Abstract
Viruses rely on attachment to specific cell surface receptors to infect host cells. Selective expression of viral receptors has the potential to attenuate infection of susceptible tissues by redirecting virus to cells that cannot support viral replication. We propose that erythrocytes are an ideal instrument for this strategy, because they are present in vast numbers, permeate every organ, and cannot serve as hosts for viral propagation. To test this hypothesis, we generated a transgenic mouse, termed globin transcription factor 1 (GATA1)- coxsackie and adenovirus receptor (CAR), that expressed the CAR on erythrocytes. Coxsackievirus group B (CVB) adhered to the surface of CAR-expressing erythrocytes and was rendered noninfectious. Upon infection with CVB, GATA1-CAR mice had diminished viremia and reduced viral replication in heart, brain, and liver. Furthermore, when faced with a CVB challenge that was lethal to WT littermates, the survival of GATA1-CAR mice was prolonged, and their ultimate mortality was reduced. The GATA1-CAR mouse model presented here demonstrates that erythrocyte expression of CAR limits CVB pathogenesis. Erythrocytes also may be coated with a variety of receptors by nontransgenic methods, making this a very flexible model for the treatment of infectious diseases in humans.
Keywords: coxsackie and adenovirus receptor, virus receptor
Viruses infect cells through attachment to specific host cell membrane receptors. These receptors mediate adhesion of the virus and facilitate its entry into the cell. The expression pattern of the specific receptors for a virus is thus a major determinant of viral tropism. Viruses cannot attack cells that do not bear the appropriate receptors, but they will attempt to infect cells that do, even if those cells are not suitable for viral propagation. If the receptor-expressing cell cannot support replication of the virus, then the virus will spend itself fruitlessly in an attempt to infect it. This idea can be advantageously applied by using selective expression of viral receptors to redirect virus to nonproductive cells, thus protecting susceptible tissues. We propose that erythrocytes are the ideal instrument for this strategy, because these cells are present in vast numbers, permeate every organ, are easily manipulated, are relatively disposable, and cannot serve as hosts for viral replication.
Erythrocytes are simultaneously the most numerous and the simplest cells in the body. In their mature form, they lack the nuclei and organelles required to replicate nucleic acids and elaborate proteins. Because viruses depend on the use of the host cell machinery to replicate, erythrocytes are invulnerable to viral infection. The redirection of virus to erythrocytes has the potential to attenuate infection by leading virions to a dead end, leaving fewer infectious particles free to invade susceptible tissues. We hypothesized that the transgenic expression of viral receptor on erythrocytes would overwhelm the virus with decoy targets and thereby limit pathogenesis. Coxsackievirus group B (CVB) and its receptor, the coxsackie and adenovirus receptor (CAR), were chosen as models to evaluate this strategy.
CVB is a human and mouse pathogen that can cause pancreatitis, encephalitis, and hepatitis, in addition to being the leading cause of adult myocarditis (1, 2). In mice, the exocrine pancreas is extremely susceptible to CVB infection. CVB invades pancreatic acinar cells and there replicates to very high levels, leading to the shedding of large amounts of virus into the bloodstream (3). The resulting high-level viremia enables the virus to invade other organs, notably the heart, brain, and liver, and morbidity and mortality are generally the result of damage to these other organs. The pancreatic infection appears to be critical to multiorgan pathogenesis, because when the pancreatic infection is suppressed, mice are protected from consequent myocarditis (4, 5). This dependence on a viremic phase for infection of organs makes CVB an appropriate target to evaluate the efficacy of erythrocyte-bound receptor. Receptor-expressing erythrocytes could have the capacity to capture circulating CVB and dampen the viremic phase, thereby protecting organs from infection.
The essential receptor for CVB is CAR, which mediates both cellular adhesion and internalization of all six serotypes of CVB (6-10). CAR also facilitates the attachment of adenovirus, although integrins are required for adenovirus internalization (6, 11, 12). CVB binds to CAR with high affinity, and this interaction triggers an irreversible conformational change in the viral capsid that enables viral uncoating (13-16). The transgenic expression of CAR on normally nonpermissive cells is sufficient to allow CVB infection (3, 7, 8). CAR expression is widespread in both humans and mice, being present in many organs, including heart, brain, pancreas, liver, lung, kidney, small intestine, colon, and prostate, but CAR is notably absent on both WBCs and RBCs (7, 10, 17-19).
