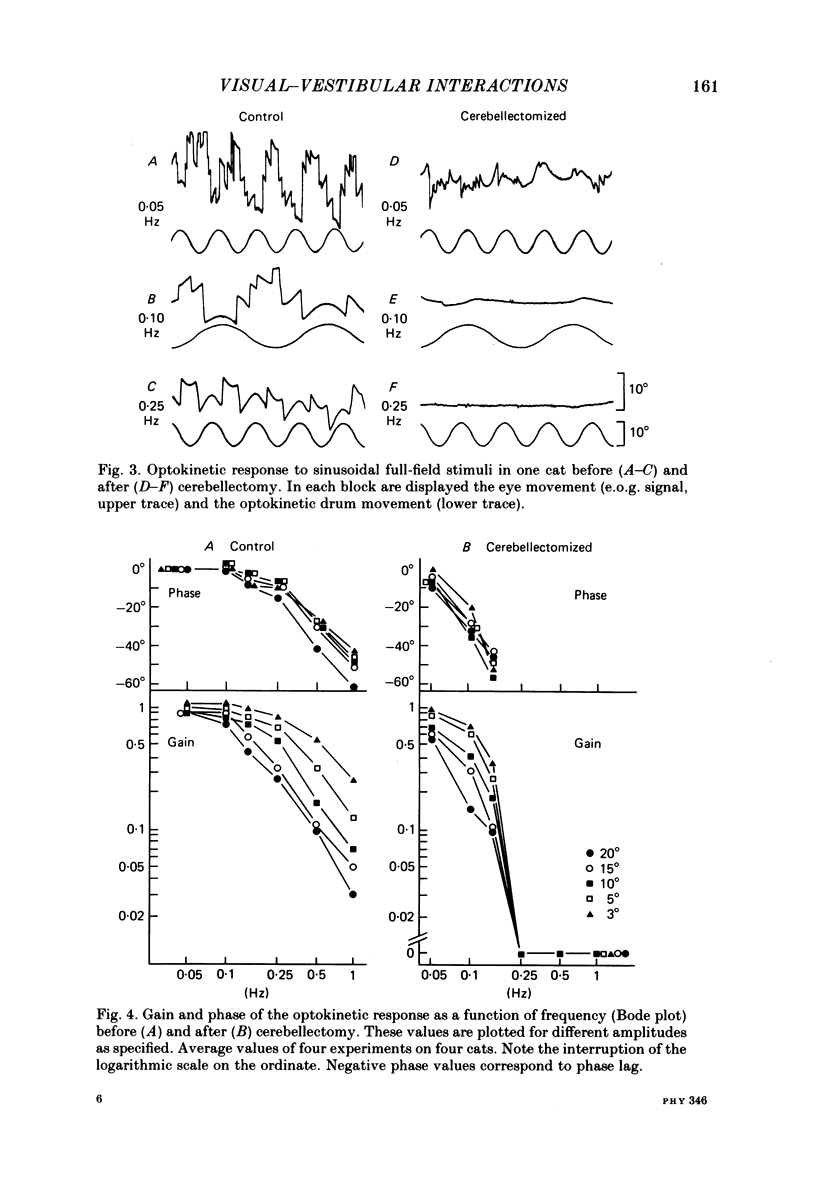
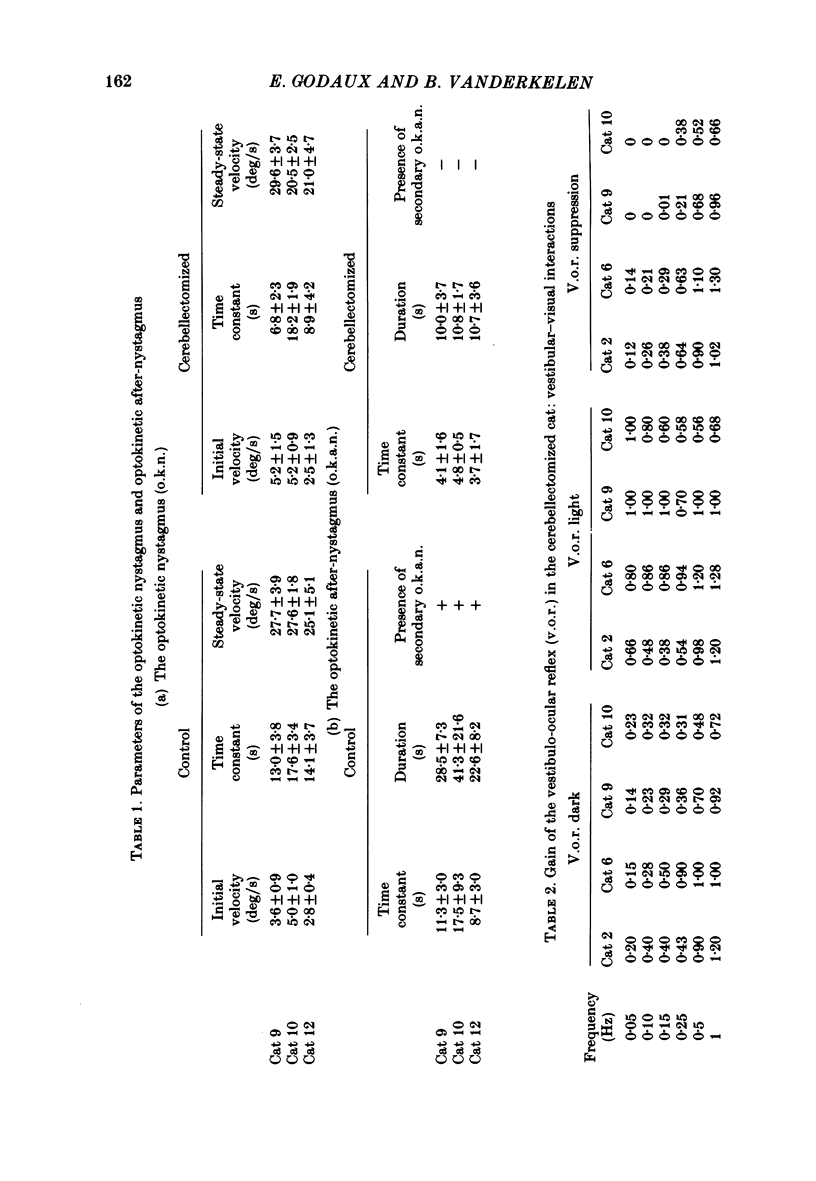
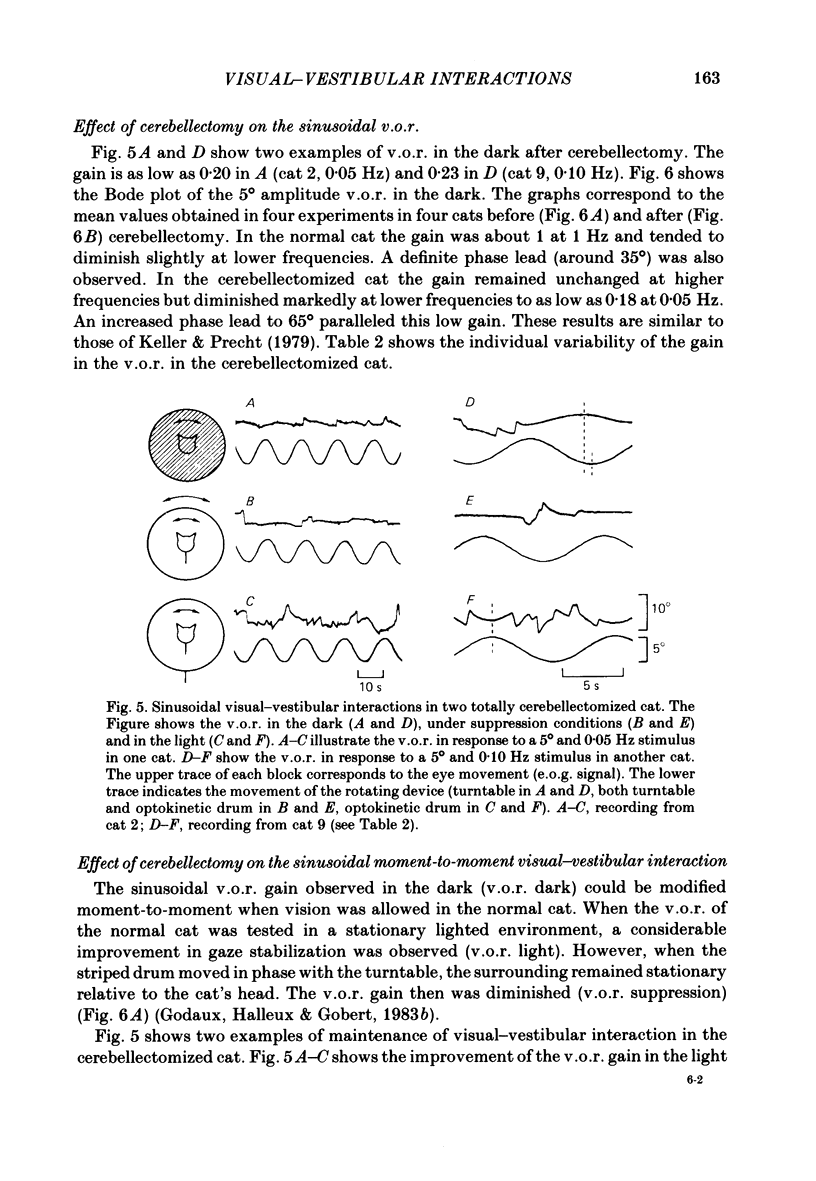
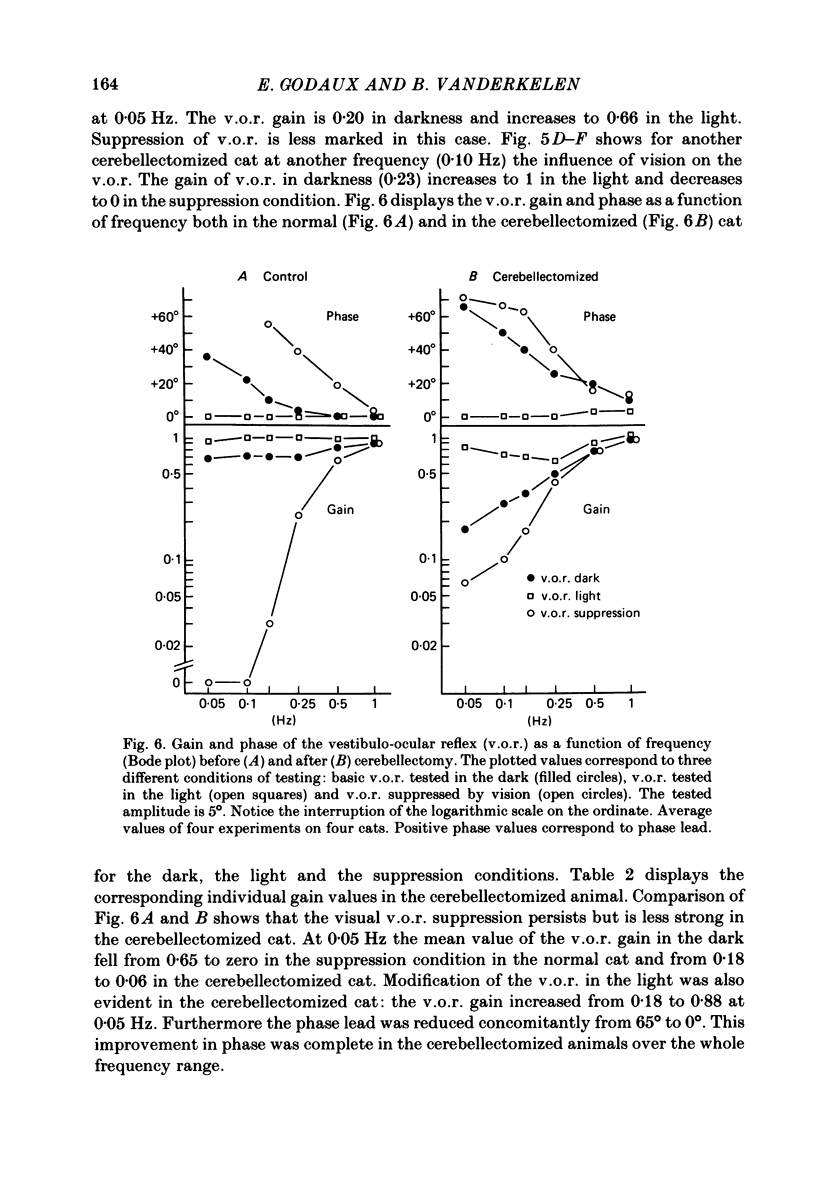
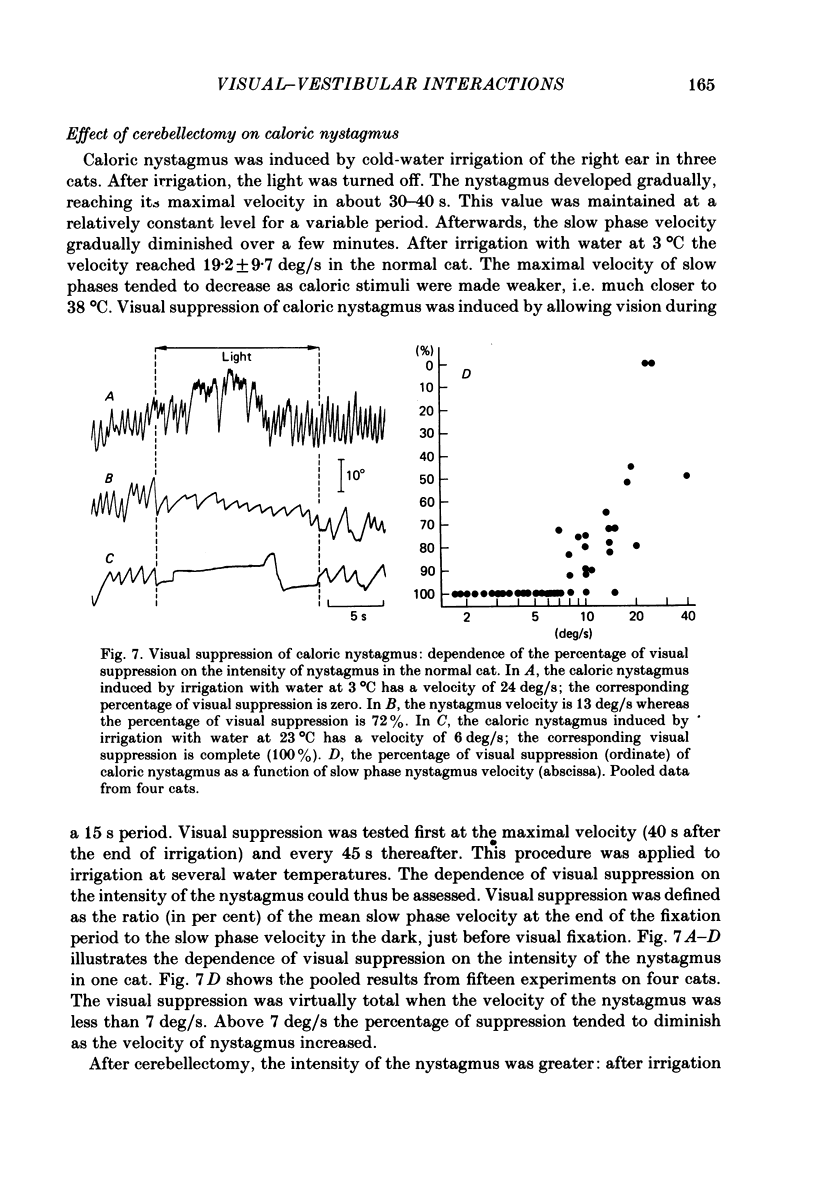
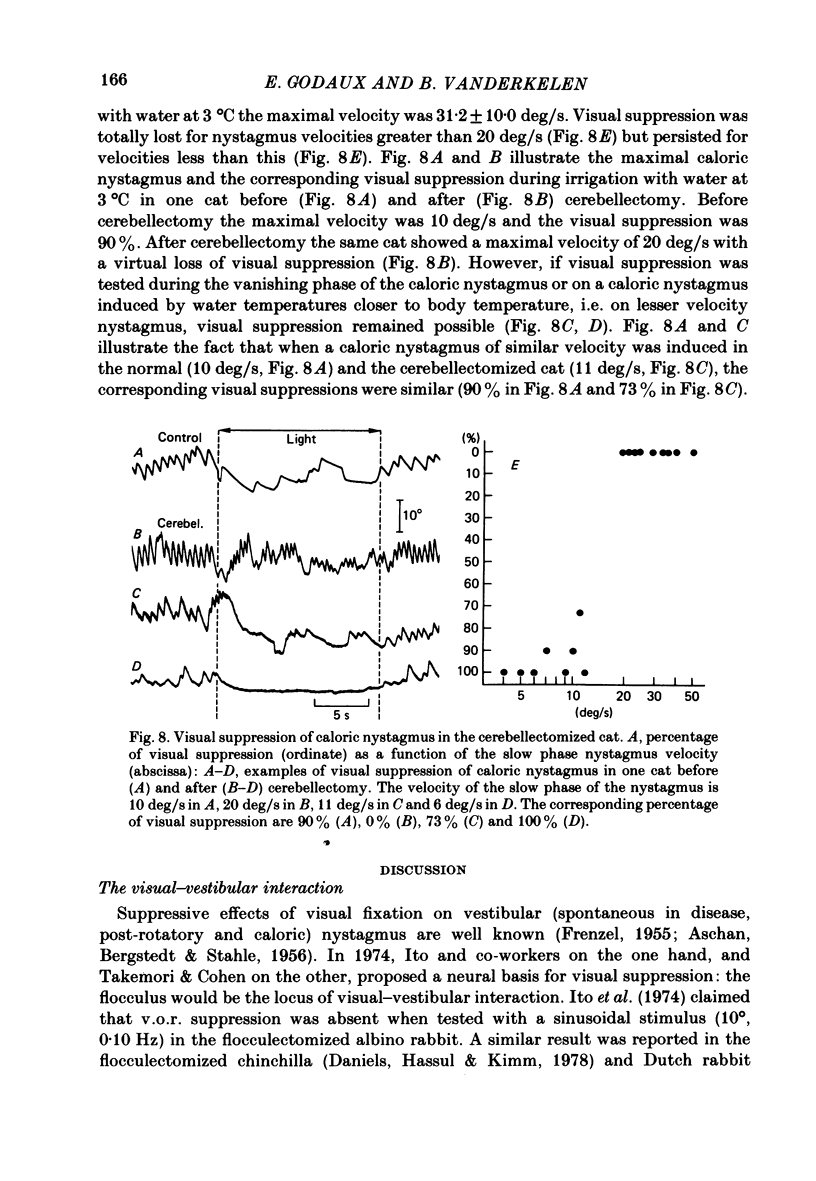
The effects of total ablation of the cerebellum on eye movements were studied in alert adult cats. The normal cat could easily hold a steady eye position after a saccadic movement in the dark. The cerebellectomized animal could not: after a saccade the eye position shifted towards a more central position. Vision reduced this 'post-saccadic drift'. The sinusoidal vestibulo-ocular reflex (v.o.r.) was strongly affected by total cerebellectomy. In darkness the v.o.r. gain remained stable at high frequencies (0.5 and 1 Hz) but decreased markedly at lower frequencies to as low as 0.18 at 0.05 Hz. A phase advance (up to 65 degrees at 0.05 Hz) paralleled this gain depression. Velocity characteristics of optokinetic nystagmus (o.k.n.) and optokinetic after-nystagmus (o.k.a.n.) induced by constant-velocity full-field rotation of 60 deg/s amplitude and 60 s duration were studied. The features of o.k.n. (initial velocity, maximal velocity and time constant) were only mildly affected by cerebellectomy. On cessation of visual stimulation when the animal was plunged into darkness, the