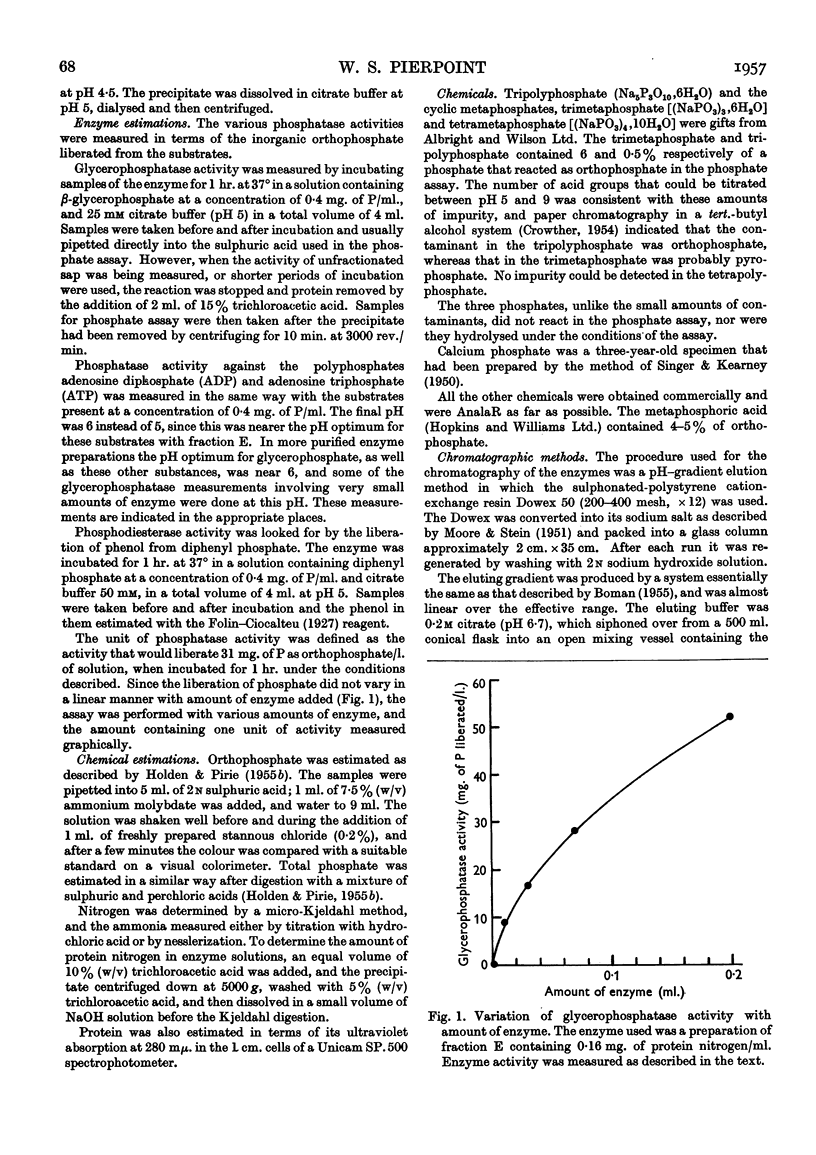
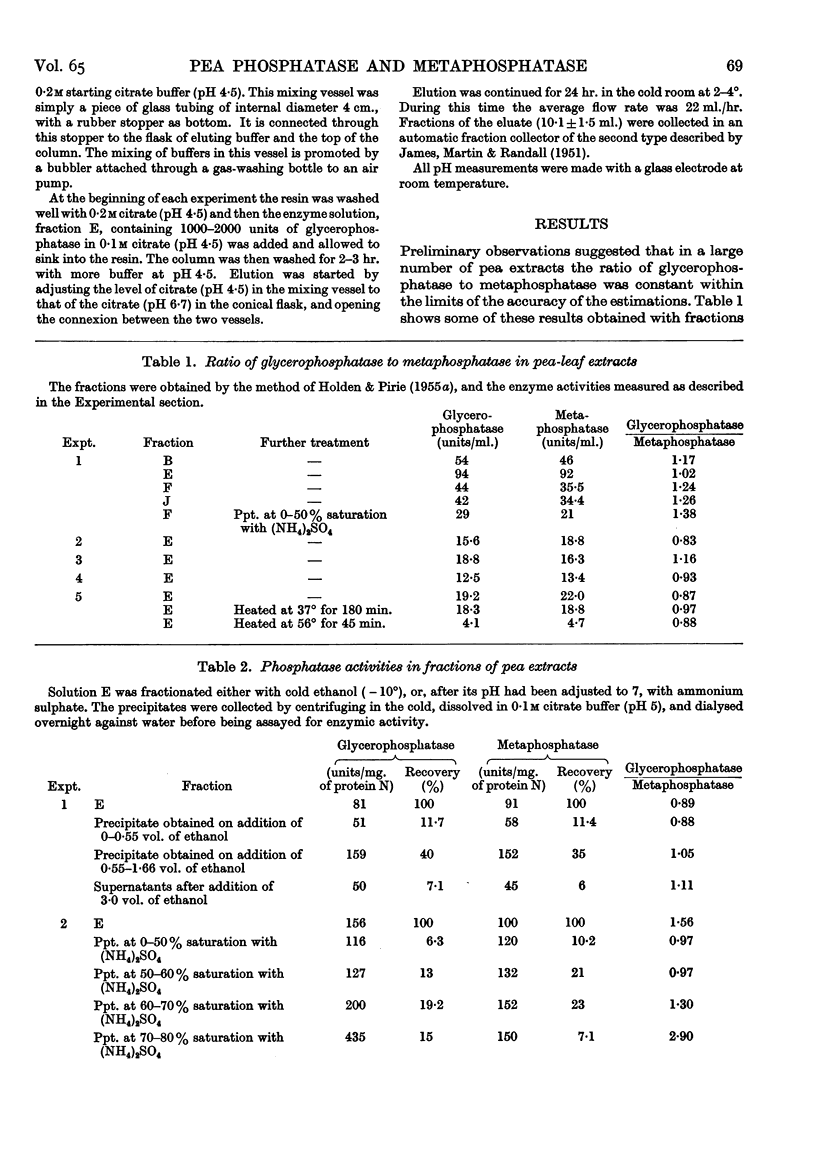
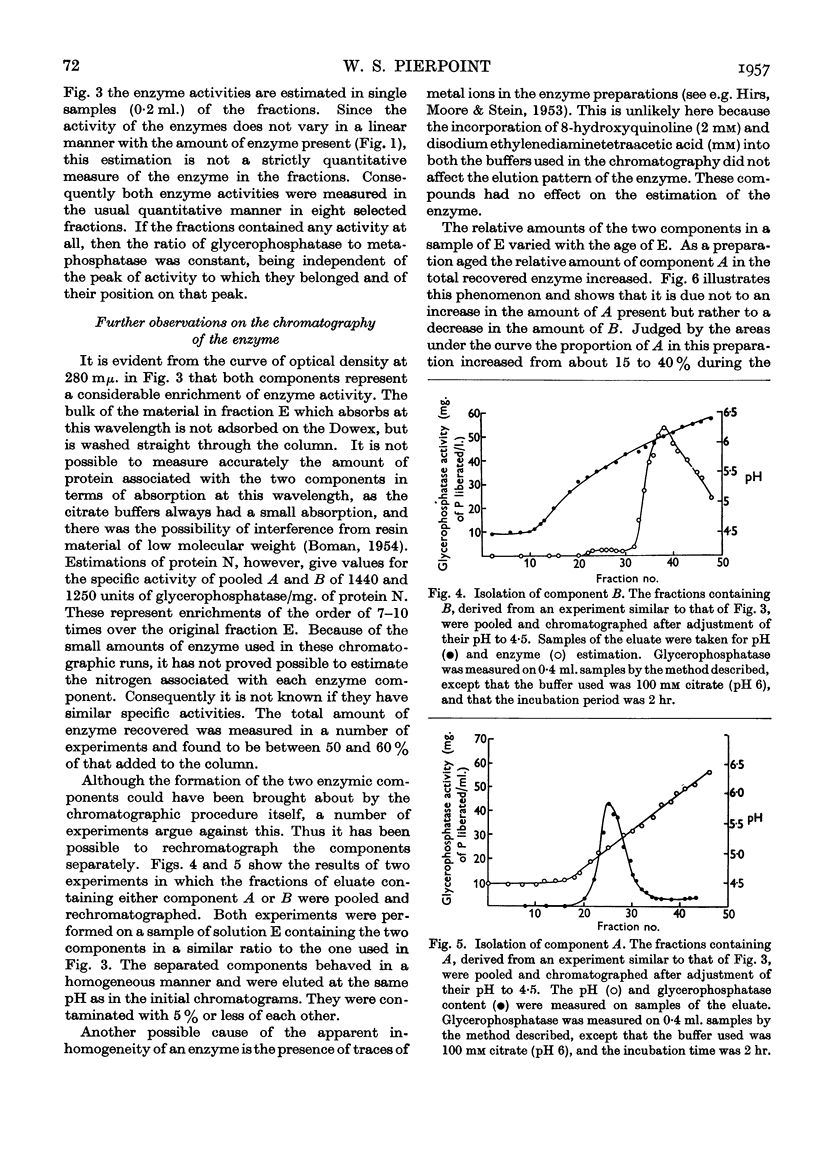
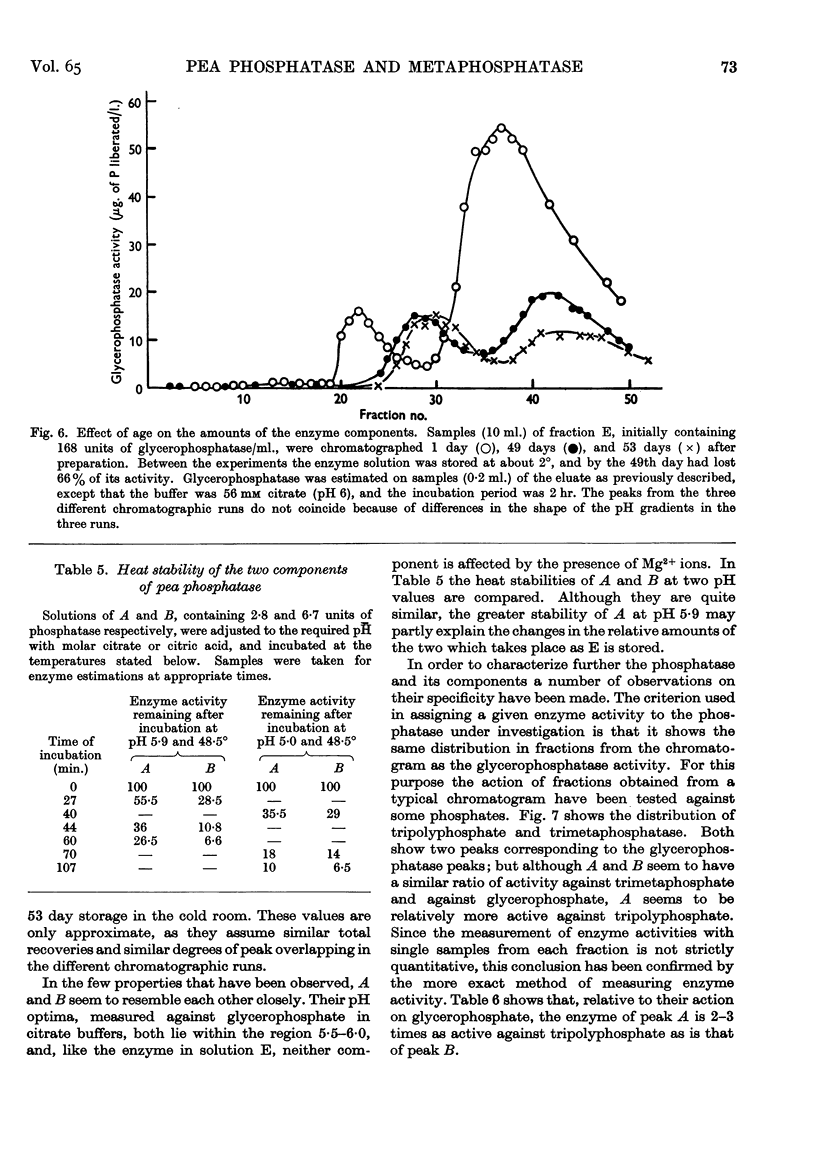
Full text
PDF


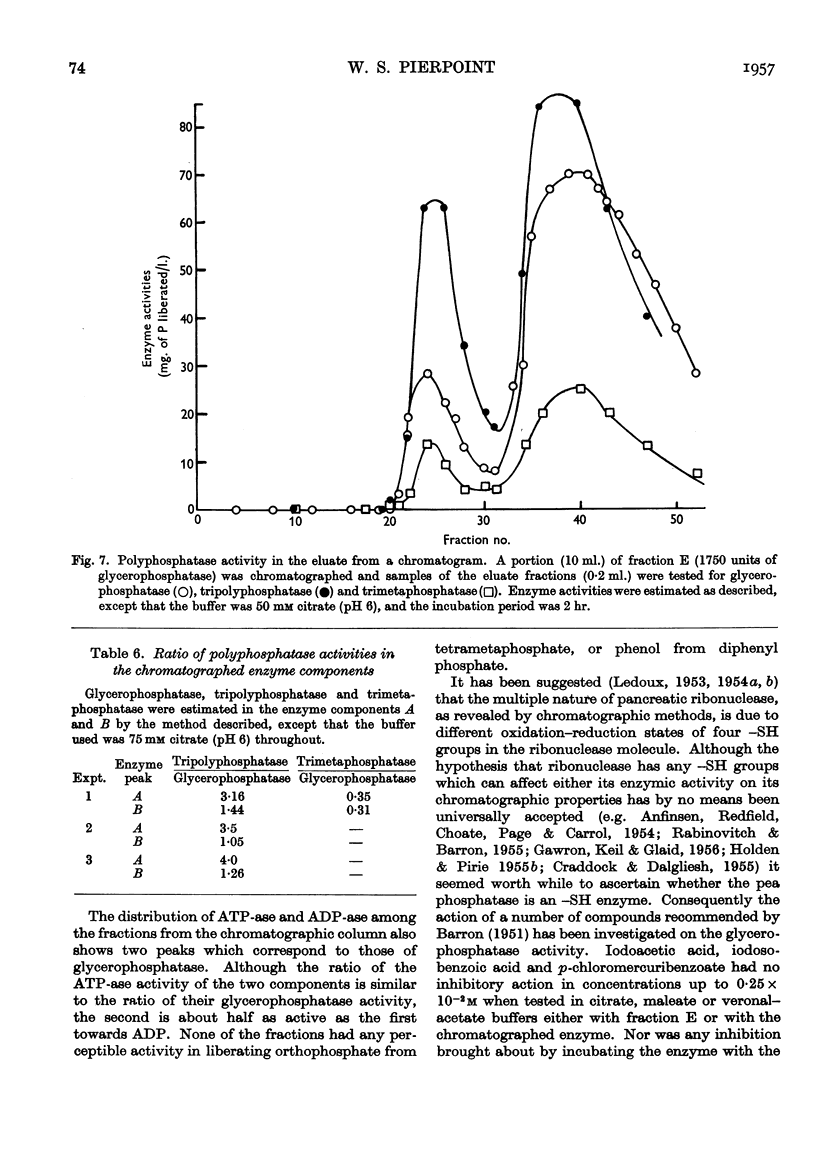


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANFINSEN C. B., REDFIELD R. R., CHOATE W. L., PAGE J., CARROLL W. R. Studies on the gross structure, cross-linkages, and terminal sequences in ribonuclease. J Biol Chem. 1954 Mar;207(1):201–210. [PubMed] [Google Scholar]
- BERRIDGE N. J., BRIGGS C. A. Electrophoresis of group-specific extracts of streptococci. Nature. 1954 Mar 13;173(4402):486–487. doi: 10.1038/173486b0. [DOI] [PubMed] [Google Scholar]
- BOARDMAN N. K. Use of a sulphonic acid ion-exchange resin for the chromatography of insulin. Biochim Biophys Acta. 1955 Oct;18(2):290–291. doi: 10.1016/0006-3002(55)90073-1. [DOI] [PubMed] [Google Scholar]
- BOMAN H. G. Adsorption of prostatic phosphatase on ion-exchange resin. Nature. 1954 Mar 6;173(4401):447–448. doi: 10.1038/173447b0. [DOI] [PubMed] [Google Scholar]
- BOMAN H. G. Chromatography of prostatic phosphatase. Biochim Biophys Acta. 1955 Feb;16(2):245–253. doi: 10.1016/0006-3002(55)90210-9. [DOI] [PubMed] [Google Scholar]