These factors advanced CVB and CAR as suitable candidates to test the efficacy of the erythrocyte-expressed receptor. CAR was placed under the control of the mouse globin transcription factor 1 (GATA-1) promoter, producing a transgenic GATA1-CAR mouse that expressed CAR on erythrocytes. Here, we show that the GATA1-CAR erythrocytes bound to and neutralized CVB, and this neutralization reduced both viremia and infection of target organs. This protection enabled the mice to survive a normally lethal viral challenge. These results validate the strategy of using the erythrocyte-bound receptor to create “decoy erythrocytes” that trap virus and improve the outcome of infection.
Materials and Methods
Mice. The GATA1-CAR transgenic vector was built by inserting the coding cDNA sequence of human CAR1 into the unique NotI site of plasmid pGATA-1. This vector placed CAR under the control of the hematopoietic cell-specific GATA-1 promoter (20). The orientation of the human CAR insert was confirmed by restriction analysis and DNA sequencing. The vector was microinjected into fertilized oocytes harvested from F1 intercrosses of SJL × C57BL/6 mice (The Jackson Laboratory). After microinjection, the oocytes were implanted into pseudo-pregnant SW female mice. The resulting progeny were screened for integration of the transgene by PCR using mouse-tail DNA and flow cytometric analysis of erythrocytes for CAR expression. The mouse line was maintained by mating with C57BL/6 mice. In all experiments, CAR-negative littermates were used as WT controls. Mice were 10-14 weeks of age at the time of infection, with a roughly equal distribution of males and females. Mice were scored as deceased either when found dead or when they were clearly imminently moribund. The experimental protocols involving animals were approved by the University of Massachusetts Medical School Institutional Animal Care and Use Committee.
Flow Cytometric Analysis. Blood samples were drawn from tail cuts or terminal heart punctures. Platelet-rich plasma was made by centrifuging heparinized blood samples at 75 × g for 20 min and drawing the platelet-rich plasma layer from the surface of the cell pellet. Platelets were identified by flow cytometry by staining the platelet-rich plasma with anti-mouse CD41-FITC mAb (553848, BD Pharmingen) and gating on this signal. Lymphocytes were isolated by homogenization of whole spleens by using a sterile wire mesh, followed by RBC lysis using ammonium chloride/Tris·HCl buffer (Sigma). CAR was stained by using anti-human CAR mFab or isotype control Fab, followed by phycorerythin-conjugated goat anti-human IgG (H + L chain-specific) AffiniPure (Fab′)2 (Jackson ImmunoResearch). CVB4 was labeled by using mAb purified from anti-CVB4 hybridoma supernatants (HB-185, American Type Culture Collection), followed by FITC-conjugated anti-mouse IgG (Sigma). Flow cytometric data were acquired using an LSR Flow Cytometer with cellquest software, both from (BD Biosciences), and were analyzed by using flowjo software (Treestar, Ashland, OR).
CVB. These experiments used our laboratory strain of CVB type 3 (CVB3) or CVB4 strain JVB (VR-184, American Type Culture Collection). Virus was grown by passage through HeLa cells and harvested from cell lysates. Virus was quantitated by plaque-forming assay as described below. Mice were inoculated with virus by i.p. injection of the stated virus quantities in 0.5 ml of saline.
Virus Neutralization Assays. Blood was drawn from GATA1-CAR and WT mice into heparin. CVB3 or CVB4 at a concentration of 105 plaque-forming units (PFU)/ml was incubated at 37°C with the erythrocytes at 3 × 109 erythrocytes per ml in RPMI medium 1640, or in medium alone. Anti-CVB4 mAb was added to virus samples in medium without erythrocytes at 100 μg/ml. Samples were flash-frozen at time points, and virus was quantitated by PFU assays on HeLa cells. The first sample was drawn within 5 s of adding virus and mixing.
PFU Assays. Blood samples from mouse infection experiments were separated into cell and plasma fractions by centrifugation at 400 × g for 15 min. Plasma fractions were drawn off, and cell pellets were washed three times in Hanks' buffered saline solution. Organs were weighed, then homogenized by using sterile pestles. All samples were frozen and thawed before analysis. Duplicate 10-fold serial dilutions of infected organ samples were added to confluent layers of HeLa cells in six-well plates. The HeLa monolayers were incubated with the virus for 45 min at room temperature, then washed with medium. The HeLa monolayers were then overlaid with 4 ml of a mixture containing 1% agar, 10 mM MgCl2, 5% FBS, and Eagle's minimal essential medium and incubated for 2 days at 37°C. PFUs were counted after staining cells with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide/iodotetrazolium chloride (MTT/INT) dye.
