Abstract
Purpose of Review
This review was conducted to discuss the etiology of autism in the light of current information, to draw attention to the fact that defects in different biological mechanisms cause autism, and to examine the effectiveness of dietary interventions and supplements in relieving ASD symptoms.
Recent Findings.
Autism spectrum disorder (ASD) is an extremely heterogeneous condition characterized by delays in reciprocal social interaction and communication skills, stereotyped behaviors, and a narrowed range of interests and limited activities. Comorbid conditions such as cognitive impairment, epilepsy, psychiatric diseases, and behavioral symptoms such as impaired social communication, repetitive behaviors, lack of interest in the environment, nutritional disorders, gastrointestinal diseases and abnormal (dysbiotic) states, sleep disorders, and dysmorphism are frequently encountered in individuals with ASD. Although nutrition is one of the environmental factors affecting ASD, it can also be effective in alleviating the behavioral and gastrointestinal symptoms of ASD. Various dietary models (GFCF diet, low glycemic index diet, ketogenic diet, specific carbohydrate diet, Mediterranean diet, GAPS, Feingold, Candida body ecology, allergy elimination diets, etc.) and supplements (vitamin D, polyunsaturated fatty acids, probiotics and prebiotics, phytochemicals) can be used to alleviate symptoms in individuals with ASD.
Summary
The effectiveness and reliability of dietary interventions in individuals with ASD are a matter of significant debate, and the evidence for these practices is limited. Furthermore, there is no consensus on establishing an ideal nutritional model for individuals with ASD.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13668-025-00655-y.
Keywords: Autism spectrum disorder, Gluten; Free; Casein; Free diet; Ketogenic diet; Probiotics, Gut; Brain axis
Introduction
Autism spectrum disorder (ASD) is characterized by repetitive and restricted behaviors and deficits in social communication [1]. DSM- 5 criteria are used to easily diagnose ASD. DSM- 5 defines ASD according to degrees of severity based on deficits in social communication and restricted and repetitive behaviors [2–4]. According to data from the National Center for Health Statistics, it has been suggested that 1 in 36 children may have autism spectrum disorder [5]. WHO reports that 1 in every 100 children is diagnosed with ASD [6]. According to estimates from the Centers for Disease Control and Prevention’s Autism and Developmental Disability Monitoring Network, an average of 1 in 44 children is diagnosed with ASD [4]. This rate is thought to be the same across all racial, ethnic, or socioeconomic backgrounds; however, gender differences exist. ASD is four times more common in boys than girls [7] and it incurs significant costs for managing or supporting individuals with ASD, whether children or adults [8].
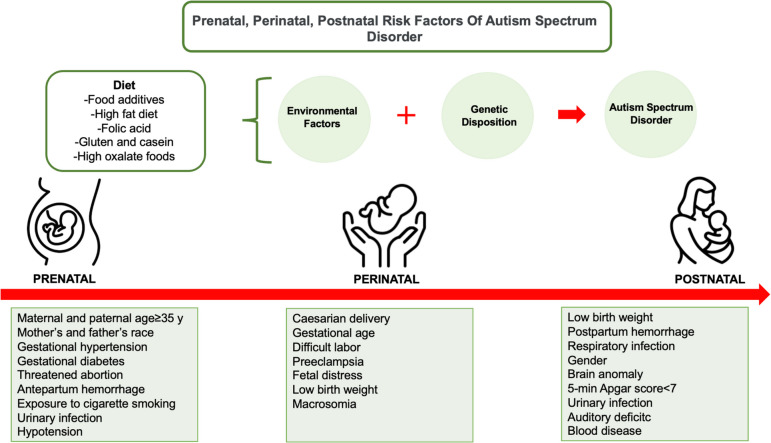
Although the exact cause of ASD is not fully known, the most widely accepted causal hypothesis is that it arises from the interaction of multiple factors. The underlying causes of autism include genetic predisposition, epigenetics, neurological factors, metabolic factors, psychosocial factors, prenatal/postnatal factors, mitochondrial dysfunction, cellular stress, environmental and immunological-inflammatory factors, and the interactions of these factors with each other [9, 10]. These are thought to lead to dysfunctional neuronal pathways, abnormal synaptogenesis, neurotransmitter imbalance and neuronal connectivity [11]. The etiology of ASD is complex and still not fully understood [12]. Prenatal, perinatal, and postnatal risk factors in the etiology of ASD are shown in Fig. 1 [12, 14–16]. It is highlighted that generally some factors related to the mother’s nutrition during pregnancy (such as folic acid and fatty acids) may be effective in the development of ASD [12]. In autism spectrum disorder (ASD), the gut-brain axis theory suggests that the nutritional program extending from early period of life to pregnancy may affect cognitive function and cause ASD in genetically susceptible individuals. Even though the impact of the microbiota-gut-brain axis in ASD has been frequently discussed, people have paid a great interest in nutritional interventions [13].
Fig. 1.
Prenatal, perinatal, postnatal risk factors of autism spectrum disorder [11, 13–15]
This review was conducted to discuss the etiology of autism in the light of current information, to draw attention to the fact that defects in different biological mechanisms cause autism, and to examine the effectiveness and side effects of dietary interventions applied in alleviating ASD symptoms, their applicability and dietary supplements.
Nutritional Approaches in Autism Spectrum Disorder
Many children with ASD may prefer to consume only certain foods and may have food selectivity [17]. A study reported that 30–84% of children with ASD had difficulties in food intake [18]. Zinc, calcium, iron, selenium, iodine, methyl-cobalamin, vitamin A, thiamine, pantothenic acid and niacin are the most common nutrient deficiencies in individuals with ASD [19]. Individuals with ASD suffer from many gastrointestinal system complaints such as bloating, abdominal pain, constipation, chronic diarrhea, leaky gut syndrome and gastroesophageal reflux [17]. Gastrointestinal complaints are observed in 9% to 91% of individuals with ASD [20].
Nutrition is thought to be one of the environmental factors that influence the symptoms of ASD [21]. Various dietary interventions including GFCF diet, GAPS, FODMAP diet, ketogenic diet, Mediterranean diet, Feingold, Candida body ecology, allergy elimination diets, specific carbohydrate diet, etc.) and supplements (vitamin D, polyunsaturated fatty acids, probiotics and prebiotics, phytochemicals) are used to alleviate these symptoms. Additionally, some nutrients such as camel milk and nutritional supplements such as omega- 3 fatty acids and probiotics may have an effect in alleviating the symptoms of ASD. Some nutritional approaches are shown in Fig. 2 [13, 19, 22, 23]. The gut microbiota of children with ASD differs from the microbiota of children without ASD. Gut microbiota affects gastrointestinal symptoms, behavioral change and mood. Diet affects gastrointestinal functions through its ability to alter the gut microbiome. These nutritional interventions bring both advantages and disadvantages. Restrictive diet may cause food selectivity and nutrient deficiency in individuals with ASD [24].
Fig. 2.
Nutritional approaches in autism spectrum disorder [13, 19, 22, 23]
Gluten-Free-Casein-Free Diet
Gluten is found in rye, wheat, and barley, while casein proteins are found in milk. Both can have inflammatory effects on the immune system [25]. Gluten and casein induce the expression of lgA and lgG antibodies in children with ASD and may exacerbate its symptoms. Another theory is the “opioid excess” theory, which suggests that gluten and casein peptides are metabolized in the intestine into structurally similar short-chain peptides called gluteomorphins and casomorphins in the behavioral problems seen in these children [16]. The small intestinal mucosa acts as a luminal barrier that prevents such peptides from passing into the bloodstream. In individuals with ASD, intestinal permeability increases due to inflammation and the luminal barrier is altered. The passage of casein, gluten and their metabolites into the bloodstream and central nervous system increases. Increased passage of these metabolites contributes to the development of symptoms of ASD [26].
For all these reasons, some hypotheses suggest that nutritional interventions can improve cognitive function, communication skills, social behaviors, and learning ability [13]. Removal of gluten and casein peptides from the diet in GFCF diet intervention will decrease the possibility of stimulation of the opioid system and alleviate the symptoms of ASD [27].
Casein-free diets involve no milk and dairy products or products containing lactose. In gluten-free diet, gluten-free foods (such as vegetables, fruits, legumes, meat, eggs, and fish) are preferred instead of gluten-containing grains [28, 29]. On the other hand, GFCF diet is a diet protocol that combines casein-free diet and gluten-free diet [29]. Adams et al. [30] applied a gluten-free, soy-free, casein-free diet and vitamin and mineral supplements to individuals with ASD for 12 months. An increase in biomarkers of vitamin A, folic acid, B2, B5, B6, B12, EPA, DHA and coenzyme Q10 was detected in the intervention group. Dietary intervention has been reported to be effective in improving nonverbal IQ, ASD symptoms, and nutritional status in most individuals with ASD [30]. In another study, it was observed that applying a normal diet for six months and then a GFCF diet for another six months did not cause any behavioral changes in individuals with ASD [31]. Bavykina et al. [32] applied a GFCF diet to children with ASD for six months. They observed increased levels of specific IgG antibodies against casein in 79.5% of the children. They stated that there was an increase in IgG antigliadin antibodies in 19.3% of children who did not follow a gluten-free diet, and 40–50% of individuals with ASD had gluten intolerance [32]. Piwowarczyk et al. [26] applied a gluten-free diet to children with ASD for six months and reported that the intervention was not effective on their ASD symptoms, behavioral disorders, and intellectual abilities. Matthews and Adams [33] stated that the ketogenic diet and the GFCF diet were the dietary interventions that scored best on ASD symptoms compared to other dietary patterns. It is suggested that diets high in gluten and casein may be effective in the pathogenesis of leaky gut syndrome through the gut-brain axis and may synergistically exacerbate dysbiosis as a comorbidity in ASD [33]. However, this study is a retrospective study and is limited to parent-reported data. These results need to be supported by data from larger, prospective studies. In another study, male rats were given diets high in gluten and casein and it was reported that diets rich in gluten and casein may synergistically exacerbate dysbiosis by worsening leaky gut syndrome, by affecting the brain through the gut-brain axis. Both dietary patterns negatively affected the intestinal microbiota and increased intestinal permeability [34]. Another meta-analysis study reported that GFCF dietary intervention may reduce stereotyped behaviors in ASD and elevate the cognition level of children with ASD [35]. In another meta-analysis study, it was determined that GFCF diet had no effect on the basic symptoms, functional level and behavioral problems of children with ASD. Additionally, it was emphasized that dietary intervention may have negative effects on gastrointestinal symptoms [36]. This study provides stronger findings because it is a meta-analysis of randomized controlled trials and the sample size (n = 143) is relatively higher than other studies.