- GAWRON O., KEIL J., GLAID A. J., 3rd On the action of cyanide on ribonuclease. Biochim Biophys Acta. 1956 Jan;19(1):170–171. doi: 10.1016/0006-3002(56)90401-2. [DOI] [PubMed] [Google Scholar]
- HILL R. L., SMITH E. L. Crystalline papain. VI. Extensive stepwise hydrolysis of mercuripapain by leucine aminopeptidase without loss of proteolytic activity. Biochim Biophys Acta. 1956 Feb;19(2):376–377. doi: 10.1016/0006-3002(56)90444-9. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. W., MOORE S., STEIN W. H. A chromatographic investigation of pancreatic ribonuclease. J Biol Chem. 1953 Feb;200(2):493–506. [PubMed] [Google Scholar]
- HOLDEN M., PIRIE N. W. The partial purification of leaf ribonuclease. Biochem J. 1955 May;60(1):39–46. doi: 10.1042/bj0600039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLDEN M., PIRIE N. W. The preparation of ribonucleic acid from yeast, tobacco leaves and tobacco mosaic virus. Biochem J. 1955 May;60(1):46–53. doi: 10.1042/bj0600046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAMES A. T., MARTIN A. J. P., RANDALL S. S. Automatic fraction collectors and a conductivity recorder. Biochem J. 1951 Aug;49(3):293–299. doi: 10.1042/bj0490293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG S. R. Tripolyphosphate and trimetaphosphate in yeast extracts. J Biol Chem. 1956 Jan;218(1):23–31. [PubMed] [Google Scholar]