velocity of the eyes decreased progressively (o.k.a.n.). The time constant of o.k.a.n. was 12.5 s in the normal cat and 4.2 s in the cerebellectomized cat. Furthermore cerebellectomy abolished the secondary o.k.a.n. Optokinetic response was also tested by a set of sinusoidal (0.05-1 Hz; 3-20 degrees) full-field stimuli. The o.k.n. was not abolished but dramatically decreased, especially at higher frequencies. No response could be detected above 0.15 Hz. Visual suppression of inappropriate vestibulo-ocular reflex was still possible but was mildly impaired after cerebellectomy. Visual suppression could only be detected with stimuli below 0.25 Hz. Visual suppression of caloric nystagmus was studied in the normal cat. A clear dependence of the effectiveness of visual suppression on the velocity of the nystagmus was demonstrated. In the cerebellectomized cat, the visual suppression of caloric nystagmus was lost when tested on nystagmus velocities above 20 deg/s but remained when tested on nystagmus velocities below 20 deg/s. The relationship between cerebellectomy and the loss of visual suppression of caloric nystagmus was found to be at least partially indirect: cerebellectomy increased the velocity of caloric nystagmus, and visual suppression was usually less effective at higher velocities.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASCHAN G., BERGSTEDT M., STAHLE J. Nystagmography; recording of nystagmus in clinical neuro-otological examinations. Acta Otolaryngol Suppl. 1956;129:1–103. [PubMed] [Google Scholar]
- Allum J. H., Graf W., Dichgans J., Schmidt C. L. Visual-vestibular interactions in the vestibular nuclei of the goldfish. Exp Brain Res. 1976 Dec 22;26(5):463–485. doi: 10.1007/BF00238821. [DOI] [PubMed] [Google Scholar]
- Avanzini G., Girotti F., Crenna P., Negri S. Alterations of ocular motility in cerebellar pathology. An electro-oculographic study. Arch Neurol. 1979 May;36(5):274–280. doi: 10.1001/archneur.1979.00500410052007. [DOI] [PubMed] [Google Scholar]
- Baarsma E., Collewijn H. Vestibulo-ocular and optokinetic reactions to rotation and their interaction in the rabbit. J Physiol. 1974 May;238(3):603–625. doi: 10.1113/jphysiol.1974.sp010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloh R. W., Konrad H. R., Honrubia V. Vestibulo-ocular function in patients with cerebellar atrophy. Neurology. 1975 Feb;25(2):160–168. doi: 10.1212/wnl.25.2.160. [DOI] [PubMed] [Google Scholar]
- Batini C., Ito M., Kado R. T., Jastreboff P. J., Miyashita Y. Interaction between the horizontal vestibulo-ocular reflex and optokinetic response in rabbits. Exp Brain Res. 1979 Sep;37(1):1–15. doi: 10.1007/BF01474249. [DOI] [PubMed] [Google Scholar]