While there are studies showing that the GFCF diet offers effective results on ASD symptoms, there are also studies showing that it has no effect. These conflicting results in the literature indicate that more studies with larger sample sizes should be conducted in this area.
Ketogenic Diet
The ketogenic diet is a diet model that includes high fat, low carbohydrate, and adequate protein intake. In the classic ketogenic diet, the fat-carbohydrate ratio is 3:1/4:1. Since this ratio makes it difficult to comply with the diet, different ketogenic diet models have been developed such as modified Atkins diet, MCT ketogenic diet and low glycemic index ketogenic diet [37, 38]. Recently, the ketogenic diet has been used in the treatment of various neurological diseases [39–41]. Ketogenic diets elevate the levels of ATP and enzymes related to mitochondrial metabolic pathways and mitochondrial biogenesis. β-hydroxybutyrate, acetoacetate and acetone, known as ketone bodies, are used as the brain’s energy source in case of hunger. Ketone bodies prevent mitochondrial permeability and reduce reactive oxygen species (ROS). Therefore, ketone bodies have a neuroprotective effect on the central nervous system [42]. The ketogenic diet has been suggested to alleviate the symptoms of some neurological diseases by influencing the intestinal microbiota [38]. A systematic review reported that ketogenic diet to neurological patients positively affected the composition of the gut microbiota [43]. Mu et al. [44] applied the ketogenic diet to children with ASD for three months and observed changes in their blood parameters and behavioral problems. At baseline, the children had higher concentrations of galactose intermediates, N-acetylserotonin and trimethylamine N-oxide, and lower concentrations of selenium and 3-hydroxybutyrate than the control group. Circulating ketone bodies and acetylcarnitine and low selenium levels increased following ketogenic diet intervention. Selenium levels were negative correlated with behavior scores [44]. However, this is a pilot study, the sample size is limited and there is no control group. The results need to be confirmed by further studies. In another study, a modified ketogenic gluten-free diet intervention was applied to children with ASD for three months. Significant improvements were observed in the imitation, body use, fear and irritability subscales of the ADOS- 2 and the CARS- 2. It was emphasized that modified gluten-free ketogenic diet intervention with MCT supplementation may be an effective method to improve symptoms in ASD [45]. However, the sample size of this study is limited, and there is no control group. This makes it difficult to determine whether the results are solely attributable to the dietary intervention. More studies are needed to evaluate the effectiveness of the modified ketogenic diet. Another study conducted on BTBR rats with ASD revealed that ketogenic diet intervention changed the mitochondrial function and improved mitochondrial morphology in BTBR rats. Mitochondrial dysfunction and mitochondrial fragmentation play a role in the etiology of ASD [46]. Yu et al. [29] reported that GFCF diet and ketogenic diet significantly improved the basic symptoms of individuals with ASD. Another study revealed that ketogenic diet intervention reduced social deficits, repetitive behaviors, memory impairments, and inflammatory markers such as TNF-α, IL- 1β, and IL- 6 in BTBR rats [47]. In a case report, it was found that the patient’s behavioral problems improved in the first month of a 16-month ketogenic diet intervention in a six-year-old child with ASD symptoms, and 18 FDG PET decreased significantly in 12 months [48]. Epigenetic and synaptic abnormalities are the most important risk factors in ASD. A study conducted on rats revealed that in ASD, there was significantly lower histone acetylation and higher HDAC2 expression in the PFC. Four-week ketogenic diet intervention increased the transcription and histone acetylation of Grin2a and Grin2b in Shank3-deficient rats and ameliorate the decreased NMDAR synaptic function in PFC neurons. The ketogenic diet is reported to be a promising approach for social deficits seen in ASD by regulating gene expression and histone acetylation in the brain [49].
There are studies reporting that the ketogenic diet has a positive effect on ASD symptoms. However, some of the studies in the literature have low reliability due to limited sample size, lack of a control group, or being pilot studies. More studies with larger sample sizes are needed in this area to make a general conclusion.
Specific Carbohydrate Diet
Specific carbohydrate diet is thought to contribute to the alleviation of microbiota changes, comorbid gastrointestinal symptoms, and behavioral symptoms seen in some individuals with ASD. The specific carbohydrate diet was developed in the 1930 s for the treatment of gluten enteropathy. It has been later used for the treatment of Crohn’s disease, ulcerative colitis, chronic diarrhea and diverticulitis [50]. The aim of this dietary model is to alleviate malabsorption symptoms and prevent the proliferation of pathogenic microflora that results in dysbiosis [50, 51]. While monosaccharide sources such as fruits, some vegetables and honey are used as carbohydrate sources in the diet, complex carbohydrate sources (cereals, potatoes, dairy products, processed foods, and disaccharides including lactose, sucrose, and maltose) are limited [13, 50]. In children with ASD with gastrointestinal problems, implementation of a specific carbohydrate diet protocol has not been evaluated but is an intervention commonly used by many families without clinical guidance. However, further studies are needed to make generalizations on this subject [52]. Barnhill et al. [52] applied a specific carbohydrate diet to a four-year-old boy diagnosed with Fragile X Syndrome and ASD for four months, and they determined that this dietary intervention was well tolerated. Improvements were also observed in gastrointestinal symptoms and behavioral problems after the intervention [52]. Although the specific carbohydrate diet is a widely used dietary intervention, evidence published regarding the effectiveness or safety of this diet in patients with ASD is currently lacking [13].
Low Glutamate Diet
It is thought that some deficiencies in neurodevelopmental mechanisms may affect the symptoms of individuals with ASD. Neurotransmitters play critical roles in the development of the central and peripheral nervous system. Therefore, neurotransmitter dysfunctions take part in the pathophysiology of ASD [53]. Glutamate is one of the important stimulant neurotransmitters in the human brain. Glutamate receptors are involved in diverse cognitive and neuronal processes such as learning, memory, and synaptic system [54]. Glutamatergic system dysfunction lies at the root of neurotransmitter dysfunction in ASD. Two theories attract attention in glutamatergic system disorder. The first one is hyperglutamate theory, which accepts that glutamate levels are high in the serum and plasma of individuals with ASD, and the second one is the hypoglutamate theory, which states that there is dysfunction in glutamate receptors in ASD and glutamatergic agonists may have a positive effect on some of ASD symptoms [55].
Carvalho et al. [56] showed in their study that total N-acetylaspartate (tNAA) levels lowered in individuals with ASD and Gamma aminobutyric acid (GABA) was associated with communication skills and developmental scores, which are the main symptoms of ASD. Naaijen et al. [57] reported that N-acetylaspartate (NAA) and glutamate play a role in striatal volume in individuals with ASD, and low NAA in ASD indicates decreased neuronal integrity and impaired neuronal functioning. Siegel-Ramsay et al. [58] conducted a study of adults with ASD and found that there was a significant negative correlation between increased glutamine concentrations and decreased functional connectivity in the insular, limbic and parietal regions of the dorsal anterior cingulate cortex (dACC). A study conducted on children with ASD in Egypt reported that children with ASD had higher levels of serum glutamate and this parameter may help diagnosing ASD in the early period [59]. In a study conducted on rats with ASD, it was observed that the NAA/total creatine (tCr) ratio, glutamate/GABA ratio and tCr level in the prefrontal cortex were substantially associated with the lack of social behavior in rats [60]. A study investigating d-aspartate metabolism in the brain and serum of rats with ASD that the levels of mRNA of d-aspartate oxidase, which encodes the enzyme, responsible for d-aspartate catabolism, significantly lowered [61].
A low glutamate diet acts on N-methyl-D-aspartate receptors. It reduces the negative effects of dietary amino acids such as aspartate and glutamate, which cause excitotoxicity in the long term, and can be used in the treatment of some diseases. In a low glutamate diet, the free or esterified form of glutamate is eliminated from the diet. Foods such as soy sauce, old cheese and mushrooms can be given as examples of foods containing free glutamate. The free form of aspartate is also limited in this dietary model because it activates N-methyl-D-aspartate receptors. Also, it is recommended for individuals with ASD to consume a diet rich in antioxidants to protect them from oxidative stress caused by glutamate excitotoxicity [62]. There has been no study investigating the efficiency of a low glutamate diet in ASD in the literature. The effect of a low glutamate diet on high glutamate levels in the serum and plasma of individuals with ASD is unknown. More advanced clinical studies are needed in this field.
Low FODMAP Diet
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) are a heterogeneous group of poorly absorbed short-chain carbohydrates that can be fermented in the small intestine or large intestine. FODMAPs include lactose (milk and dairy products), fructose (fruits and high fructose corn syrup), fructans (vegetables, fruits, and grains), galactans (legumes and vegetables) and sugar alcohols (fruits, vegetables polyols, and sorbitol) [63]. The low FODMAP diet is a dietary model that has long been used to improve symptoms such as constipation, diarrhea, bloating, and abdominal pain. It is thought that gastrointestinal symptoms observed in ASD are associated with carbohydrate metabolism. In children, a low FODMAP diet may alleviate symptoms of ASD, including irritable bowel syndrome [51, 64]. Nogay et al. [65] applied a low FODMAP diet to children with ASD for two weeks. They observed that the individuals’ gastrointestinal syndromes alleviated significantly and there was no significant difference in behavioral problems [59]. In another case report, the ketogenic diet and the low FODMAP diet were applied to a 17-year-old girl with ASD. It was observed that the patient with ASD tolerated the low-FODMAP diet better than the ketogenic diet, and the low-FODMAP diet significantly improved neurological, gastrointestinal and metabolic symptoms [66].
The number of studies in the literature on the effectiveness of the FODMAP diet on ASD symptoms is quite limited. Longer-term studies with larger sample sizes are needed to make generalizations.