Statistical Methods. Viral titers from the mouse infection experiments are presented as the geometric means of the groups on a log10 scale. Statistical significance of plaque assay results from these experiments was determined by unpaired two-tailed Student's t test on the log10-transformed PFU data. Significance for survival data was calculated by log-rank χ2 test.
Results
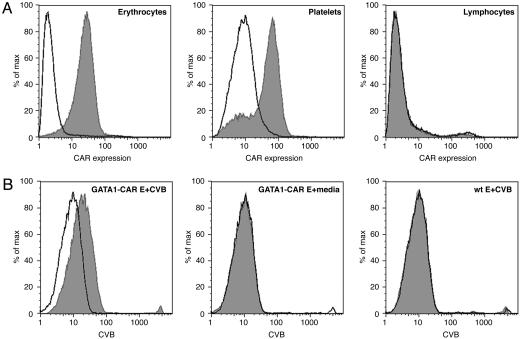
GATA1-CAR Mice Expressed CAR on Their Erythrocytes. Transgenic mice that expressed CAR on their erythrocytes were generated to serve as a model for the decoy erythrocyte technique. The transgenic vector was built by subcloning the full-length human CAR1 cDNA into a plasmid that encoded regions of the mouse GATA-1 promoter. These GATA-1 gene regulatory elements were expected to drive expression of CAR specifically in erythroblasts and megakaryocytes (20, 21). As predicted, CAR, an Ig-superfamily transmembrane receptor, was readily detectable on the surface of mature erythrocytes and platelets drawn from GATA1-CAR mice but was not expressed by splenocytes (Fig. 1A). The GATA1-CAR mice exhibited no overt phenotype as a consequence of the transgene, and no abnormalities such as atypical hematocrit or blood coagulation were noted. These mice were backcrossed to C57BL/6, and equally backbred, non-CAR-expressing littermates were used as WT controls throughout these experiments.
Fig. 1.
The erythrocytes of GATA1-CAR mice expressed CAR and bound CVB. (A) Flow cytometric analysis of samples drawn from GATA1-CAR mice demonstrated expression of CAR on erythrocytes (Left) and platelets (Center) but not lymphocytes (Right). WT mice did not express CAR in any of these fractions (data not shown). Erythrocytes were stained by using anti-CAR mFab (shaded peaks) or isotype control Fab (unshaded peaks), followed by a phycoerythrin-labeled secondary mAb. (B) Flow cytometric analysis revealed that CVB adhered to the surface of GATA1-CAR erythrocytes (E) (Left) but not WT E (Right). No shift was detected on GATA1-CAR E incubated with medium alone (Center). Erythrocytes were incubated with CVB4, then stained by using an anti-CVB4 mAb (shaded peaks) or isotype control mAb (unshaded peaks), followed by a FITC-labeled secondary mAb.
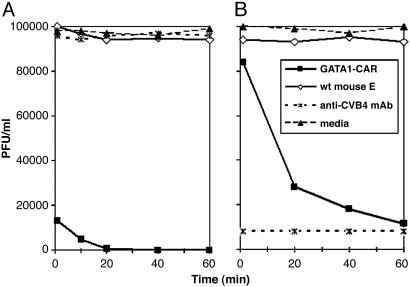
GATA1-CAR Erythrocytes Bound and Neutralized Multiple CVB Serotypes. In vitro experiments established that CVB adhered to CAR expressed by GATA1-CAR erythrocytes and that this interaction abolished viral infectivity. To test for receptor binding, CVB4 was incubated with blood drawn from GATA1-CAR or WT mice. Anti-CVB4 mAb subsequently stained only GATA1-CAR erythrocytes, indicating that the virus had adhered to the surface of these blood cells (Fig. 1B). Furthermore, exposure to the GATA1-CAR erythrocytes rendered multiple serotypes of the virus noninfectious. CVB3 incubated with GATA1-CAR erythrocytes rapidly lost infectivity, with a 10-fold reduction within 10 min and nearly complete neutralization by 1 h (Fig. 2A). GATA1-CAR erythrocytes also curtailed CVB4 infectivity, albeit at a slower rate, achieving almost 90% inhibition by 1 h (Fig. 2B). The level of CVB4 inhibition achieved by the CAR-expressing erythrocytes was ultimately equivalent to that of serotype-specific neutralizing mAb. This CVB4-specific mAb was ineffective against CVB3, as expected (Fig. 2 A). These experiments demonstrated that erythrocyte-expressed CAR matched the inhibitory potency of neutralizing antibody while maintaining the distinct advantage of being effective against multiple CVB serotypes.
Fig. 2.
