Full text
PDF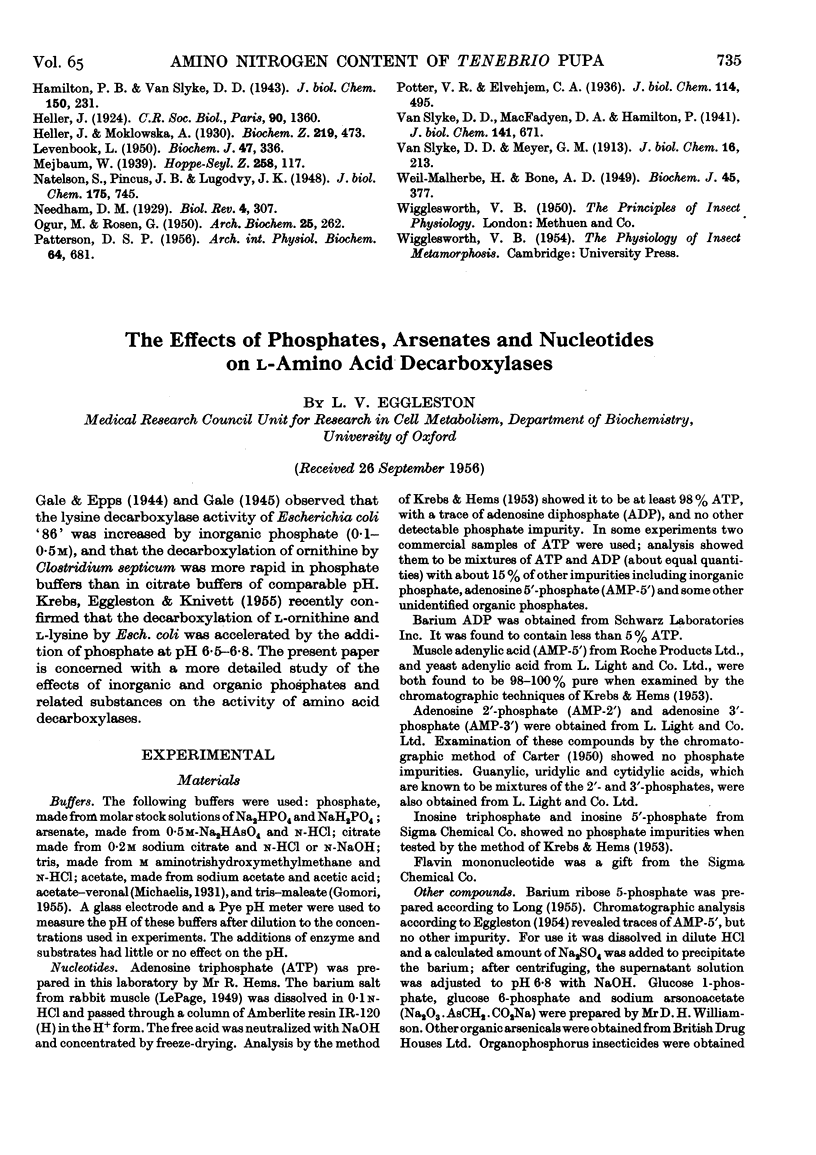






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLEY W. Efficiency of oxidative phosphorylation during the oxidation of pyruvate. Biochem J. 1953 Jul;54(4):677–682. doi: 10.1042/bj0540677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGERET B., CHATAGNER F., FROMAGEOT C. Quelques relations entre le phosphate de pyridoxal et la décarboxylation de l'acide cystéinesulfinique par divers organes du rat normal ou du rat carencé en vitamine B6. Biochim Biophys Acta. 1955 May;17(1):128–135. doi: 10.1016/0006-3002(55)90327-9. [DOI] [PubMed] [Google Scholar]
- Berenblum I., Chain E. An improved method for the colorimetric determination of phosphate. Biochem J. 1938 Feb;32(2):295–298. doi: 10.1042/bj0320295. [DOI] [PMC free article] [PubMed] [Google Scholar]
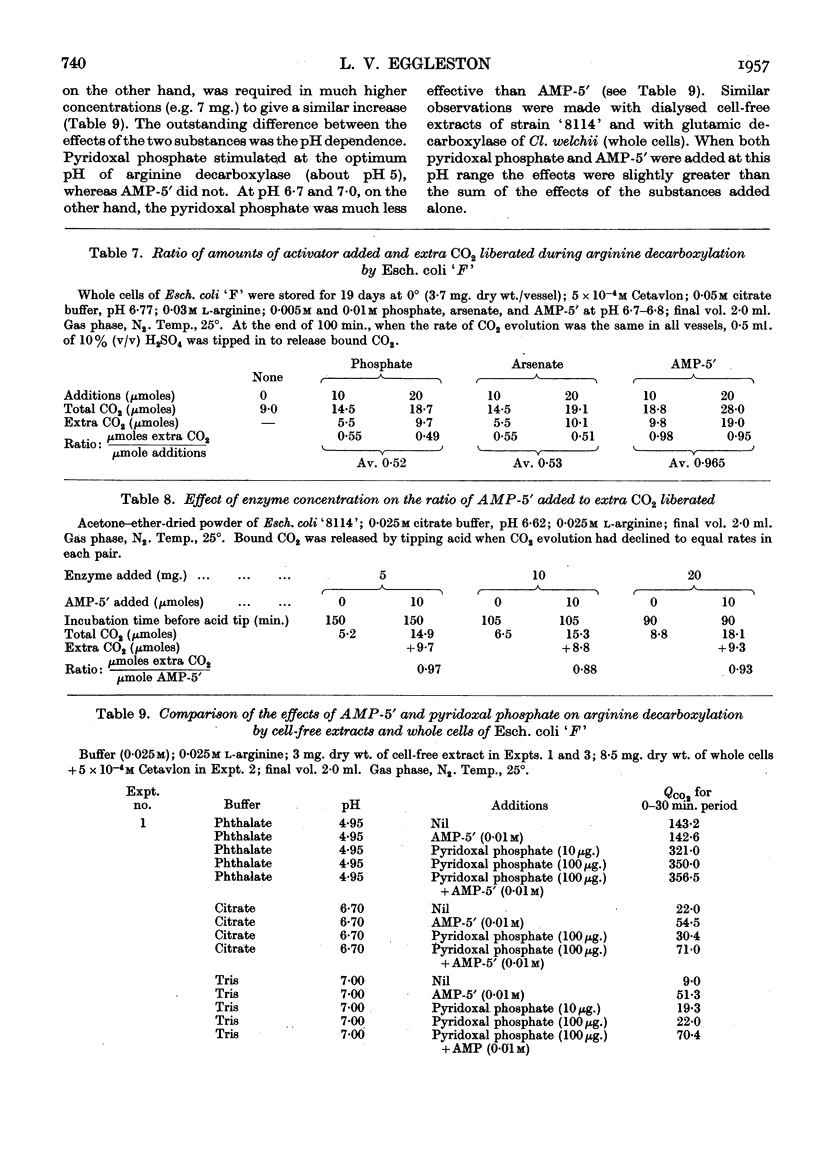