Mediterranean Diet
The Mediterranean Diet contains high amounts of vegetables, fruits, olive oil, legumes, and oilseeds, moderate amounts of fish, dairy products, and wine, and small amounts of red meat and saturated fat [67]. This diet ensures adequate intake of n- 3 fatty acids and B vitamins, which have protective effects on neurodevelopment in individuals with depression or ASD [64]. There has been no study investigating the relationship between the Mediterranean diet and ASD in the literature. However, some studies have examined the effect of the Mediterranean diet on the neurodevelopmental system and its effect on attention deficit and hyperactivity disorder (ADHD).
In a study conducted on 1471 mother–child pairs in China, it was observed that the mother’s adherence to the Mediterranean diet during pregnancy was associated with lower neurodevelopmental risk in children [68]. In another study, high adherence to the Mediterranean diet in the first trimester of pregnancy was associated with higher communication skills in 6-month-old infants [69]. Ríos-Hernández et al. [70] showed that adherence to the Mediterranean diet was lower in children with ADHD. In another study, the Mediterranean diet and n- 3 fatty acid supplements were given to the individuals with ADHD. It was observed that n- 3 fatty acid was associated with less pronounced impulsive behavior. Applying the Mediterranean diet may improve the results, but further studies are needed [71].
The number of studies on the effect of the Mediterranean diet on ASD symptoms is limited. In addition, studies are mostly on compliance with the Mediterranean diet. Further studies should be conducted to investigate the effectiveness of the Mediterranean diet on ASD.
Low Glycemic Index Diet
The glycemic index is defined as a scale that ranks the effect of a carbohydrate-containing food on blood glucose compared to a reference food (glucose). If the glycemic index of a food is ≤ 55, it is grouped as low glycemic index, and ≥ 70 as high glycemic index [72]. Low glycemic index diet is used for body weight regulation and glycemia control in individuals with diabetes mellitus. In addition, this dietary pattern has an effect on brain functions. For this reason, it is used to improve cognitive functions (such as attention and memory) in individuals or to modulate abnormal brain functions in ASD, epilepsy and other neurological disorders [73].
Currais et al. [74] applied low-and high-glycemic index diets to BTBR rats. They reported that while the low glycemic index diet lowered levels of advanced glycation end products (AGEs) and other inflammatory markers in plasma and brain, the high glycemic index diet affected many parameters that were thought to lead to ASD.
There are very few studies in the literature investigating the effect of low glycemic index on ASD. This makes it very difficult to draw any conclusions about the effect of low glycemic index diets on ASD. More studies with larger samples are needed in this area.
Other Dietary Interventions, Nutrients and Food Additives
In individuals with ASD, some substances such as oxalates may be associated with gastrointestinal dysfunction, neurological development, and disruption of the central nervous system structure and functionality in the nervous system [16, 75]. The high level of oxalate in the blood serum and urine of individuals with ASD suggests that oxalate may be effective in in neurobiological mechanisms of ASD [16, 22]. ADI (acceptable daily intake) of oxalates for an adult is 250 mg/g. In the Western diet, daily dietary intake of oxalate can reach up to 1000 mg/g. For individuals with ASD, it is recommended to restrict foods with high oxalate content and daily dietary oxalate intake to 40–50 mg. Since limiting foods such as figs, green apples, kiwis, beets, spinach, and tangerines, which are rich in oxalates, may cause micronutrient deficiency, vitamins A and E, magnesium, calcium and zinc supplements are recommended for individuals with ASD who follow a low oxalate diet [22]. Although dietary models that restrict oxalate have been developed for ASD, there is no evidence-based study yet on the effectiveness of this diet in ASD [19]
It has been said for many years that yeasts play a role in the behavior and learning problems of children with ASD. Excessive antibiotic exposure causes intestinal dysbiosis and leads to the proliferation of C. albicans yeasts in the intestine. Candida can cause ASD by allowing toxic substances to pass into the bloodstream and affecting the brain functions and immune system. However, this condition is not seen in all children with ASD. For this reason, it is not correct to conclude that Candida causes ASD. Candida species proliferate excessively in the intestines of individuals with ASD [76]. Iovene et al. [77] found in their study that 57.5% of individuals with ASD had the aggressive form of Candida species. They emphasized that carbohydrate consumption was also effective in the increase of Candida species in children with ASD. In a study including children with ASD, C. albicans, C. dubliniensis and C. tropicalis species were detected in the stools of the majority of children with ASD who consumed a carbohydrate-free diet. C. parapsilosis and C. albicans were found in the stools of children with ASD fed a carbohydrate-containing diet, and it was reported that carbohydrate consumption may differ the Candida species in the stools of children with ASD [78]. A study conducted on rats indicated that carbohydrates and sugar alcohols caused growth of C. Albicans [79]. The anti-candida diet aims mainly to reduce carbohydrates. This diet is a dietary pattern that limits highly refined carbohydrates, added sugars (including sugar, honey, jam), dairy products, and cured and red meats. However, there is no study on whether this dietary model can alleviate symptoms in individuals with autism spectrum disorder [76].
The Feingold diet does not include foods containing grapes, almonds, oranges, apples, peaches, strawberries, pickled cucumbers, artificial food colorings, sweeteners, flavorings and preservatives as well as foods containing BHA, TBHQ, BHT and salicylates [23, 33]. The Feingold diet is one of the oldest diet models used in the management of ADHD [80]. This is an elimination diet used for children with ASD, as well [23]. Abdallah et al. [81] applied the Feingold diet to children with ASD and gave them a language education. Insignificant improvements in receptive language skills and language training performance were seen after the intervention [81]. In a study comparing 13 dietary interventions in ASD, it was found that the Feingold diet did not have any side effect and ranked first in six areas related to behavior, including aggression, sensory sensitivity, hyperactivity, irritability, falling asleep and staying asleep, anxiety, attention, and sleep. In terms of cognition, it was in the top three when compared to other dietary interventions [33].
The basic principles of the GAPS diet are the formation of an optimal microbiota for the proper functioning of the body [82]. This dietary pattern is a nutritional model that aims to improve the damaged intestinal microbiota and is used in ASD, ADHD, depression, dyspraxia, dyslexia, schizophrenia, obsessive compulsive disorder, epilepsy, bipolar disorder, and many other conditions that affect brain functions [83]. GAPS diet frequently includes high-density, easily digestible foods with high nutritional values. All foods that are difficult to digest and negatively affect the microbiota are eliminated from this diet. All food is prepared in house with fresh natural ingredients. Fermented foods are widely used in this nutritional model [82], in which grains (wheat, barley, oats, corn, rice), grain products (bread, pasta, etc.), starchy vegetables, milk, sugar, artificial sweeteners, and packaged and processed products are prohibited and home-cooked meat, fish, poultry, juices and soups of vegetables with low starch content, homemade yoghurt, kefir, ginger and chamomile tea are recommended. As symptoms alleviate, organic eggs, ghee oil, avocado, cold-pressed olive oil, freshly squeezed fruit juices, oilseeds and fruit purees are added to the diet [83].
Ābele et al. [84] applied a specific carbohydrate diet/GAPS diet to children with ASD and those having an unclear diagnosis, and n- 3 essential fatty acids, probiotics, ascorbyl palmitate vitamin C and vitamin D were supplemented. An important decrease was observed in the gastrointestinal system symptoms of the intervention and control groups. Specific carbohydrate diet/GAPS and dietary supplements may be a secure method to improve some symptoms of ASD in children [84]. However, there is no enough evidence in the literature for the GAPS diet and further randomized controlled studies are needed.
The number of studies investigating the effectiveness of other dietary interventions such as anti-candida, Feingold, GAPS diet on ASD symptoms is quite limited. More comprehensive studies are needed to investigate the long-term effects of these diets and interventions and to what extent they are useful in clinical practice.
While the lactose content of camel milk is lower than the content of cow milk, its MCT, vitamin (A, E C) and mineral (Ca, Fe, Mg, Cu, Zn, K) content is higher [19, 85, 86]. Low plasma glutathione (GSH) and cysteine levels are associated with ASD. It has been shown that camel milk alleviates the clinical symptoms of ASD by elevating glutathione peroxidase (GSH-Px) and superoxide dismutase levels [87]. In addition, it may have beneficial effects by reducing oxidative stress, which is considered one of the etiologies of ASD [86].
In the meta-analysis study conducted by Kandeel et al. [88], it was reported that camel milk had a positive effect on the social behavior of children with ASD, but did not cause a statistically significant difference in CARS scores. This study offers stronger results because it is a meta-analysis of randomized controlled trials and the sample size is higher than other studies. In another study, children with ASD were supplemented with raw camel milk, cooked camel milk, and placebo (cow’s milk) for two weeks. Both raw and cooked camel milk reduced neuroinflammation in these children and increased their Social Responsiveness Scale (SRS) score and gastrointestinal symptoms [89]. Hamzawy et al. [90] stated that camel milk supplementation in pregnant female rats regulated inflammatory and apoptotic pathways and may be a potential beneficial agent for ASD.
Food additives are substances added to foods to provide taste, appearance and other desirable properties [91]. One of the most current debates in this field is the negative impact of artificial food dyes on children’s behaviors [92]. One concern the researchers have about the adverse neurobehavioral effects of food additives is that artificial food dyes are among the etiological factors in childhood hyperactivity. In a study conducted on individuals with ADHD, subjects in the intervention group consumed chocolate chip cookies containing artificial food dyes three days a week for two weeks. It was observed that artificial food dyes caused an increase in inattention symptoms in these individuals [93]. It has not been fully proven that food dyes cause ASD. However, high doses of food additives may cause pharmacologically an adverse effect in a small percentage of children with ASD [92].
Dietary Supplements
In some children with ASD, inadequate nutritional intake, vitamin-mineral and trace element deficiencies, low vitamin levels in the body and brain, and the formation of antibodies against vitamin carriers in the blood–brain barrier are observed. Gastrointestinal inflammation seen in ASD increases the risk of nutrient deficiency [94]. Esteban-Figuerola et al. [95] reported that individuals with ASD consume lower amounts of vitamin D, calcium and dairy products and higher amounts of fruits, vegetables, protein, selenium, phosphorus, riboflavin, thiamine, and vitamin B12. Nutritional interventions to alleviate symptoms of ASD include vitamins, minerals, polyunsaturated fatty acids and probiotic supplements in addition to dietary approaches [23].