- Cohen B., Matsuo V., Raphan T. Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol. 1977 Sep;270(2):321–344. doi: 10.1113/jphysiol.1977.sp011955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H. An analog model of the rabbit's optokinetic system. Brain Res. 1972 Jan 14;36(1):71–88. doi: 10.1016/0006-8993(72)90767-6. [DOI] [PubMed] [Google Scholar]
- Daniels P. D., Hassul M., Kimm J. Dynamic analysis of the vestibulo-ocular reflex in the normal and flocculectomized chinchilla. Exp Neurol. 1978 Jan 1;58(1):32–45. doi: 10.1016/0014-4886(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Dichgans J., Schmidt C. L., Graf W. Visual input improves the speedometer function of the vestibular nuclei in the goldfish. Exp Brain Res. 1973 Oct 26;18(3):319–322. doi: 10.1007/BF00234602. [DOI] [PubMed] [Google Scholar]
- Estanol B., Romero R., Corvera J. Effects of cerebellectomy on eye movements in man. Arch Neurol. 1979 May;36(5):281–284. doi: 10.1001/archneur.1979.00500410059008. [DOI] [PubMed] [Google Scholar]
- Evinger C., Fuchs A. F. Saccadic, smooth pursuit, and optokinetic eye movements of the trained cat. J Physiol. 1978 Dec;285:209–229. doi: 10.1113/jphysiol.1978.sp012568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godaux E., Gobert C., Halleux J. Vestibuloocular reflex, optokinetic response, and their interactions in the alert cat. Exp Neurol. 1983 Apr;80(1):42–54. doi: 10.1016/0014-4886(83)90005-5. [DOI] [PubMed] [Google Scholar]