GATA1-CAR erythrocytes neutralized CVB types 3 and 4. CVB3 (A) or CVB4 (B) at an initial concentration of 105 PFU/ml was incubated with GATA1-CAR or WT erythrocytes (E) at 3 × 109 erythrocytes per ml. Virus also was incubated with anti-CVB4 mAb or in medium only. Samples were taken at the indicated time points, flash-frozen, and then quantitated by plaque-forming assay.
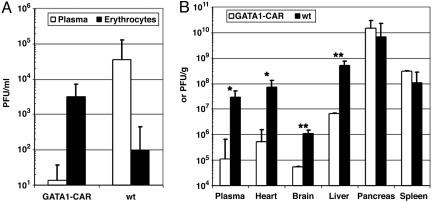
GATA1-CAR Mice Had Diminished Viremia, and Several Target Organs Were Protected from CVB. The ability of GATA1-CAR erythrocytes to neutralize CVB in vitro translated into significant decreases in viral pathogenicity in vivo. Groups of GATA1-CAR and WT mice were inoculated i.p. with CVB3 at 104 PFU per mouse. Twenty-four hours later, blood was drawn from the animals, and the cell and plasma fractions were individually titrated for CVB3 by plaque-forming assay. The absolute amount of virus in the plasma fraction was significantly reduced in the GATA1-CAR mice, compared with WT (GATA1-CAR 1.13 ± 0.43 vs. WT 4.55 ± 0.56; P ≤ 0.01, based on log10-transformed PFU/ml data) (Fig. 3A). In the GATA1-CAR blood, 100-fold more virus was found in the cell fraction, which consisted mainly of erythrocytes, than in the plasma (P < 0.02). The opposite pattern was found in the WT blood, where there was more virus present in the plasma than on the cells (P < 0.05). These results strongly suggested that adherence of CVB to the GATA1-CAR erythrocytes resulted in the depletion of free virus from the plasma and decreased overall viremia.
Fig. 3.
Viremia was reduced, and several target organs were protected from infection in GATA1-CAR mice. (A) CVB was depleted from the plasma and adhered to GATA1-CAR erythrocytes in vivo. Mice were inoculated with 104 PFU of CVB3 per mouse by i.p. injection. Blood samples were drawn by tail cuts 24 h later, and virus in erythrocyte and plasma fractions was quantitated by plaque-forming assay. (B) Several target organs were protected from CVB infection in the GATA1-CAR mice. Three days after infection with 103 PFU of CVB3 per mouse, blood and organs were harvested. Viral titers in the plasma, hearts, brains, and livers were reduced by 10- to 100-fold in the GATA1-CAR mice, compared with WT controls. Plasma titers are represented as PFU/ml, whereas the solid organs are PFU/g(*, 0.01 < P ≤ 0.05 and **, P ≤ 0.01; n = 3 for each strain). Error bars represent standard error of the means of the log10-transformed data.
Erythrocyte CAR-mediated diminution of viremia was accompanied by decreased viral replication in several vulnerable tissues. GATA1-CAR and WT mice were challenged with CVB3 at a dose of 103 PFU per mouse. At 3 days after infection, during the typical time of peak viral production (3, 22-24), organs were harvested, and virus in the plasma, heart, brain, liver, pancreas, and spleen was quantitated. The GATA1-CAR plasma titers again revealed a dramatic reduction in circulating infectious virus. More importantly, several susceptible target organs also had considerably decreased viral loads (Fig. 3B). There were 10- to 100-fold reductions in infectious virus in the heart, brain, and liver in the GATA1-CAR mice, compared with the WT group. When the mice were challenged with a 10-fold greater initial inoculum of 104 PFU per mouse, the heart and brain were similarly protected and had significant reductions in their viral titers (liver not tested, data not shown).
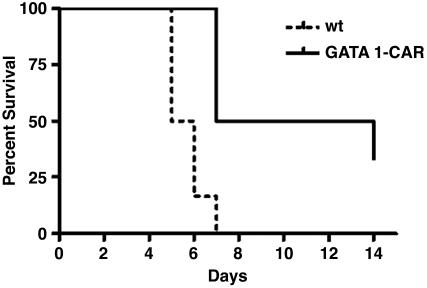
GATA1-CAR Mice Had Improved Survival When Faced with a Lethal Viral Challenge. Suppression of infection by erythrocyte-expressed CAR enabled GATA1-CAR mice to survive a CVB3 challenge that was invariably lethal to WT animals. A 4 × 103 PFU per mouse dose of CVB3 given to WT mice caused 50% mortality by day 5 after infection and left no survivors by day 7 (Fig. 4). The GATA1-CAR mice were much more resilient, with no deaths before day 7, 50% survival through 2 weeks, and 33% survival at the termination of the experiment on day 14. Only mice from the GATA1-CAR group survived the experiment, and these had resolved all outward signs of sickness, such as altered posture, ruffled fur, and lethargy, and maintained only a very low level of viremia (mean 350 ± 300 PFU/ml of whole blood).