Vitamin D
Vitamin D is a fat-soluble steroid hormone that is essential for the human body. Vitamin D3 is synthesized in the skin as a result of the reaction of 7-dehydrocholesterol with ultraviolet B (UVB) rays [96, 97]. In recent years, it has been frequently discussed that vitamin D deficiency is one of the environmental factors that cause ASD. Although the underlying mechanisms between vitamin D and ASD are not clearly clarified, there is some biological evidence showing the relationship between them [96]. Vitamin D plays an important role in neurodevelopment. Neurotransmission, neural cell proliferation, immune functions and oxidative stress are among the main mechanisms mediated by vitamin D in the central nervous system [98]. Vitamin D has important effects on brain development and functions, such as proliferation, immune modulation, and regulation of synaptic plasticity. Considering all these factors, vitamin D deficiency during pregnancy and early childhood can negatively affect brain development of children and cause many neuropsychological disorder, especially ASD [96].
Petruzzelli et al. [99] found that serum 25-hydroxylvitamin D (25(OH)D) concentration was lower in children with ASD than in those without ASD. They stated that vitamin D deficiency may be a risk factor for ASD [99]. In a cohort study including families having children with ASD, a strong correlation was observed between one of the VDR (Vitamin D receptor) polymorphisms and hyperactivity behavior in children with ASD. It was reported that the same VDR polymorphism in mothers without ASD may increase the risk of giving birth to a child with ASD [100]. Another meta-analysis study reported that vitamin D supplementation in children with ASD reduced SRS and CARS- 2 scores and alleviated the symptoms of ASD [101]. This study, being a meta-analysis and including randomized controlled trials, makes the findings stronger compared to other studies. Moradi et al. [102] applied vitamin D supplementation and perceptual motor exercises to children with ASD. They observed that the combination of vitamin D3 supplementation and perceptual-motor exercises led to a significant decrease in stereotyped behaviors [102]. In another study where vitamin D (300 IU/kg/g) supplementation was applied to children with ASD for 15 weeks, it was observed that 86% of children with ASD had vitamin D deficiency. Vitamin D supplementation reduced CARS and Autism treatment Checklist (ATEC) scores, but did not change IL- 6 and ABC scores, and may have a beneficial effect on ASD symptoms [103].
There are some studies in the literature showing that serum vitamin D levels are low in individuals with ASD and that supplementation may improve symptoms. However, larger sample sizes and longer-term studies should be conducted to make generalization from this area.
Polyunsaturated fatty acids
Polyunsaturated fatty acids (PUFAs) have at least two carbon–carbon double bonds in their carboxylic chains. PUFAs are classified as n- 3, n- 6, n- 9 according to their distance from the methyl group located at the end of the molecule in the first double bond [104]. The most abundant PUFAs in brain tissue are n- 3 and n- 6. Both groups of fatty acids play an important role in the development of the central nervous system. n- 3 and n- 6 fatty acids are involved in the regulation of brain processes such as neurite outgrowth, neurogenesis and synaptogenesis [105]. EPA and DHA act on the synaptic membrane and signal transmission [104]. Recently, the effect of PUFAs on the cognition and behavioral disorders of individuals with ASD has been frequently investigated. The benefits of PUFAs on these individuals are explained as increasing the efficiency of synaptic transmission and modulating neurotransmitter release [105]. Doaei et al. [106] reported that daily 1000 mg omega- 3 fatty acid supplementation to children with ASD alleviated ASD-related symptoms, including social communication. In a study conducted in New Zealand, where children with ASD were supplemented with vitamin D (2000 IU/day) and n- 3 (722 mg/day DHA) for 12 months, it was observed that their irritability and hyperactivity symptoms considerably alleviated [107]. In another study, premature infants (18–38 months) were given n- 3, n- 6 and n- 9 fatty acid supplements for 3 months. Fatty acid supplementation improved socio-emotional parameters, especially in children suffering from symptoms common in ASD [108]. A meta-analysis study investigating the effect of n- 3 fatty acid supplementation on the symptoms of children with ASD reported that n- 3 and n- 6 supplementation alleviated ASD symptoms according to the ABC, but had no effect on irritability, inappropriate speech, and hyperactivity, which are subgroups of this scale [109]. However, the dosage of n- 3 fatty acids varies significantly based on various important factors such as duration of supplementation, severity of ASD, and type of fatty acid supplemented. Large-scale clinical studies are needed before definitive conclusions can be drawn.
Probiotic and Prebiotic
Probiotics are live microorganisms that provide health benefits to the host. Prebiotics, on the other hand, are substrates that are selectively used by host microorganisms and have a beneficial effect on health [110]. Although probiotics have therapeutic properties in gastrointestinal system diseases, they have also recently been used in the treatment of psychological symptoms such as depression and anxiety. Gut microbiota is involved in modulating the gut-brain axis. Dysbiosis causes psychological symptoms along with gastrointestinal system symptoms [111]. There is evidence that probiotics and prebiotics alleviate gastrointestinal tract symptoms and behavioral problems in ASD [112]. Grimaldi et al. [113] applied prebiotic supplementation to children with ASD for 6 weeks and observed that prebiotic supplementation provided an important reduction in gastrointestinal complaints such as abdominal pain and bowel movements. Another study conducted on children with ASD reported that probiotic supplementation increased Lactobacillus species in the intestines and alleviated gastrointestinal symptoms, while no side effects were observed [114]. In their study, Li et al. [115] determined that probiotic supplementation to individuals with ASD for 3 months provided a significant decrease in ATEC score. The relative abundance of Bifidobacterium, Lactobacillus, Coprobacillus, Ruminococcus, Prevotella, and Blautia was significantly higher after supplementation, and the abundance of Shigella and Clostridium was significantly lower, meaning that probiotic supplementation regulated the intestinal flora [115]. In another study, bovine colostrum and probiotic supplements with high prebiotic content were given to children with ASD aged between 2 and 11 months, and it was stated that the supplement reduced the frequency of gastrointestinal symptoms and abnormal behaviors [116]. In a study including probiotic and fructooligosaccharide supplementation to children with ASD, it was reported that the intervention was associated with ASD symptoms, including dopamine metabolism disorder and hyper-serotonergic state, and modulated the gut microbiota [117]. In addition to the mentioned dietary supplements, it has been reported that micronutrients such as vitamin B6, vitamin B12, vitamin C, vitamin A, folate and magnesium may be associated with ASD [13]. Although many dietary interventions have been investigated for the management of ASD, the effect of dietary interventions or supplements on ASD is unclear. It would not be correct to make a definitive recommendation for a specific nutritional intervention.
Other Nutrients and Components
Phytochemicals
Dietary phytochemicals such as carotenoids, isoprenoids, polyphenols, phytosterols and saponins are associated with many diseases such as obesity, cancer and cardiovascular diseases [118]. Additionally, the potential beneficial effect of phytochemicals in neurodevelopmental disorders such as ASD has recently attracted attention [119]. Sulforaphane increases detoxification, regulates cytoprotective enzymes, and removes toxic substances and free radicals from the body. Twelve weeks of sulforaphane supplementation to children with ASD and related neurodevelopmental disorders significantly improved ABC and SRS scale scores, and urinary metabolites (amino acid/gut microbiome, neurotransmitters, oxidative stress, sphingomyelin and hormones) related to changes in symptoms can be used to determine pathways playing an role in action mechanism of interventions aimed at alleviate ASD symptoms [120]. In another study, significant improvements were observed in the ABC scale score of 36-week sulforaphane supplementation in children with ASD compared to the placebo group [121]. Lynch et al. [122] reported that sulforaphane supplementation to individuals with ASD for 3 years could alleviate the symptoms of ASD.
Curcumin is the bioactive compound of turmeric and has a neuroprotective effect [123]. It has been shown that 20 mg/kg/day curcumin supplementation to BTBR rats reduces repetitive behaviors and improves ASD-related symptoms and cognitive impairment [124]. Jayaprakash et al. [125] reported that curcumin supplementation strengthened α7-nAChRs in central nervous system neurons, reduced oxidative stress, and improved ASD symptoms in BTBR rats.
Lutein is a fat-soluble xanthophyll carotenoid that is found in the brain of newborns and plays a significant role in neurodevelopment in their first years of life. It may be effective in alleviating the symptoms of ASD. In a study, lutein supplementation was administered to rats showing ASD-like symptoms caused by valproic acid exposure for 14 days. As a result of the intervention, reductions were observed in ASD symptoms such as social memory deficit, repetitive behaviors, and biomarkers of oxidative stress and apoptosis in the hippocampus [126]. In another study in which beta-carotene supplementation was performed on BTBR rats, it was reported that oral beta-carotene supplementation to infants of families prone to ASD after birth may be effective in preventing and/or alleviating ASD symptoms [127].
Naringenin is a flavonoid compound that is abundant in grapefruit, orange and tomato peels, has antioxidant properties [86] and is a potent neuroprotective through its direct or indirect effect on various pathological pathways. In a study in which naringenin supplementation was administered for 29 days to rats exhibiting ASD-like behavior due to propanoic acid exposure, naringenin showed beneficial effects by alleviating neuropsychopathology of ASD [128].
Quercetin is a polyphenol belonging to the flavonoid group found in vegetables and fruits and significantly reduces ROS, neuroinflammation, anxiety risk, and stress-related mood swings [129]. Owing to its antioxidant properties Quercetin can reduce oxidative stress associated with ASD. It was observed in a study that 40 mg/kg/g quercetin supplementation to rats could reduce ASD symptoms and oxidative and apoptotic damages [130]. Supplementation of 50 mg/kg/g quercetin to rats showing ASD-like behavior with the application of valproic acid showed a neuroprotective effect [131].