- Godaux E., Halleux J., Gobert C. Adaptive change of the vestibulo-ocular reflex in the cat: the effects of a long-term frequency-selective procedure. Exp Brain Res. 1983;49(1):28–34. doi: 10.1007/BF00235538. [DOI] [PubMed] [Google Scholar]
- Honrubia V., Koehn W. W., Jenkins H. A., Fenton W. H., Jr Effect of bilateral ablation of the vestibular cerebellum on visual-vestibular interaction. Exp Neurol. 1982 Mar;75(3):616–626. doi: 10.1016/0014-4886(82)90029-2. [DOI] [PubMed] [Google Scholar]
- Ito M., Jastreboff P. J., Miyashita Y. Specific effects of unilateral lesions in the flocculus upon eye movements in albino rabbits. Exp Brain Res. 1982;45(1-2):233–242. doi: 10.1007/BF00235783. [DOI] [PubMed] [Google Scholar]
- Ito M., Shiida T., Yagi N., Yamamoto M. Visual influence on rabbit horizontal vestibulo-ocular reflex presumably effected via the cerebellar flocculus. Brain Res. 1974 Jan 4;65(1):170–174. doi: 10.1016/0006-8993(74)90344-8. [DOI] [PubMed] [Google Scholar]
- Keller E. L. Participation of medial pontine reticular formation in eye movement generation in monkey. J Neurophysiol. 1974 Mar;37(2):316–332. doi: 10.1152/jn.1974.37.2.316. [DOI] [PubMed] [Google Scholar]