Fig. 4.
GATA1-CAR mice had prolonged survival upon lethal CVB challenge. GATA1-CAR mice and WT mice were injected i.p. with 4 × 103 PFU of CVB3 per mouse and scored for survival. Thirty-three percent of the GATA1-CAR mice survived through day 14 and appeared healthy. The GATA1-CAR survival advantage was statistically significant (P < 0.01; n = 6 for each strain).
Discussion
Expression of viral receptor on normally nonpermissive cells has the potential to attenuate pathogenesis by diverting virus away from vulnerable tissues. Erythrocytes are uniquely suited for service as viral decoy targets, because they perfuse all tissues of the body but cannot themselves act as hosts for viral replication. CVB and CAR were chosen as appropriate candidates for the evaluation of this strategy. The GATA1-CAR model presented here demonstrated that CAR-expressing erythrocytes protected target tissues from CVB and ultimately conferred a survival advantage.
The GATA1-CAR mouse was generated by putting a CAR transgene under the control of the hematopoietic cell-specific GATA-1 promoter. This transgene resulted in the expression of transgenic CAR specifically on erythrocytes and platelets. CVB recognized this CAR and adhered to the erythrocyte surface (Fig. 1), and this interaction directly neutralized multiple serotypes of CVB (Fig. 2). When GATA1-CAR mice were challenged with CVB, virus was captured by circulating erythrocytes, and viremia was consequently reduced. The abatement of viremia correlated with significant reductions in the viral loads of the heart, brain, and liver (Fig. 3). This protection of susceptible organs granted the GATA1-CAR mice a survival advantage, enabling them to resist a lethal viral challenge (Fig. 4).
Whereas the GATA1-CAR decoy erythrocytes diminished the viral titers in the blood and several organs, no reductions in virus were observed in the pancreas or spleen (Fig. 3). A possible explanation for this is that the exocrine pancreas expresses relatively high levels of CAR and is very susceptible to CVB (3). This susceptibility was reflected in our experiments, where the pancreas produced at least one order of magnitude more virus per gram than any other organ in both GATA1-CAR and WT mice. The GATA1-CAR erythrocytes were apparently unable to attenuate the spread of the virus through the exceptionally vulnerable acinar tissue. The spleen, on the other hand, is not thought to be particularly susceptible to CVB, but it is known to serve as a reservoir for erythrocytes (25). CVB titers may have been elevated there due to the large number of virus-coated RBCs within. Another possibility is that erythrocytes bearing CVB may have been captured by resident macrophages in the spleen, resulting in the concentration of virus there.
The variable success of the GATA1-CAR erythrocytes in protecting various organs revealed some interesting aspects of the pathogenesis of CVB in mice. The devastation of the exocrine pancreas apparently was not the proximal cause of death in these animals, because both groups had equal amounts of virus in this tissue, and histopathologic analysis confirmed destruction of the acinar tissue in all animals (data not shown). Rather, it appears that high-level infection of other organs such as heart, liver, and brain correlated with early mortality. These findings illustrate how this type of transgenic model can yield insights into the importance of viremia and the impact of viral dissemination to various target organs.
Data presented here suggest that GATA1-CAR erythrocytes decrease CVB pathogenicity principally by direct viral neutralization (Fig. 2). This finding corresponds to studies of poliovirus, an enterovirus that is closely related to CVB, that show that the receptor-binding site of the poliovirus capsid undergoes an irreversible conformational change upon interaction with a soluble form of poliovirus receptor. This conversion prevents subsequent infection of target cells (26, 27). Soluble anti-CAR Fc fragment has similarly been found to neutralize CVB (28). The transgenic receptors present on GATA1-CAR erythrocytes likely function in an analogous manner to trigger irreversible conversion of the recognition sites on the viral capsid. In vivo, resident macrophages of the liver and spleen also may contribute to the clearance of the virus, either by stripping virus from erythrocyte surfaces or by phagocytosing virus-coated erythrocytes. However, if reticuloendothelial clearance did occur, this did not result in any measurable anemia, because hematocrits were equivalent across infected GATA1-CAR and WT groups (data not shown).