Resveratrol is a polyphenolic stilbenoid found in grapes, pine nuts, peanuts and red wine and shows antioxidant, anti-inflammatory and neuroprotective properties [132, 133]. It is known that individuals with ASD have a high level of oxidative stress and an impaired immune system. Therefore, resveratrol may contribute to ASD-related mechanisms [133]. It was shown that 200 mg/g resveratrol supplementation to children with ASD for 3 months significantly reduced the ABC score, and resveratrol supplementation modulated behavioral changes and immune system-related markers [134]. Hendouei et al. [135] reported that children with ASD received 250 mg resveratrol supplements twice a day for 10 weeks and this supplementation did not have any effect on their irritable behaviors, but could recover hyperactivity and noncompliance. Rats experiencing ASD-like symptoms were supplemented with propanoic acid and resveratrol (5, 10, and 15 mg/kg) for 28 days. Resveratrol alleviated these symptoms by suppressing mitochondrial dysfunction, oxidative-nitrosative stress, TNF-α and MMP- 9 expression in rats. Resveratrol can be used to manage neurobehavioral and biochemical disorders in ASD [136].
Bioactive compounds such as sulforaphane, curcumin, lutein, resveratrol, quercetin and naringenin may have potential positive effects on ASD. There are also studies in the literature reporting that these compounds do not show any effect. In addition, there is not yet sufficient information on the long-term effects of phytochemicals. Large-scale and long-term studies should be conducted to better understand the role of phytochemicals in ASD.
Major clinical studies conducted to evaluate the effects of various dietary interventions and supplements on the symptoms of ASD are summarized in Table 1.
Table 1.
Dietary interventions and supplements in autism spectrum disorder: animal and clinical studies
| Sample | Intervention | Outcomes | Reference |
|---|---|---|---|
| BTBR and C57BL/6 J (B6) rat (control group) |
Group 1: standard diet B6 rats Group 2: KD B6 rat Group 3: standard diet BTBR rat Group 4: KD BTBR rat KD: 6.3:1 |
It was observed that KD caused lower movement threshold and higher excitation/inhibition in BTBR rats compared to C57BL/6 J (B6) controls. KD increased cortical excitability in rats | [137] |
| 3–8 year old child with ASD (n = 45) |
Group 1: modified Atkins diet (KD) Group 2: GFCF Group 3: control Intervention time: 6 months |
Both diet groups showed significant improvement in ATEC and CARS scores compared to the control group. KD provided better results in terms of cognition and sociability than the GFCF diet group | [138] |
| Juvenile male C57BL/6 (B6) and BTBR rat |
BTBR rat: KD C57BL/6 (B6) rat: standard diet Response time: 10–14 days |
KD ameliorated some neurological symptoms associated with ASD by reducing the total intestinal microbial count and compositional restructuring in BTBR rats | [139] |
| Children with ASD aged 4–16 (n = 80) |
Intervention group: gluten-free diet Control group: standard diet Intervention time: 6 weeks |
Gluten-free diet intervention resulted in significant reductions in gastrointestinal symptoms and behavioral problems | [140] |
| 3–5 year old child with ASD (n = 14) |
After 6 weeks of GCFC intervention 12 weeks gluten, casein, gluten + casein and placebo groups |
The intervention showed no significant effects on physiological functioning, behavioral problems, and ASD symptoms | [141] |
| 2–12 year old child with ASD (n = 45) |
Group 1: cooked camel milk, 500 mL Group 2: raw camel milk, 500 mL Group 3: placebo |
Serum TARC levels decreased significantly in group 1 and group 2. Significant improvements were seen in the CARS score only in the raw camel milk supplement group | [142] |
| Child with ASD (n = 83) | Vitamin D3 (300 IU/kg/day, max: 5000 IU) for 3 months | Vitamin D3 supplementation resulted in significant improvement in 80.72% of children with ASD on sections of the CARS and ABC subscales measuring behavior, stereotypy, eye contact, and attention span | [143] |
| 7–18 year old child with ASD (n = 41) | 1 g omega- 3 fatty acid supplement twice daily, 12 weeks | Significant improvements were seen in all subscales of the SRS and the CBCL scale | [144] |
| 2.5–8 year old child with ASD (n = 73) |
Group 1: Vitamin D (2000 IU/day) Group 2: omega- 3 LCPUFA (722 mg/day docosahexaenoic acid) Group 3: Vitamin D (2000 IU/day) + omega- 3 LCPUFA (722 mg/day docosahexaenoic acid) Group 4: placebo Intervention period: 12 months |
The intervention modulated the inflammatory status (IL- 1β) in children with ASD and improvements in SRS scores were observed | [145] |
| 4–9 year old child with ASD (n = 13) | Prebiotic (partially hydrolyzed guar gum), 6 g/day | Partially hydrolyzed guar gum supplementation to children with ASD who experienced constipation alleviated the symptoms of constipation and intestinal dysbiosis. A significant decrease was observed in serum inflammatory cytokines and the behavioral irritability subscale of ABC | [146] |
| 5–9 year old child with ASD (n = 30) | Probiotic supplement (Lactobacillus acidophilus, Lactobacillus rhamnosus and Bifidobacteria longum), powder form, 5 g/day, 3 months | Improvements were seen in ATEC and gastrointestinal symptoms (6-GSI) | [147] |
| 18–72 month old child with ASD |
Group 1: GI probiotic (n = 9) Group 2: NGI probiotic (n = 22) Group 3: GI placebo (n = 8) Group 4: NGI placebo (n = 24) Intervention time: 6 months |
There was a significant decrease in ADOS-CSS scores in Group 2 compared to the placebo group. Greater improvements in some GI symptoms, adaptive functioning, and sensory profiles were seen in group 1 compared to group 3 | [148] |
| Male Sprague–Dawley rat |
With the application of 1 M propanoic acid, rats were caused to show ASD-like symptoms curcumin supplement (50, 100 and 200 mg/kg), 4 weeks |
Curcumin supplementation improved the core of the autistic phenotype and associated symptoms by suppressing mitochondrial dysfunction, oxidative-nitrosative stress, TNF-α and MMP- 9 in rats | [149] |
| 4–12 year old child with ASD (n = 60) |
Sulforaphane daily 50 μmol (≤ 45 kg) 100 μmol (> 45 kg) |
Sulforaphane supplementation improved symptoms of irritability and hyperactivity in children with ASD | [150] |
| 4–10 year old child with ASD (n = 40) | Product supplement containing lutein and quercetin, 26 weeks | After the intervention, a decrease in serum inflammatory cytokine levels (IL- 6 and TNF) and an improvement in the general symptoms of ASD were observed | [151] |
| Female pregnant wistar rat |
Rats were divided into 4 groups: Control group (n = 7), RSV group (n = 7), VPA group (n = 12–19) and VPA + RSV group (n = 9–10) Response time: 13 days Prenatal VPA application to rats was made to exhibit ASD-like behavior |
Prenatal administration of RSV prevented VPA-induced social impairments | [152] |
| Children with ASD aged 2–18 (n = 247 with ASD, n = 267 healthy controls) | In a case–control study, the prevalence and types of gastrointestinal symptoms, frequency of food selectivity, and mealtime difficulties were investigated | A gastrointestinal severity index questionnaire was administered. A higher prevalence of gastrointestinal symptoms was observed in girls with ASD than in boys. The incidence of mealtime difficulties (64.3%) and food selectivity (69.1%) is high. Behavioral traits had weak but significant correlations with gastrointestinal symptom frequency | [153] |
Abbreviations ASD: Autism spectrum disorder, KD: Ketogenic diet, GFCF: gluten-free-casein-free diet, ATEC: Autism Treatment Evaluation Test, CARS: Childhood Autism Rating Scale, ABC: Abnormal behavior checklist, 6-GSI: Gastrointestinal Severity Index, GI: Gastrointestinal symptoms, NGI: Non-gastrointestinal, ADOS-CSS: Total Autism Diagnostic Observation Schedule—Calibrated Severity Score, TNF-α: Tumor necrosis factor-α, MMP- 9: Matrix metalloproteinases, IL- 6: Interleukin- 6, SRS: Social Responsiveness Scale, CBCL: Social and Attention Problems syndrome of Child Behavior Checklist, IL- 1β: Interleukin- 1β, TARC: Thymus and activation-regulated chemokine, RSV: Resveratrol, VPA: Valproic acid, LCPUFA: Long-chain polyunsaturated fatty acid
Conclusion and Recommendations
There is no nutritional intervention that has a definitive and clear effect in managing ASD. Although nutritional interventions have shown a positive effect on symptoms in some individuals with ASD, the literature does not provide a definitive result due to reasons such as limited sample size in the studies conducted. Since the symptoms of ASD are specific to the individual, nutritional interventions differ for each individual. Nutrition of individuals with ASD should focus on reducing behavioral symptoms and eliminating troubles. In order to create the ideal diet model that can be used for its management, to determine the effect of nutrition on autism behaviors and to make correct nutrition plans, prospective epidemiological studies with large samples and clinical studies with in vivo, in vitro, animals and humans are needed.
Future Perspective
In light of these reviews, the efficiency of dietary interventions and supplements in ASD appears to be a strong and evolving topic of debate. No clear conclusion can be made as to which nutritional intervention is more appropriate for alleviating ASD symptoms. Future research initiatives in dietary patterns need to focus on increasing the bioavailability of nutrients. Larger-scale and longer-term clinical studies are needed to target and fill existing gaps in the relevant literature. Substantial evidence is needed to reveal clinical practices and protective effects on health, and to evaluate potential risks from nutrition in individuals with ASD. However, current scientific evidence is still insufficient to create a consensus regarding the use of foods and dietary patterns in reducing and managing ASD symptoms.
Since the nutritional patterns and consequences of children with autism are not yet fully understood and clarified, it is important to conduct further studies that will include the process of shaping the eating habits of these individuals, their eating behaviors, the effects of the applied diet on their daily eating habits and the formation of food selectivity. In addition, it is needed to conduct comprehensive, large-scale, longer-term randomized controlled in vivo, in vitro, human and animal clinical studies in order to identify, evaluate, and improve the effectiveness of dietary interventions and supplements in ASD, to ensure safety, and to provide a better understanding of potential health effects.
Key References
- Olivito I, Avolio E, Minervini D, Soda T, Rocca C, Angelone T, et al. Ketogenic diet ameliorates autism spectrum disorders-like behaviors via reduced inflammatory factors and microbiota remodeling in BTBR T+Itpr3tf/J mice. Exp Neurol. 2023;366:114,432.10.1016/j.expneurol.2023.114432.
- This article investigated the effects of ketogenic diet on rats. It was reported that ketogenic diet reduces social deficits, repetitive behaviors, memory impairments and inflammatory markers such as TNF-α, IL- 1β and IL- 6 and may be a therapeutic approach in autism spectrum disorder.