- Keller E. L., Precht W. Visual-vestibular responses in vestibular nuclear neurons in the intact and cerebellectomized, alert cat. Neuroscience. 1979;4(11):1599–1613. doi: 10.1016/0306-4522(79)90023-x. [DOI] [PubMed] [Google Scholar]
- Luschei E. S., Fuchs A. F. Activity of brain stem neurons during eye movements of alert monkeys. J Neurophysiol. 1972 Jul;35(4):445–461. doi: 10.1152/jn.1972.35.4.445. [DOI] [PubMed] [Google Scholar]
- Ritchie L. Effects of cerebellar lesions on saccadic eye movements. J Neurophysiol. 1976 Nov;39(6):1246–1256. doi: 10.1152/jn.1976.39.6.1246. [DOI] [PubMed] [Google Scholar]
- Robinson D. A. The effect of cerebellectomy on the cat's bestibulo-ocular integrator. Brain Res. 1974 May 17;71(2-3):195–207. doi: 10.1016/0006-8993(74)90961-5. [DOI] [PubMed] [Google Scholar]
- Skavenski A. A., Robinson D. A. Role of abducens neurons in vestibuloocular reflex. J Neurophysiol. 1973 Jul;36(4):724–738. doi: 10.1152/jn.1973.36.4.724. [DOI] [PubMed] [Google Scholar]
- Takemori S., Cohen B. Loss of visual suppression of vestibular nystagmus after flocculus lesions. Brain Res. 1974 Jun 7;72(2):213–224. doi: 10.1016/0006-8993(74)90860-9. [DOI] [PubMed] [Google Scholar]
- Takemori S., Cohen B. Visual suppression of vestibular nystagmus in rhesus monkeys. Brain Res. 1974 Jun 7;72(2):203–212. doi: 10.1016/0006-8993(74)90859-2. [DOI] [PubMed] [Google Scholar]