This study was designed to evaluate whether expression of viral receptor by erythrocytes could attenuate viral infection. These data show that the GATA1-CAR transgene was successful in creating a pervasive and perpetual viral sink that diverted virus from target organs and thus impeded CVB pathogenesis. This approach has several advantages over comparable techniques such as antibody- or soluble receptor-mediated neutralization. The use of viral receptor as the capture reagent, rather than a specific antibody, circumvents the problems of serotype exclusivity and antigenic shift. Although viruses often alter their antigens to avoid recognition by antibodies, a mutation that would evade capture by erythrocyte-expressed receptor would require modification of the viral receptor recognition site, a change that would likely foil infection of host cells. Moreover, the stable expression of receptor on the surface of erythrocytes yields a dramatic improvement over the relatively short half-life of soluble viral receptors. Injected, soluble anti-CAR Fc fragment that is optimized for longevity has an estimated half-life of only 33 h (28); compare this with the average erythrocyte lifespan of 120 days. Furthermore, although soluble receptor has the potential to diffuse into tissues and interfere with the normal functions of the protein, erythrocyte-expressed receptor will be largely confined to the interior of blood vessels and therefore be less likely to cause incidental complications.
The strategy of redirecting virus to erythrocytes through receptor presentation may prove to be broadly effective, and models that exchange CAR for other viral receptors should be explored. Although the mechanism of action in the case of the CAR and CVB model appears to be direct viral inactivation, the technique could prove effective against other viruses, bacteria, and toxins that firmly bind, even if they are not inactivated by their receptor. Erythrocyte-expressed receptor could sequester these invaders from their target tissues and mark them for clearance by the reticuloendothelial system.
The most apparent barrier to clinical implementation of this technique as presented is that it required creation of a transgenic animal. Although emerging methods for the growth of genetically modified erythrocytes (29, 30) may eventually enable production of receptor-bearing erythrocytes for infusion, the primary purpose of the GATA1-CAR mouse was not to propose genetic manipulation as an antiviral therapy. Rather, the principal goal was to evaluate the efficacy of erythrocyte-presented receptors. The major asset of this model is that it set a benchmark for the potential of receptor-bearing erythrocytes to attenuate disease. This same effect should be achievable by nontransgenic methods. An advantage of using erythrocytes is that they are relatively easy to collect, manipulate, and transfuse into subjects, and a variety of methods will allow the coating of WT erythrocytes with viral receptors either ex vivo or in vivo. As has been shown for CVB and many other viruses, including poliovirus, HIV, Epstein-Barr virus, measles, rhinovirus, and retrovirus (27, 31-36), transmembrane expression of the host receptor is not required for viral binding. Receptor that is mounted on the erythrocyte surface by external methods should prove, in many cases, to be as effective as transgenically expressed protein.
Recombinant receptor proteins may be directly affixed to erythrocyte surfaces by using chemical crosslinkers (37) or be made to externally insert themselves into erythrocyte membranes by fusing them to a glycosyl-phosphatidylinisotol anchor (38, 39). Biotinylation of erythrocytes is also readily achieved, and biotinylated erythrocytes can be safely reinfused into humans (40-42). This procedure would allow specific conjugation and multimerization of biotinylated viral receptors on the erythrocyte surface by using avidin. Another promising technique is to use heteropolymers that comprise viral receptor fused to an antibody fragment specific for erythrocyte-expressed membrane proteins. These could be injected intravenously to mount the viral receptor on the surface of RBCs. Deliberate construction of these reagents should avoid clearance by the immune system and permit the receptor to persist on the erythrocyte surface for the normal life of the cell. We have successfully used heteropolymers composed of two antibodies to clear phage from the bloodstream (43) and expect that receptor-antibody heteropolymer also will be effective.
Regardless of the method used to coat the erythrocytes, transfusion of these receptor-bearing cells could be used as an acute treatment for infection and poisoning, or they could be administered prophylactically to create a long-lived barrier to pathogens and toxins. The aforementioned techniques should be investigated with the goal of reproducing the GATA1-CAR antiviral effects in WT animals. These methods will, we hope, emulate the efficacy and persistence of transgenically expressed receptor and ultimately allow clinically beneficial implementation of this technique in humans.
Acknowledgments
We thank S. Orkin (Children's Hospital, Dana-Farber Cancer Institute, Harvard Medical School, Boston) for his advice and the gift of the pGATA-1 plasmid; S. Jones and the UMassMed Transgenic Animal Modeling Core for their work in production of the transgenic chimeras; S. Pincus (Elusys Therapeutics, Pine Brook, NJ) for production of the anti-CVB4 antibody; and N. Shenoy for critical review of this manuscript. This work was supported by National Institutes of Health Grant AI 49309 (to R.W.F.), National Institute of Allergy and Infectious Diseases Regional Center of Excellence Grant AI 057159 (to R.W.F.), and Diabetes Endocrinology Research Grant DK 32520 (to R.W.F.).