- Kandeel M, Morsy MA, Al Khodair KM, Alhojaily S. Meta-analysis of the efficacy of camel milk consumption for improving autism symptoms in children in randomized clinical trials. Open Vet J. 2024;14(9):2441–52.10.5455/OVJ.2024.v14.i9.33.
- This article is a meta-analysis study examining the effect of camel milk on autism spectrum disorder. It has been reported that camel milk may have positive effects on the social behavior of children with autism spectrum disorder.
- Viana CE, Bortolotto VC, Araujo SM, Dahleh MMM, Machado FR, de Souza Pereira A, et al. Lutein-loaded nanoparticles reverse oxidative stress, apoptosis, and autism spectrum disorder-like behaviors induced by prenatal valproic acid exposure in female rats. Neurotoxicology. 2023;94:223–34. 10.1016/j.neuro.2022.12.006.
- This article is an animal study examining the effect of lutein on ASD symptoms. Lutein-loaded nanoparticles were reported to reduce ASD symptoms such as social memory deficit, repetitive behaviors, and biomarkers of oxidative stress and apoptosis in the hippocampus after valproic acid exposure in female rats.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- ABS
Abnormal behavior checklist
- ADI
Acceptable daily intake
- ADOS- 2
Autism diagnostic observation schedule
- AGEs
Glycation end products
- ASD
Autism spectrum disorder
- ATEC
Autism treatment evaluation test
- ATP
Adenosine triphosphate
- BHA
Butylated hydroxyanisole
- BHT
Butylated hydroxytoluene
- CARS- 2
Childhood autism rating scale
- dACC
Dorsal anterior cingulate cortex
- DHA
Docosahexaenoic acid
- DSM- 5
Diagnostic and Statistical Manual of Mental Disorders fifth edition
- EPA
Eicosapentaenoic acid
- FODMAP
Fermented soluble oligosaccharides disaccharides monosaccharides and polyols
- GABA
Gamma aminobutyric acid
- GARS- 2
Gilliam autism rating scale
- GFCF
Gluten-free-casein-free
- GSH
Glutathione
- GSH-Px
Glutathione peroxidase
- HDAC2
Histone deacetylase 2
- IQ
Intelligence quotient
- IL- 6
Interleukin-6
- IL- 1β
Interleukin-1β
- lgG
Immunoglobulin G
- MCT
Medium chain triglyceride
- MMP- 9
Matrix metalloproteinases
- NAA
N-acetylaspartate
- PFC
Prefrontal cortex
- PUFA
Polyunsaturated fatty acids
- ROS
Reactive oxygen species
- SRS
Social sensitivity scale
- TBHQ
Tert-butylhydroquinone
- tCr
Total creatine
- tNAA
Total N-acetylaspartate
- TNF-α
Tumor necrosis factor-α
- UVB
Ultraviolet B
- VDR
Vitamin D receptor
- 18 FDG PET
18 Fluoro-deoxyglucos
Author Contributions
N.S. and E.O. wrote the main manuscript text and table. N.S. and E.O. prepared the figure. N.S. overviewed the selection of the literature and reviewed the draft, finalized the final version of the manuscript.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability
All data needed to evaluate the conclusions in this article are included in the article. Additional data related to this article may be requested from the authors.
Declarations
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hirota T, King BH. Autism spectrum disorder: a review. JAMA. 2023;329(2):157–68. 10.1001/jama.2022.23661. [DOI] [PubMed] [Google Scholar]
- 2.Lord C, Brugha TS, Charman T, Cusack J, Dumas G, Frazier T, et al. Autism spectrum disorder. Nat Rev Dis Primers. 2020;6(1):5. 10.1038/s41572-019-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman MM, Usman OL, Muniyandi RC, Sahran S, Mohamed S, Razak RA. A review of machine learning methods of feature selection and classification for autism spectrum disorder. Brain Sci. 2020;10(12):949. 10.3390/brainsci10120949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maenner MJ, Shaw KA, Bakian AV, Bilder DA, Durkin MS, Esler A, et al. Prevalence and characteristics of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, united states, 2018. MMWR Surveill Summ. 2021;70(11):1–16. 10.15585/mmwr.ss7202a1 [DOI] [PMC free article] [PubMed]
- 5.Zablotsky B, Black LI, Blumberg SJ. Estimated prevalence of children with diagnosed developmental disabilities in the united states, 2014–2016. NCHS Data Brief. 2017;291:1–8. [PubMed] [Google Scholar]
- 6.WHO. World Health Organization. 2023. Autism. https://www.who.int/news-room/fact-sheets/detail/autism-spectrum-disorders. Accessed 10 Nov 2023
- 7.Green RM, Travers AM, Howe Y, McDougle CJ. Women and autism spectrum disorder: diagnosis and ımplications for treatment of adolescents and adults. Curr Psychiatry Rep. 2019;21(4):22. 10.1007/s11920-019-1006-3. [DOI] [PubMed] [Google Scholar]
- 8.Salari N, Rasoulpoor S, Rasoulpoor S, Shohaimi S, Jafarpour S, Abdoli N, et al. The global prevalence of autism spectrum disorder: a comprehensive systematic review and meta-analysis. Ital J Pediatr. 2022;48(1):112. 10.1186/s13052-022-01310-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodges H, Fealko C, Soares N. Autism spectrum disorder: definition, epidemiology, causes, and clinical evaluation. Transl Pediatr. 2020;9(Suppl 1):S55–S65. 10.21037/tp.2019.09.09 [DOI] [PMC free article] [PubMed]
- 10.Singhi P, Malhi P. Early Diagnosis of Autism spectrum disorder: what the pediatricians should know. Indian J Pediatr. 2023;90(4):364–8. 10.1007/s12098-022-04363-1. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Wang B, Wu C, Wang J, Sun M. Autism spectrum disorder: neurodevelopmental risk factors, biological mechanism, and precision therapy. Int J Mol Sci. 2023;24(3):1819. 10.3390/ijms24031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peretti S, Mariano M, Mazzocchetti C, Mazza M, Pino MC, Verrotti Di Pianella A, et al. Diet: the keystone of autism spectrum disorder? Nutr Neurosci. 2019;22(12):825–39. 10.1080/1028415X.2018.1464819. [DOI] [PubMed] [Google Scholar]
- 13.Karhu E, Zukerman R, Eshraghi RS, Mittal J, Deth RC, Castejon AM, et al. Nutritional interventions for autism spectrum disorder. Nutr Rev. 2020;78(7):515–31. 10.1093/nutrit/nuz092. [DOI] [PubMed] [Google Scholar]
- 14.Hadjkacem I, Ayadi H, Turki M, Yaich S, Khemekhem K, Walha A, et al. Prenatal perinatal and postnatal factors associated with autism spectrum disorder. J Pediatr (Rio J). 2016;92(6):595–601. 10.1016/j.jped.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Geng H, Liu W, Zhang G. Prenatal, perinatal, and postnatal factors associated with autism: a meta-analysis. Medicine (Baltimore). 2017;96(18):e6696. 10.1097/MD.0000000000006696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartman RE, Patel D. Dietary approaches to the management of autism spectrum disorders. Adv Neurobiol. 2020;24:547–71. 10.1007/978-3-030-30402-7_19. [DOI] [PubMed] [Google Scholar]
- 17.Vartanian C. Overview of nutritional therapy for autism spectrum disorder. Adv Neurobiol. 2020;24:527–34. 10.1007/978-3-030-30402-7_17. [DOI] [PubMed] [Google Scholar]
- 18.Mayes SD, Zickgraf H. Atypical eating behaviors in children and adolescents with autism, ADHD, other disorders, and typical development. Res Autism Spectr Disord. 2019;64:76–83. 10.1016/j.rasd.2019.04.002. [Google Scholar]
- 19.Cekici H, Sanlier N. Current nutritional approaches in managing autism spectrum disorder: a review. Nutr Neurosci. 2019;22(3):145–55. 10.1080/1028415X.2017.1358481. [DOI] [PubMed] [Google Scholar]
- 20.Leader G, Abberton C, Cunningham S, Gilmartin K, Grudzien M, Higgins E, et al. Gastrointestinal symptoms in autism spectrum disorder: a systematic review. Nutrients. 2022;14(7):1471. 10.3390/nu14071471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emberti Gialloreti L, Mazzone L, Benvenuto A, Fasano A, Garcia Alcon A, Kraneveld A, et al. Risk and Protective environmental factors associated with autism spectrum disorder: evidence-based principles and recommendations. J Clin Med. 2019;8(2):217. 10.3390/jcm8020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodop BB, Başkaya E, Altuntaş İ, Erbaş O. Nutrition effect on autism spectrum disorders. J Exp Med Sci. 2021;2(1):7–17. 10.5606/jebms.2021.75633. [Google Scholar]
- 23.Onal S, Sachadyn-Król M, Kostecka M. A review of the nutritional approach and the role of dietary components in children with autism spectrum disorders in light of the latest scientific research. Nutrients. 2023;15(23):4852. 10.3390/nu15234852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madra M, Ringel R, Margolis KG. Gastrointestinal ıssues and autism spectrum disorder. Child Adolesc Psychiatr Clin N Am. 2020;29(3):501–13. 10.1016/j.chc.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González-Domenech PJ, Diaz-Atienza F, Gutiérrez-Rojas L, Fernández-Soto ML, González-Domenech CM. A narrative review about autism spectrum disorders and exclusion of gluten and casein from the diet. Nutrients. 2022;14(9):1797. 10.3390/nu14091797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piwowarczyk A, Horvath A, Pisula E, Kawa R, Szajewska H. Gluten-Free diet in children with autism spectrum disorders: a randomized, controlled, single-blinded trial. J Autism Dev Disord. 2020;50(2):482–90. 10.1007/s10803-019-04266-9. [DOI] [PubMed] [Google Scholar]
- 27.Baspinar B, Yardimci H. Gluten-Free casein-free diet for autism spectrum disorders: can ıt be effective in solving behavioural and gastrointestinal problems? Eurasian J Med. 2020;52(3):292–7. 10.5152/eurasianjmed.2020.19230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pistollato F, Forbes-Hernández TY, Calderón Iglesias R, Ruiz R, Elexpuru Zabaleta M, Cianciosi D, et al. Pharmacological, non-pharmacological and stem cell therapies for the management of autism spectrum disorders: a focus on human studies. Pharmacol Res. 2020;152:104579. 10.1016/j.phrs.2019.104579. [DOI] [PubMed] [Google Scholar]