- LEDOUX L. Les groupes actifs de la ribonucléase. I. Action du parachloromercuribenzoate de sodium sur le système ARN-RNase. Biochim Biophys Acta. 1953 Aug;11(4):517–523. doi: 10.1016/0006-3002(53)90089-4. [DOI] [PubMed] [Google Scholar]
- LEDOUX L. Les groupes actifs de la ribonucléase. II. Oxydo-réduction. Biochim Biophys Acta. 1954 Jan;13(1):121–134. doi: 10.1016/0006-3002(54)90280-2. [DOI] [PubMed] [Google Scholar]
- LEDOUX L. Les groupes actifs de la ribonucléase. IV. Sur l'homogénéité des solutions de protéines. Biochim Biophys Acta. 1954 Jun;14(2):267–273. doi: 10.1016/0006-3002(54)90167-5. [DOI] [PubMed] [Google Scholar]
- LI C. H., PAPKOFF H., FONSS-BECH P., CONDLIFFE P. G. Action of chymotrypsin on hypophyseal growth hormone. J Biol Chem. 1956 Jan;218(1):41–52. [PubMed] [Google Scholar]
- LONDON M., HUDSON P. B. Identity of phosphotransferase and phosphomonoesterase of human prostate and of sera from patients with prostatic cancer. Biochim Biophys Acta. 1955 Aug;17(4):485–493. doi: 10.1016/0006-3002(55)90411-x. [DOI] [PubMed] [Google Scholar]
