Abstract
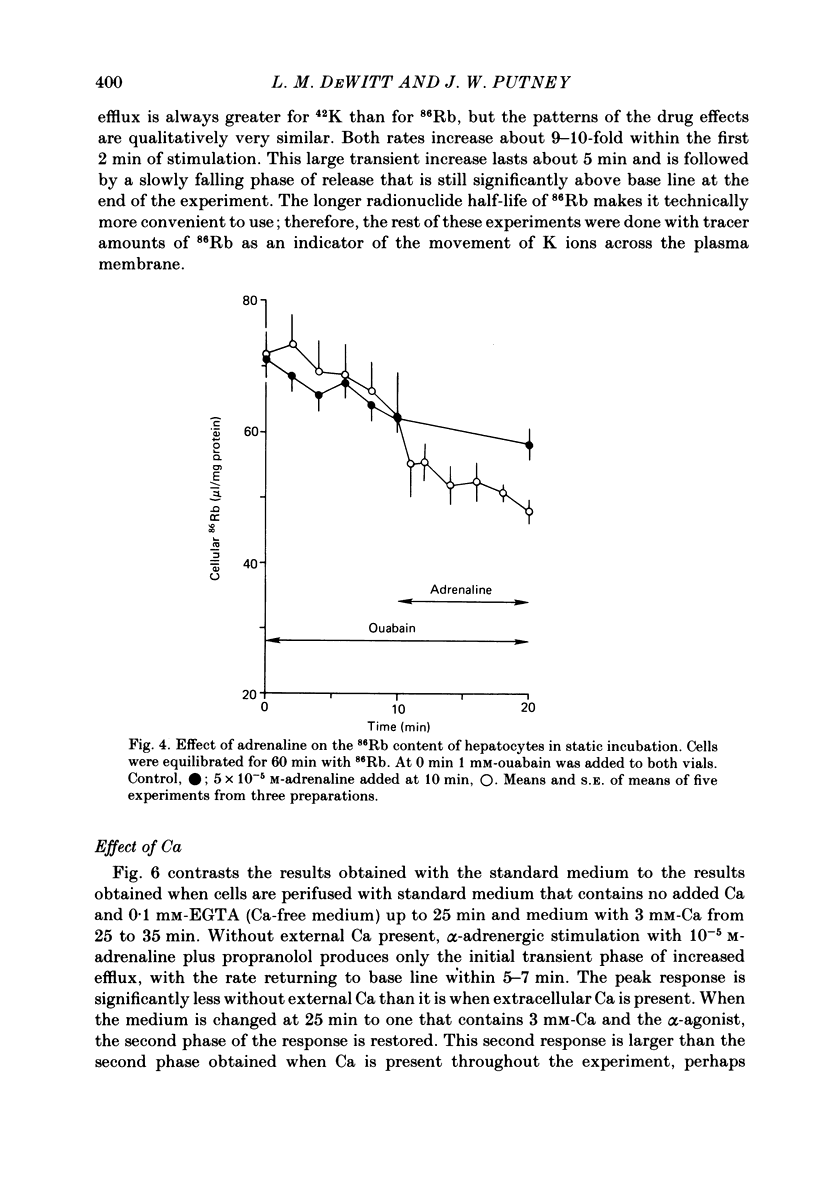
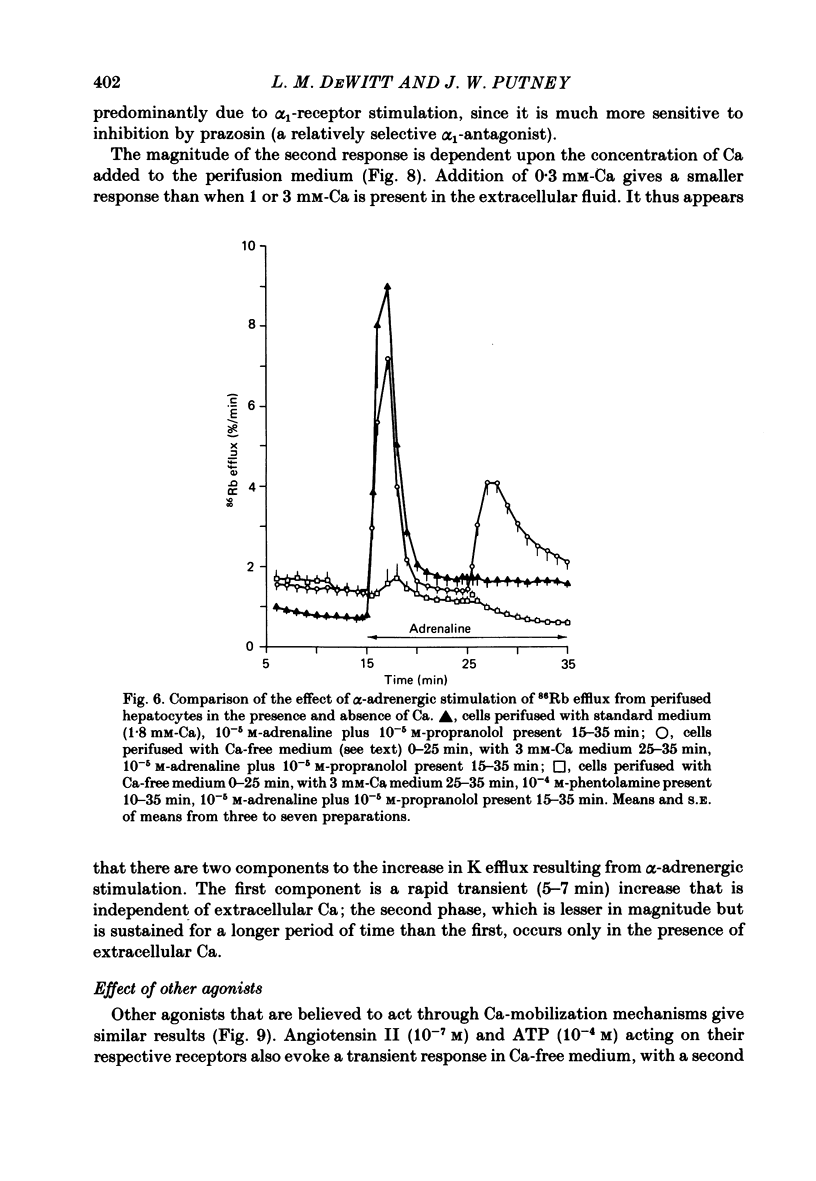
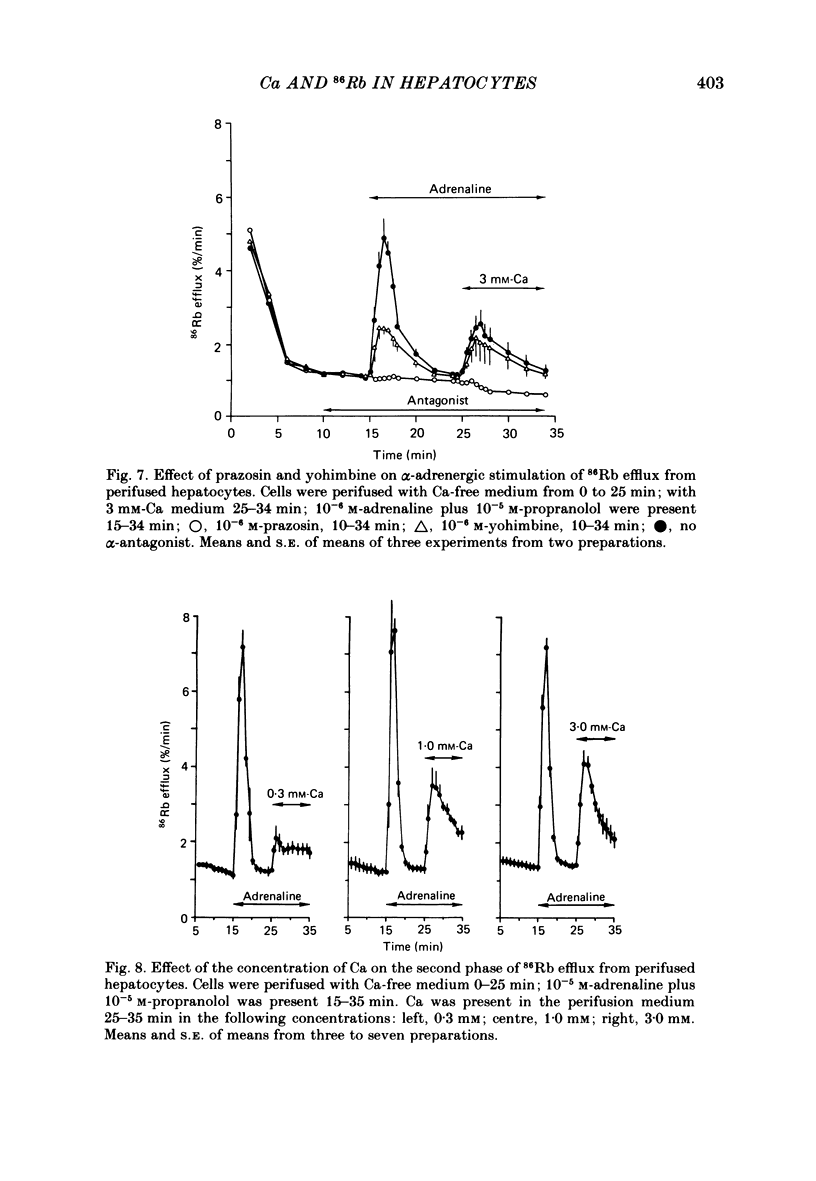
Either 86Rb or 42K appears to be a useful marker for monitoring the movement of cellular K in dispersed guinea-pig hepatocytes. Alpha-adrenergic stimulation of perifused hepatocytes causes a biphasic increase in 86Rb or 42K efflux from hepatocytes previously equilibrated with radio-isotope. The first phase is a large transient (about 5 min) increase which is followed by a slowly falling phase of release. Alpha-adrenergic stimulation of hepatocytes perifused with medium containing no added Ca plus 0.1 mM-EGTA evokes only the transient increase in 86Rb efflux. The addition of Ca to the medium in the continued presence of agonist restores the second phase of the response. Both phases of the response appear to be mediated by alpha 1-receptors. The magnitude of the second phase is dependent upon the concentration of Ca added to the perifusion medium. Other agonists that are believed to act by mobilizing Ca give similar results in this system. Angiotensin II, ATP and A23187 stimulate a transient increase in 86Rb efflux without extracellular Ca present, with the second phase of the response appearing upon the addition of Ca to the medium. These results suggest that the initial transient phase of 86Rb efflux, which is independent of extracellular Ca, is stimulated by Ca released from an intracellular pool. The second phase, which occurs only in the presence of extracellular Ca, is probably a result of Ca influx into the cell.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assimacopoulos-Jeannet F. D., Blackmore P. F., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Studies on role of calcium in alpha-adrenergic activation of phosphorylase. J Biol Chem. 1977 Apr 25;252(8):2662–2669. [PubMed] [Google Scholar]