- 29.Yu Y, Huang J, Chen X, Fu J, Wang X, Pu L, et al. Efficacy and safety of diet therapies in children with autism spectrum disorder: a systematic literature review and meta-analysis. Front Neurol. 2022;13:844117. 10.3389/fneur.2022.844117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams JB, Audhya T, Geis E, Gehn E, Fimbres V, Pollard EL, et al. Comprehensive nutritional and dietary ıntervention for autism spectrum disorder-a randomized, controlled 12-month trial. Nutrients. 2018;10(3):369. 10.3390/nu10030369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.González-Domenech PJ, Díaz Atienza F, García Pablos C, Fernández Soto ML, Martínez-Ortega JM, Gutiérrez-Rojas L. Influence of a combined gluten-free and casein-free diet on behavior disorders in children and adolescents diagnosed with autism spectrum disorder: a 12-month follow-up clinical trial. J Autism Dev Disord. 2020;50(3):935–48. 10.1007/s10803-019-04333-1. [DOI] [PubMed] [Google Scholar]
- 32.Bavykina IA, Popov VI, Zvyagin AA, Bavykin DV. 2019 [Frequency of determining markers of casein's inhability and gluten in children with disorders of autistic spectrum]. Vopr Pitan. 88(4):41–7. 10.24411/0042-8833-2019-10040 [DOI] [PubMed]
- 33.Matthews JS, Adams JB. Ratings of the effectiveness of 13 therapeutic diets for autism spectrum disorder: results of a national survey. J Pers Med. 2023;13(10):1448. 10.3390/jpm13101448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al Dera H, Alrafaei B, Al Tamimi MI, Alfawaz HA, Bhat RS, Soliman DA, et al. Leaky gut biomarkers in casein- and gluten-rich diet fed rat model of autism. Transl Neurosci. 2021;12(1):601–10. 10.1515/tnsci-2020-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quan L, Xu X, Cui Y, Han H, Hendren RL, Zhao L, et al. A systematic review and meta-analysis of the benefits of a gluten-free diet and/or casein-free diet for children with autism spectrum disorder. Nutr Rev. 2022;80(5):1237–46. 10.1093/nutrit/nuab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keller A, Rimestad ML, Friis Rohde J, Holm Petersen B, Bruun Korfitsen C, Tarp S, et al. The effect of a combined gluten- and casein-free diet on children and adolescents with autism spectrum disorders: a systematic review and meta-analysis. Nutrients. 2021;13(2):470. 10.3390/nu13020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varesio C, Grumi S, Zanaboni MP, Mensi MM, Chiappedi M, Pasca L, et al. Ketogenic dietary therapies in patients with autism spectrum disorder: facts or fads? a scoping review and a proposal for a shared protocol. Nutrients. 2021;13(6):2057. 10.3390/nu13062057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim JM, Letchumanan V, Tan LT, Hong KW, Wong SH, Ab Mutalib NS, et al. Ketogenic diet: a dietary ıntervention via gut microbiome modulation for the treatment of neurological and nutritional disorders (a narrative review). Nutrients. 2022;14(17):3566. 10.3390/nu14173566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi AH, Delgado M, Chen KY, Chung ST, Courville A, Turner SA, et al. A randomized feasibility trial of medium chain triglyceride-supplemented ketogenic diet in people with Parkinson’s disease. BMC Neurol. 2024;24(1):106. 10.1186/s12883-024-03603-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoeler NE, Marston L, Lyons L, Halsall S, Jain R, Titre-Johnson S, et al. Classic ketogenic diet versus further antiseizure medicine in infants with drug-resistant epilepsy (KIWE): a UK, multicentre, open-label, randomised clinical trial. Lancet Neurol. 2023;22(12):1113–24. 10.1016/S1474-4422(23)00370-8. [DOI] [PubMed] [Google Scholar]
- 41.El-Shafie AM, Bahbah WA, Abd El Naby SA, Omar ZA, Basma EM, Hegazy AAA, et al. Impact of two ketogenic diet types in refractory childhood epilepsy. Pediatr Res. 2023;94(6):1978–89. 10.1038/s41390-023-02554-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Q, Liang J, Fu N, Han Y, Qin J. A ketogenic diet and the treatment of autism spectrum disorder. Front Pediatr. 2021;9:650624. 10.3389/fped.2021.650624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazandarani M, Lashkarbolouk N, Ejtahed HS, Qorbani M. Does the ketogenic diet improve neurological disorders by influencing gut microbiota? A systematic review. Nutr J. 2023;22(1):61. 10.1186/s12937-023-00893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mu C, Corley MJ, Lee RWY, Wong M, Pang A, Arakaki G, et al. Metabolic framework for the ımprovement of autism spectrum disorders by a modified ketogenic diet: a pilot study. J Proteome Res. 2020;19(1):382–90. 10.1021/acs.jproteome.9b00581. [DOI] [PubMed] [Google Scholar]
- 45.Lee RWY, Corley MJ, Pang A, Arakaki G, Abbott L, Nishimoto M, et al. A modified ketogenic gluten-free diet with MCT improves behavior in children with autism spectrum disorder. Physiol Behav. 2018;188:205–11. 10.1016/j.physbeh.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahn Y, Sabouny R, Villa BR, Yee NC, Mychasiuk R, Uddin GM, et al. Aberrant mitochondrial morphology and function in the btbr mouse model of autism ıs ımproved by two weeks of ketogenic diet. Int J Mol Sci. 2020;21(9):3266. 10.3390/ijms21093266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olivito I, Avolio E, Minervini D, Soda T, Rocca C, Angelone T, et al. Ketogenic diet ameliorates autism spectrum disorders-like behaviors via reduced inflammatory factors and microbiota remodeling in BTBR T+Itpr3tf/J mice. Exp Neurol. 2023;366:114432. 10.1016/j.expneurol.2023.114432. [DOI] [PubMed] [Google Scholar]
- 48.Żarnowska I, Chrapko B, Gwizda G, Nocuń A, Mitosek-Szewczyk K, Gasior M. Therapeutic use of carbohydrate-restricted diets in an autistic child; a case report of clinical and 18FDG PET findings. Metab Brain Dis. 2018;33(4):1187–92. 10.1007/s11011-018-0219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin L, Ma K, Yan Z. Rescue of histone hypoacetylation and social deficits by ketogenic diet in a Shank3 mouse model of autism. Neuropsychopharmacology. 2022;47(6):1271–9. 10.1038/s41386-021-01212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ristori MV, Quagliariello A, Reddel S, Ianiro G, Vicari S, Gasbarrini A, et al. Autism, gastrointestinal symptoms and modulation of gut microbiota by nutritional ınterventions. Nutrients. 2019;11(11):2812. 10.3390/nu11112812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandecka A, Regulska-Ilow B. 2022 The importance of nutritional management and education in the treatment of autism. Rocz Panstw Zakl Hig. 73(3):247–58. 10.32394/rpzh.2022.0218 [DOI] [PubMed]
- 52.Barnhill K, Devlin M, Moreno HT, Potts A, Richardson W, Schutte C, et al. Brief report: ımplementation of a specific carbohydrate diet for a child with autism spectrum disorder and fragile x syndrome. J Autism Dev Disord. 2020;50(5):1800–8. 10.1007/s10803-018-3704-9. [DOI] [PubMed] [Google Scholar]
- 53.Montanari M, Martella G, Bonsi P, Meringolo M. Autism spectrum disorder: focus on glutamatergic neurotransmission. Int J Mol Sci. 2022;23(7):3861. 10.3390/ijms23073861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nisar S, Bhat AA, Masoodi T, Hashem S, Akhtar S, Ali TA, et al. Genetics of glutamate and its receptors in autism spectrum disorder. Mol Psychiatry. 2022;27(5):2380–92. 10.1038/s41380-022-01506-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eltokhi A, Santuy A, Merchan-Perez A, Sprengel R. Glutamatergic dysfunction and synaptic ultrastructural alterations in schizophrenia and autism spectrum disorder: evidence from human and rodent studies. Int J Mol Sci. 2020;22(1):59. 10.3390/ijms22010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carvalho Pereira A, Violante IR, Mouga S, Oliveira G, Castelo-Branco M. medial frontal lobe neurochemistry in autism spectrum disorder is marked by reduced N-acetylaspartate and unchanged gamma-aminobutyric acid and glutamate + glutamine levels. J Autism Dev Disord. 2018;48(5):1467–82. 10.1007/s10803-017-3406-8. [DOI] [PubMed] [Google Scholar]
- 57.Naaijen J, Zwiers MP, Forde NJ, Williams SC, Durston S, Brandeis D, et al. Striatal structure and its association with N-Acetylaspartate and glutamate in autism spectrum disorder and obsessive compulsive disorder. Eur Neuropsychopharmacol. 2018;28(1):118–29. 10.1016/j.euroneuro.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 58.Siegel-Ramsay JE, Romaniuk L, Whalley HC, Roberts N, Branigan H, Stanfield AC, et al. Glutamate and functional connectivity - support for the excitatory-inhibitory imbalance hypothesis in autism spectrum disorders. Psychiatry Res Neuroimaging. 2021;313:111302. 10.1016/j.pscychresns.2021.111302. [DOI] [PubMed] [Google Scholar]
- 59.Khalifa D, Shahin O, Salem D, Raafat O. Serum glutamate was elevated in children aged 3–10 years with autism spectrum disorders when they were compared with controls. Acta Paediatr. 2019;108(2):295–9. 10.1111/apa.14477. [DOI] [PubMed] [Google Scholar]