Author contributions: D.R.A. and R.W.F. designed research; D.R.A. and A.M.C. performed research; D.R.A. and A.M.C. contributed new reagents/analytic tools; D.R.A. and R.W.F. analyzed data; and D.R.A. wrote the paper.
Abbreviations: CAR, coxsackie and adenovirus receptor; CVB, coxsackievirus group B; GATA-1, globin transcription factor 1; PFU, plaque-forming unit.
References
- 1.Kearney, M. T., Cotton, J. M., Richardson, P. J. & Shah, A. M. (2001) Postgrad. Med. J. 77, 4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitton, J. L. (2002) Springer Semin. Immunopathol. 24, 201-213. [DOI] [PubMed] [Google Scholar]
- 3.Mena, I., Fischer, C., Gebhard, J. R., Perry, C. M., Harkins, S. & Whitton, J. L. (2000) Virology 271, 276-288. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz, M. S., La Cava, A., Fine, C., Rodriguez, E., Ilic, A. & Sarvetnick, N. (2000) Nat. Med. 6, 693-697. [DOI] [PubMed] [Google Scholar]
- 5.Tracy, S., Hofling, K., Pirruccello, S., Lane, P. H., Reyna, S. M. & Gauntt, C. J. (2000) J. Med. Virol. 62, 70-81. [DOI] [PubMed] [Google Scholar]
- 6.Bergelson, J. M., Modlin, J. F., Wieland-Alter, W., Cunningham, J. A., Crowell, R. L. & Finberg, R. W. (1997) J. Infect. Dis. 175, 697-700. [DOI] [PubMed] [Google Scholar]
- 7.Bergelson, J. M., Krithivas, A., Celi, L., Droguett, G., Horwitz, M. S., Wickham, T., Crowell, R. L. & Finberg, R. W. (1998) J. Virol. 72, 415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergelson, J. M., Cunningham, J. A., Droguett, G., Kurt-Jones, E. A., Krithivas, A., Hong, J. S., Horwitz, M. S., Crowell, R. L. & Finberg, R. W. (1997) Science 275, 1320-1323. [DOI] [PubMed] [Google Scholar]
- 9.Carson, S. D., Chapman, N. N. & Tracy, S. M. (1997) Biochem. Biophys. Res. Commun. 233, 325-328. [DOI] [PubMed] [Google Scholar]
- 10.Tomko, R. P., Xu, R. & Philipson, L. (1997) Proc. Natl. Acad. Sci. USA 94, 3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einfeld, D. A., Schroeder, R., Roelvink, P. W., Lizonova, A., King, C. R., Kovesdi, I. & Wickham, T. J. (2001) J. Virol. 75, 11284-11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wickham, T. J., Mathias, P., Cheresh, D. A. & Nemerow, G. R. (1993) Cell 73, 309-319. [DOI] [PubMed] [Google Scholar]
- 13.Crowell, R. L. & Philipson, L. (1971) J. Virol. 8, 509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smyth, M. S. & Martin, J. H. (2002) Mol. Pathol. 55, 214-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossmann, M. G. (1994) Protein Sci. 3, 1712-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He, Y., Chipman, P. R., Howitt, J., Bator, C. M., Whitt, M. A., Baker, T. S., Kuhn, R. J., Anderson, C. W., Freimuth, P. & Rossmann, M. G. (2001) Nat. Struct. Biol. 8, 874-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fechner, H., Haack, A., Wang, H., Wang, X., Eizema, K., Pauschinger, M., Schoemaker, R., Veghel, R., Houtsmuller, A., Schultheiss, H. P., Lamers, J. & Poller, W. (1999) Gene Ther. 6, 1520-1535. [DOI] [PubMed] [Google Scholar]
- 18.Nagai, M., Yaoita, E., Yoshida, Y., Kuwano, R., Nameta, M., Ohshiro, K., Isome, M., Fujinaka, H., Suzuki, S., Suzuki, J., et al. (2003) Lab. Invest. 83, 901-911. [DOI] [PubMed] [Google Scholar]
- 19.Okegawa, T., Pong, R. C., Li, Y., Bergelson, J. M., Sagalowsky, A. I. & Hsieh, J. T. (2001) Cancer Res. 61, 6592-6600. [PubMed] [Google Scholar]