- Blackmore P. F., Brumley F. T., Marks J. L., Exton J. H. Studies on alpha-adrenergic activation of hepatic glucose output. Relationship between alpha-adrenergic stimulation of calcium efflux and activation of phosphorylase in isolated rat liver parenchymal cells. J Biol Chem. 1978 Jul 25;253(14):4851–4858. [PubMed] [Google Scholar]
- Blackmore P. F., Exton J. H. Alpha 1-Adrenergic stimulation of Ca2+ mobilization without phosphorylase activation in hepatocytes from phosphorylase b kinase-deficient gsd/gsd rats. Biochem J. 1981 Aug 15;198(2):379–383. doi: 10.1042/bj1980379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore P. F., Hughes B. P., Shuman E. A., Exton J. H. alpha-Adrenergic activation of phosphorylase in liver cells involves mobilization of intracellular calcium without influx of extracellular calcium. J Biol Chem. 1982 Jan 10;257(1):190–197. [PubMed] [Google Scholar]
- Burgess G. M., Claret M., Jenkinson D. H. Effects of catecholamines, ATP and ionophore A23187 on potassium and calcium movements in isolated hepatocytes. Nature. 1979 Jun 7;279(5713):544–546. doi: 10.1038/279544a0. [DOI] [PubMed] [Google Scholar]
- Egashira K. Hyperpolarization by noradrenaline in guinea pig liver cells: effects of ouabain and external Ca2+. Jpn J Physiol. 1980;30(3):473–485. doi: 10.2170/jjphysiol.30.473. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic phenomena: role of calcium ions in actions of catecholamines in liver and other tissues. Am J Physiol. 1980 Jan;238(1):E3–12. doi: 10.1152/ajpendo.1980.238.1.E3. [DOI] [PubMed] [Google Scholar]