- Takemori S. Visual suppression of vestibular nystagmus after cerebellar lesions. Ann Otol Rhinol Laryngol. 1975 May-Jun;84(3 Pt 1):318–326. doi: 10.1177/000348947508400306. [DOI] [PubMed] [Google Scholar]
- Waespe W., Henn V. Neuronal activity in the vestibular nuclei of the alert monkey during vestibular and optokinetic stimulation. Exp Brain Res. 1977 Apr 21;27(5):523–538. doi: 10.1007/BF00239041. [DOI] [PubMed] [Google Scholar]
- Waespe W., Henn V. Visual-vestibular interaction in the flocculus of the alert monkey. II. Purkinje cell activity. Exp Brain Res. 1981;43(3-4):349–360. doi: 10.1007/BF00238377. [DOI] [PubMed] [Google Scholar]
- Westheimer G., Blair S. M. Function Organization of primate oculomotor system revealed by cerebellectomy. Exp Brain Res. 1974;21(5):463–472. doi: 10.1007/BF00237165. [DOI] [PubMed] [Google Scholar]
- Zee D. S., Yamazaki A., Butler P. H., Gücer G. Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol. 1981 Oct;46(4):878–899. doi: 10.1152/jn.1981.46.4.878. [DOI] [PubMed] [Google Scholar]
- Zee D. S., Yee R. D., Cogan D. G., Robinson D. A., Engel W. K. Ocular motor abnormalities in hereditary cerebellar ataxia. Brain. 1976 Jun;99(2):207–234. doi: 10.1093/brain/99.2.207. [DOI] [PubMed] [Google Scholar]