- 20.Visvader, J. E., Fujiwara, Y. & Orkin, S. H. (1998) Genes Dev. 12, 473-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDevitt, M. A., Fujiwara, Y., Shivdasani, R. A. & Orkin, S. H. (1997) Proc. Natl. Acad. Sci. USA 94, 7976-7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gebhard, J. R., Perry, C. M., Harkins, S., Lane, T., Mena, I., Asensio, V. C., Campbell, I. L. & Whitton, J. L. (1998) Am. J. Pathol. 153, 417-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henke, A., Huber, S., Stelzner, A. & Whitton, J. L. (1995) J. Virol. 69, 6720-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber, S. A. & Job, L. P. (1983) Adv. Exp. Med. Biol. 161, 491-508. [DOI] [PubMed] [Google Scholar]
- 25.Neubauer, J. A. (2001) J. Appl. Physiol. 90, 1593-1599. [DOI] [PubMed] [Google Scholar]
- 26.Racaniello, V. R. (1996) Structure 4, 769-773. [DOI] [PubMed] [Google Scholar]
- 27.Zibert, A., Selinka, H. C., Elroy-Stein, O. & Wimmer, E. (1992) Virus Res. 25, 51-61. [DOI] [PubMed] [Google Scholar]
- 28.Yanagawa, B., Spiller, O. B., Proctor, D. G., Choy, J., Luo, H., Zhang, H. M., Suarez, A., Yang, D. & McManus, B. M. (2004) J. Infect. Dis. 189, 1431-1439. [DOI] [PubMed] [Google Scholar]
- 29.Daley, G. Q. (2003) Ann. N.Y. Acad. Sci. 996, 122-131. [DOI] [PubMed] [Google Scholar]
- 30.Puthenveetil, G., Scholes, J., Carbonell, D., Xia, P., Qureshi, N., Zeng, L., Li, S., Yu, Y., Hiti, A. L., Yee, J. K. & Malik, P. (2004) Blood 104, 3445-3453. [DOI] [PubMed] [Google Scholar]
- 31.Overbaugh, J., Miller, A. D. & Eiden, M. V. (2001) Microbiol. Mol. Biol. Rev. 65, 371-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varior-Krishnan, G., Trescol-Biemont, M. C., Naniche, D., Rabourdin-Combe, C. & Gerlier, D. (1994) J. Virol. 68, 7891-7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, X. & Bergelson, J. M. (1999) J. Virol. 73, 2559-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Diamond, D. C., Finberg, R., Chaudhuri, S., Sleckman, B. P. & Burakoff, S. J. (1990) Proc. Natl. Acad. Sci. USA 87, 5001-5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore, M. D., Cannon, M. J., Sewall, A., Finlayson, M., Okimoto, M. & Nemerow, G. R. (1991) J. Virol. 65, 3559-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staunton, D. E., Gaur, A., Chan, P. Y. & Springer, T. A. (1992) J. Immunol. 148, 3271-3274. [PubMed] [Google Scholar]
- 37.Coller, B. S., Springer, K. T., Beer, J. H., Mohandas, N., Scudder, L. E., Norton, K. J. & West, S. M. (1992) J. Clin. Invest. 89, 546-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Premkumar, D. R., Fukuoka, Y., Sevlever, D., Brunschwig, E., Rosenberry, T. L., Tykocinski, M. L. & Medof, M. E. (2001) J. Biol. Chem. 82, 234-245. [DOI] [PubMed] [Google Scholar]
- 39.Finberg, R. W., White, W. & Nicholson-Weller, A. (1992) J. Immunol. 149, 2055-2060. [PubMed] [Google Scholar]
- 40.Cordle, D. G., Strauss, R. G., Lankford, G. & Mock, D. M. (1999) Transfusion 39, 1065-1069. [DOI] [PubMed] [Google Scholar]
- 41.Mock, D. M., Lankford, G. L., Widness, J. A., Burmeister, L. F., Kahn, D. & Strauss, R. G. (1999) Transfusion 39, 156-162. [DOI] [PubMed] [Google Scholar]
- 42.Strauss, R. G., Mock, D. M., Widness, J. A., Johnson, K., Cress, G. & Schmidt, R. L. (2004) Transfusion 44, 871-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Repik, A., Pincus, S. E., Ghiran, I., Nicholson-Weller, A., Asher, D. R., Cerny, A., Casey, L. S., Jones, S. M., Jones, S. N., Mohamed, N., et al. (2005) Clin. Exp. Immunol. 140, 230-240. [DOI] [PMC free article] [PubMed] [Google Scholar]