- Haylett D. G. Effects of sympathomimetic amines on 45Ca efflux from liver slices. Br J Pharmacol. 1976 May;57(1):158–160. doi: 10.1111/j.1476-5381.1976.tb07668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylett D. G., Jenkinson D. H. Effects of noradrenaline on potassium reflux, membrane potential and electrolyte levels in tissue slices prepared from guinea-pig liver. J Physiol. 1972 Sep;225(3):721–750. doi: 10.1113/jphysiol.1972.sp009966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haylett D. G., Jenkinson D. H. The receptors concerned in the actions of catecholamines on glucose release, membrane potential and ion movements in guinea-pig liver. J Physiol. 1972 Sep;225(3):751–772. doi: 10.1113/jphysiol.1972.sp009967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima T. Isoprenaline- and noradrenaline-induced hyperpolarization of guinea-pig liver cells. Br J Pharmacol. 1981 Aug;73(4):867–877. doi: 10.1111/j.1476-5381.1981.tb08740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., Vandenheede J. R., De Wulf H. On the role of calcium as second messenger in liver for the hormonally induced activation of glycogen phosphorylase. Biochim Biophys Acta. 1977 Feb 28;496(2):448–457. doi: 10.1016/0304-4165(77)90327-0. [DOI] [PubMed] [Google Scholar]