- MARTIN A. J. P., PORTER R. R. The chromatographic fractionation of ribonuclease. Biochem J. 1951 Jul;49(2):215–218. doi: 10.1042/bj0490215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTENHEIMER H. Die Substratspezifität anorganischer Poly- und Metaphosphatasen. II. Trennung der Enzyme. Hoppe Seylers Z Physiol Chem. 1956 Mar 17;303(3-6):115–124. [PubMed] [Google Scholar]
- MATTENHEIMER H. Die Substratspezifität anorganischer Poly- und Metaphosphatasen. III. Papierchromatographische Untersuchungen beim enzymatischen Abbau von anorganischen Poly- und Metaphosphaten. Hoppe Seylers Z Physiol Chem. 1956 Mar 17;303(3-6):125–139. [PubMed] [Google Scholar]
- MEYERHOF O., SHATAS R., KAPLAN A. Heat of hydrolysis of trimetaphosphate. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):121–127. doi: 10.1016/0006-3002(53)90130-9. [DOI] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. Chromatography of amino acids on sulfonated polystyrene resins. J Biol Chem. 1951 Oct;192(2):663–681. [PubMed] [Google Scholar]
- PERLMANN G. E. Formation of enzymatically active, dialysable fragments during autodigestion of pepsin. Nature. 1954 Feb 27;173(4400):406–406. doi: 10.1038/173406a0. [DOI] [PubMed] [Google Scholar]