- EGGLESTON L. V. Preparation of 32P-labelled adenosine 5'-phosphate, inosine 5'-phosphate and ribose 5-phosphate. Biochem J. 1954 Nov;58(3):503–506. doi: 10.1042/bj0580503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F., Epps H. M. Studies on bacterial amino-acid decarboxylases: 1. l(+)-lysine decarboxylase. Biochem J. 1944;38(3):232–242. doi: 10.1042/bj0380232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. Studies on bacterial amino-acid decarboxylases: 5. The use of specific decarboxylase preparations in the estimation of amino-acids and in protein analysis. Biochem J. 1945;39(1):46–52. doi: 10.1042/bj0390046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. The production of amines by bacteria: The decarboxylation of amino-acids by strains of Bacterium coli. Biochem J. 1940 Mar;34(3):392–413. doi: 10.1042/bj0340392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTMAN W. J., AKAWIE R. I., CLARK W. G. Competitive inhibition of 3, 4-dihydroxyphenylalanine (DOPA) decarboxylase in vitro. J Biol Chem. 1955 Oct;216(2):507–529. [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- HUGHES D. E. Acceleration of bacterial glutamic decarboxylase and glutaminase by cetyltrimethylammonium bromide. Biochem J. 1949;45(3):325–331. doi: 10.1042/bj0450325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D. E. The effect of surface-active agents on bacterial glutamic decarboxylase and glutaminase. Biochem J. 1950 Feb;46(2):231–236. doi: 10.1042/bj0460231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIVETT V. A. The anaerobic interconversion of ornithine and citrulline by Streptococcus faecalis. Biochem J. 1954 Nov;58(3):480–486. doi: 10.1042/bj0580480. [DOI] [PMC free article] [PubMed] [Google Scholar]
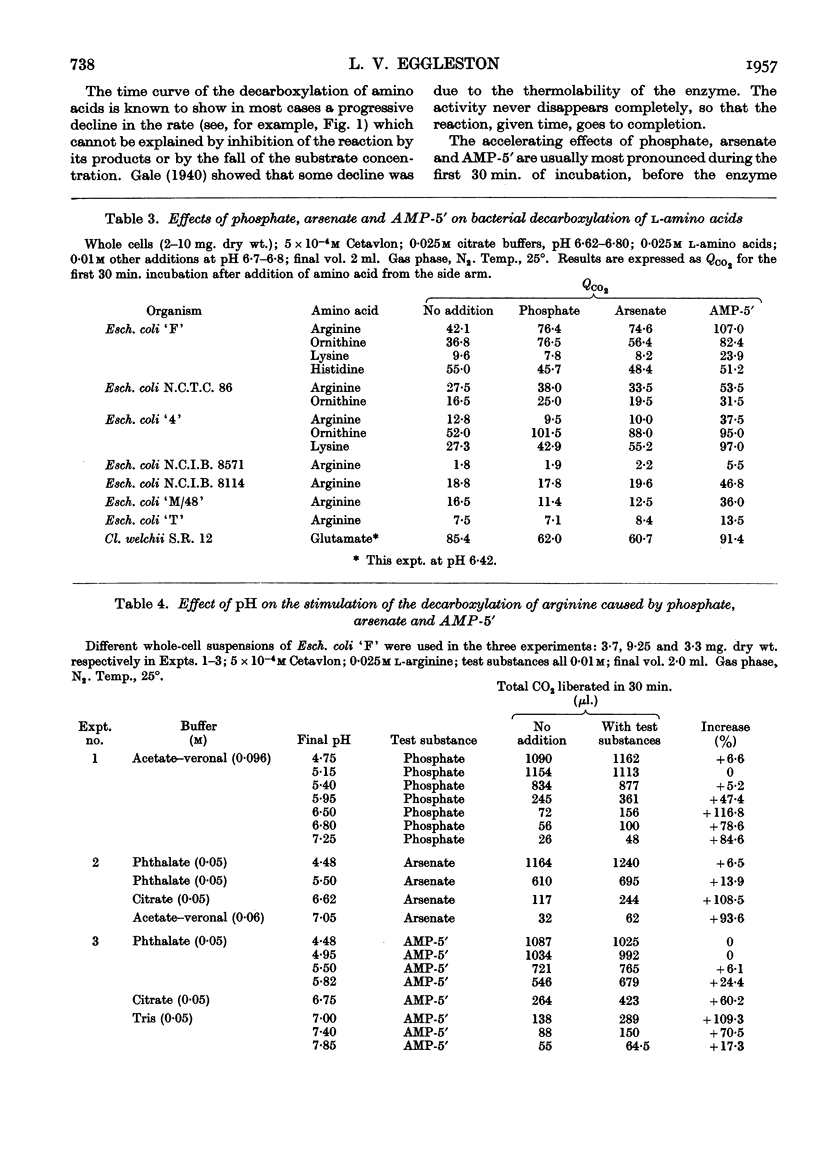
- KREBS H. A., EGGLESTON L. V., KNIVETT V. A. Arsenolysis and phosphorolysis of citrulline in mammalian liver. Biochem J. 1955 Feb;59(2):185–193. doi: 