- Kikuchi M., Rabinovitch A., Blackard W. G., Renold A. E. Perifusion of pancreas fragments. A system for the study of dynamic aspects of insulin secretion. Diabetes. 1974 Jun;23(6):550–559. doi: 10.2337/diab.23.6.550. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Parod R. J., Putney J. W., Jr An alpha-adrenergic receptor mechanism controlling potassium permeability in the rat lacrimal gland acinar cell. J Physiol. 1978 Aug;281:359–369. doi: 10.1113/jphysiol.1978.sp012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poggioli J., Berthon B., Claret M. Calcium movements in in situ mitochondria following activation of alpha-adrenergic receptors in rat liver cells. FEBS Lett. 1980 Jun 30;115(2):243–246. doi: 10.1016/0014-5793(80)81178-1. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr Biphasic modulation of potassium release in rat parotid gland by carbachol and phenylephrine. J Pharmacol Exp Ther. 1976 Aug;198(2):375–384. [PubMed] [Google Scholar]
- Putney J. W., Jr Role of calcium in the fade of the potassium release response in the rat parotid gland. J Physiol. 1978 Aug;281:383–394. doi: 10.1113/jphysiol.1978.sp012429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J. W., Jr Stimulus-permeability coupling: role of calcium in the receptor regulation of membrane permeability. Pharmacol Rev. 1978 Jun;30(2):209–245. [PubMed] [Google Scholar]
- Seglen P. O. Preparation of rat liver cells. II. Effects of ions and chelators on tissue dispersion. Exp Cell Res. 1973 Jan;76(1):25–30. doi: 10.1016/0014-4827(73)90414-x. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., Putney J. W., Jr Does calcium mediate the increase in potassium permeability due to phenylephrine or angiotensin II in the liver? J Pharmacol Exp Ther. 1978 Dec;207(3):669–676. [PubMed] [Google Scholar]