- PIERPOINT W. S. The chromatography of leaf ribonuclease. Biochim Biophys Acta. 1956 Jul;21(1):136–141. doi: 10.1016/0006-3002(56)90103-2. [DOI] [PubMed] [Google Scholar]
- RABINOVITCH M., BARRON E. S. G. The effect of -SH reagents on the activity of ribonuclease. Biochim Biophys Acta. 1955 Oct;18(2):316–317. doi: 10.1016/0006-3002(55)90090-1. [DOI] [PubMed] [Google Scholar]
- REINER J. M., TSUBOI K. K., HUDSON P. B. Acid phosphatase. IV. Fluoride inhibition of prostatic acid phosphatase. Arch Biochem Biophys. 1955 May;56(1):165–183. doi: 10.1016/0003-9861(55)90346-5. [DOI] [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. The L-amino acid oxidases of snake venom. II. Isolation and characterization of homogeneous L-amino acid oxidase. Arch Biochem. 1950 Nov;29(1):190–209. [PubMed] [Google Scholar]
- TALLAN H. H., STEIN W. H. Chromatographic studies on lysozyme. J Biol Chem. 1953 Feb;200(2):507–514. [PubMed] [Google Scholar]
- TANFORD C., HAUENSTEIN J. D. Identification of the chemical difference between chromatographic components of ribonuclease. Biochim Biophys Acta. 1956 Mar;19(3):535–539. doi: 10.1016/0006-3002(56)90477-2. [DOI] [PubMed] [Google Scholar]