10.1042/bj0590185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS H. A., HEMS R. Some reactions of adenosine and inosine phosphates in animal tissues. Biochim Biophys Acta. 1953 Sep-Oct;12(1-2):172–180. doi: 10.1016/0006-3002(53)90136-x. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. Quantitative determination of glutamine and glutamic acid. Biochem J. 1948;43(1):51–57. doi: 10.1042/bj0430051. [DOI] [PMC free article] [PubMed] [Google Scholar]
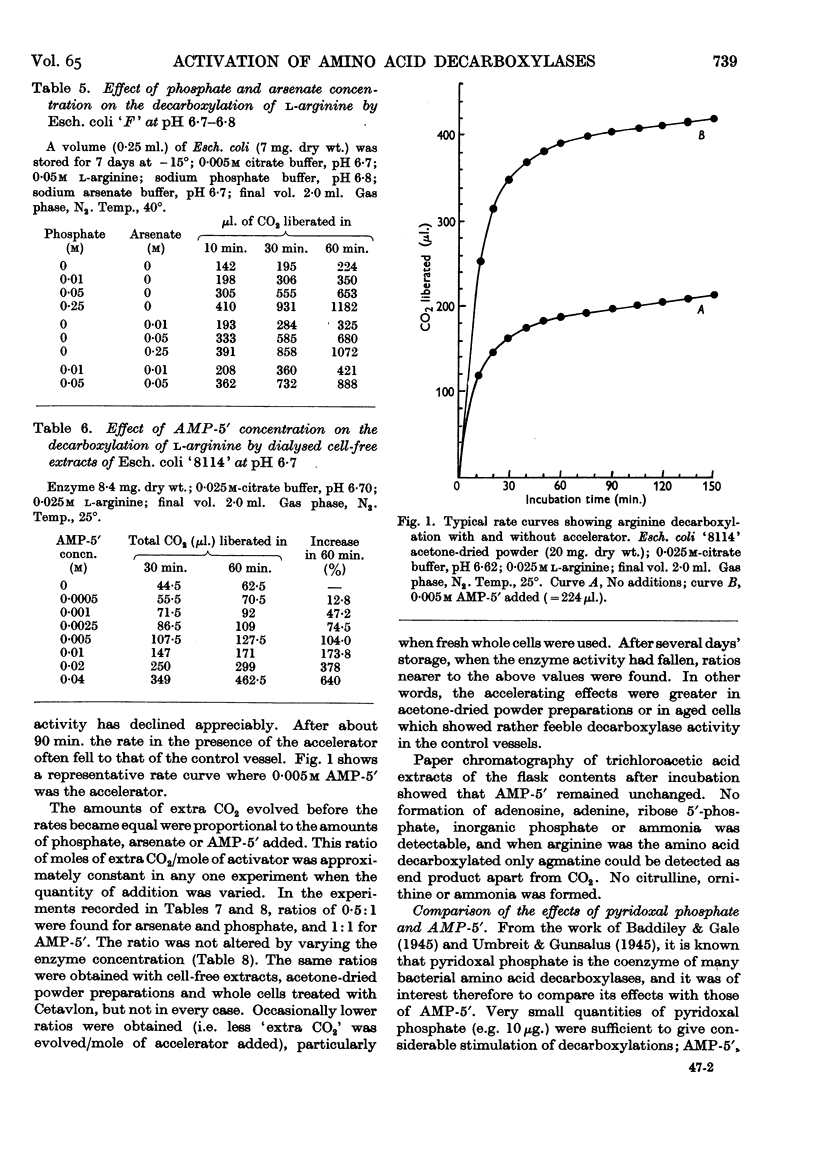
- LONG C. Studies involving enzymic phosphorylation. 4. The conversion of D-ribose into D-ribose 5-phosphate by extracts of Escherichia coli. Biochem J. 1955 Feb;59(2):322–329. doi: 10.1042/bj0590322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAGER J. M., RAUTANEN N. Sulphite oxidation by a plant mitochondrial system. I. Preliminary observations. Biochim Biophys Acta. 1955 Sep;18(1):111–121. doi: 10.1016/0006-3002(55)90014-7. [DOI] [PubMed] [Google Scholar]