- 60.Park G, Jeon SJ, Ko IO, Park JH, Lee KC, Kim MS, et al. Decreased in vivo glutamate/GABA ratio correlates with the social behavior deficit in a mouse model of autism spectrum disorder. Mol Brain. 2022;15(1):19. 10.1186/s13041-022-00904-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nuzzo T, Sekine M, Punzo D, Miroballo M, Katane M, Saitoh Y, et al. Dysfunctional d-aspartate metabolism in BTBR mouse model of idiopathic autism. Biochim Biophys Acta Proteins Proteom. 2020;1868(12):140531. 10.1016/j.bbapap.2020.140531. [DOI] [PubMed] [Google Scholar]
- 62.Holton KF, Kirkland AE, Baron M, Ramachandra SS, Langan MT, Brandley ET, et al. The low glutamate diet effectively ımproves pain and other symptoms of gulf war ıllness. Nutrients. 2020;12(9):2593. 10.3390/nu12092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bellini M, Tonarelli S, Nagy AG, Pancetti A, Costa F, Ricchiuti A, et al. Low FODMAP diet: evidence, doubts, and hopes. Nutrients. 2020;12(1):148. 10.3390/nu12010148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rizwan AAM, Anosh I, Juweria A, Sehar I. Dietary approaches and nutritional complexities of autism spectrum disorder. Int J nutr Pharmacol Neurol Dis. 2022;12(4):221–41. 10.4103/ijnpnd.ijnpnd_65_22. [Google Scholar]
- 65.Nogay NH, Walton J, Roberts KM, Nahikian-Nelms M, Witwer AN. The effect of the low FODMAP diet on gastrointestinal symptoms, behavioral problems and nutrient ıntake in children with autism spectrum disorder: a randomized controlled pilot trial. J Autism Dev Disord. 2021;51(8):2800–11. 10.1007/s10803-020-04717-8. [DOI] [PubMed] [Google Scholar]
- 66.Bertuccioli A, Cardinali M, Di Pierro F, Zonzini GB, Matera MR. Ketogenic and low FODMAP diet in therapeutic management of a young autistic patient with epilepsy and dysmetabolism poorly responsive to therapies: clinical response and effects of ıntestinal microbiota. Int J Mol Sci. 2022;23(15):8829. 10.3390/ijms23158829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guasch-Ferré M, Willett WC. The Mediterranean diet and health: a comprehensive overview. J Intern Med. 2021;290(3):549–66. 10.1111/joim.13333. [DOI] [PubMed] [Google Scholar]
- 68.Dai FC, Wang P, Li Q, Zhang L, Yu LJ, Wu L, et al. Mediterranean diet during pregnancy and infant neurodevelopment: a prospective birth cohort study. Front Nutr. 2023;9:1078481. 10.3389/fnut.2022.1078481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ganjeh BJ, Mirrafiei A, Jayedi A, Mirmohammadkhani M, Emadi A, Ehsani F, et al. The relationship between adherence to the Mediterranean dietary pattern during early pregnancy and behavioral, mood and cognitive development in children under 1 year of age: a prospective cohort study. Nutr Neurosci. 2023;25:1–8. 10.1080/1028415X.2023.2249635. [DOI] [PubMed] [Google Scholar]
- 70.Ríos-Hernández A, Alda JA, Farran-Codina A, Ferreira-García E, Izquierdo-Pulido M. The Mediterranean diet and ADHD in children and adolescents. Pediatrics. 2017;139(2):e20162027. 10.1542/peds.2016-2027. [DOI] [PubMed] [Google Scholar]
- 71.San Mauro Martin I, SanzRojo S, González Cosano L, Conty de la Campa R, GaricanoVilar E, Blumenfeld Olivares JA. Impulsiveness in children with attention-deficit/hyperactivity disorder after an 8-week intervention with the Mediterranean diet and/or omega-3 fatty acids A randomised clinical trial. Neurologia (Engl Ed). 2022;37(7):513–23. 10.1016/j.nrleng.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 72.Quarta A, Guarino M, Tripodi R, Giannini C, Chiarelli F, Blasetti A. Diet and glycemic ındex in children with type 1 diabetes. Nutrients. 2023;15(16):3507. 10.3390/nu15163507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carneiro L, Leloup C. Mens sana in corpore sano: does the glycemic ındex have a role to play? Nutrients. 2020;12(10):2989. 10.3390/nu12102989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Currais A, Farrokhi C, Dargusch R, Goujon-Svrzic M, Maher P. Dietary glycemic index modulates the behavioral and biochemical abnormalities associated with autism spectrum disorder. Mol Psychiatry. 2016;21(3):426–36. 10.1038/mp.2015.64. [DOI] [PubMed] [Google Scholar]
- 75.Cristiano C, Lama A, Lembo F, Mollica MP, Calignano A, Mattace RG. Interplay between peripheral and central ınflammation in autism spectrum disorders: possible nutritional and therapeutic strategies. Front Physiol. 2018;9:184. 10.3389/fphys.2018.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Herman A, Herman AP. Could candida overgrowth be ınvolved in the pathophysiology of autism? J Clin Med. 2022;11(2):442. 10.3390/jcm11020442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Iovene MR, Bombace F, Maresca R, Sapone A, Iardino P, Picardi A, et al. Intestinal dysbiosis and yeast ısolation in stool of subjects with autism spectrum disorders. Mycopathologia. 2017;182(3–4):349–63. 10.1007/s11046-016-0068-6. [DOI] [PubMed] [Google Scholar]
- 78.Retnaningtyas Y, Dianasari D, Elavia LLG, Munawaroh U. Identification of candida species in faeces of children with autism spectrum disorder (asd) carbohydrate and noncarbohydrates diets. Aceh Nutri J. 2022;7(2):197–204. 10.30867/action.v7i2.746. [Google Scholar]
- 79.Gutierrez D, Weinstock A, Antharam VC, Gu H, Jasbi P, Shi X, et al. 2020 Antibiotic-induced gut metabolome and microbiome alterations increase the susceptibility to candida albicans colonization in the gastrointestinal tract. FEMS Microbiol Ecol. 96(1):fiz187. 10.1093/femsec/fiz187 [DOI] [PMC free article] [PubMed]
- 80.Madzhidova S, Sedrakyan L. The use of dietary ınterventions in pediatric patients. Pharmacy (Basel). 2019;7(1):10. 10.3390/pharmacy7010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abdallah H, Soliman S, El-kader A. Language training program coupled with feingold diet for caregivers to develop autisticreceptive language skills. Mansoura Nurs J. 2019;6:107–25. 10.21608/mnj.2019.176375. [Google Scholar]
- 82.Delaunay-Vagliasindi S, Seneff S, Campbell-McBride N. GAPS Nutritional Protocol - how healing the gut removes the basis for all chronic diseases. J Orthomol Med. 2021;36(3):1–7. [Google Scholar]
- 83.Campbell-McBride N. Gut and psychology syndrome: natural treatment for autism, dyspraxia, A.D.D., dyslexia, A.D.H.D., depression, schizophrenia. Revised & enlarged edition. England. 2010
- 84.Ābele S, Meija L, Folkmanis V, Tzivian L. Specific carbohydrate diet (SCD/GAPS) and dietary supplements for children with autistic spectrum disorder. Proc Latv Acad Sci. 2021;6(735):417–25. 10.2478/prolas-2021-0062. [Google Scholar]
- 85.Ho TM, Zou Z, Bansal N. Camel milk: A review of its nutritional value, heat stability, and potential food products. Food Res Int. 2022;153:110870. 10.1016/j.foodres.2021.110870. [DOI] [PubMed] [Google Scholar]
- 86.Mondal A, Sharma R, Abiha U, Ahmad F, Karan A, Jayaraj RL, et al. A spectrum of solutions: unveiling non-pharmacological approaches to manage autism spectrum disorder. Medicina (Kaunas). 2023;59(9):1584. 10.3390/medicina59091584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Amadi CN, Orish CN, Frazzoli C, Orisakwe OE. 2022 Dietary interventions for autism spectrum disorder: an updated systematic review of human studies. Psychiatriki. 33(3):228–42 10.22365/jpsych.2022.073 [DOI] [PubMed]
- 88.Kandeel M, Morsy MA, Al Khodair KM, Alhojaily S. Meta-analysis of the efficacy of camel milk consumption for improving autism symptoms in children in randomized clinical trials. Open Vet J. 2024;14(9):2441–52. 10.5455/OVJ.2024.v14.i9.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Al-Ayadhi L, Alhowikan AM, Bhat RS, El-Ansary A. Comparative study on the ameliorating effects of camel milk as a dairy product on ınflammatory response in autism spectrum disorders. Neurochem J. 2022;16:99–108. 10.1134/S1819712422010020. [Google Scholar]
- 90.Hamzawy MA, El-Ghandour YB, Abdel-Aziem SH, Ali ZH. Leptin and camel milk abate oxidative stress status, genotoxicity induced in valproic acid rat model of autism. Int J Immunopathol Pharmacol. 2018;32:2058738418785514. 10.1177/2058738418785514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu L, Zhang C, Long Y, Chen Q, Zhang W, Liu G. Food additives: from functions to analytical methods. Crit Rev Food Sci Nutr. 2022;62(30):8497–517. 10.1080/10408398.2021.1929823. [DOI] [PubMed] [Google Scholar]
- 92.Bakthavachalu P, Kannan SM, Qoronfleh MW. Food color and autism: a meta-analysis. Adv Neurobiol. 2020;24:481–504. 10.1007/978-3-030-30402-7_15. [DOI] [PubMed] [Google Scholar]
- 93.Kirkland AE, Langan MT, Holton KF. Artificial food coloring affects EEG power and ADHD symptoms in college students with ADHD: a pilot study. Nutr Neurosci. 2022;25(1):159–68. 10.1080/1028415X.2020.1730614. [DOI] [PubMed] [Google Scholar]
- 94.Indika NR, Frye RE, Rossignol DA, Owens SC, Senarathne UD, Grabrucker AM, et al. The rationale for vitamin, mineral, and cofactor treatment in the precision medical care of autism spectrum disorder. J Pers Med. 2023;13(2):252. 10.3390/jpm13020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Esteban-Figuerola P, Canals J, Fernández-Cao JC, Arija VV. Differences in food consumption and nutritional intake between children with autism spectrum disorders and typically developing children: A meta-analysis. Autism. 2019;23(5):1079–95. 10.1177/1362361318794179. [DOI] [PubMed] [Google Scholar]
- 96.Wang Z, Ding R, Wang J. The association between Vitamin D status and autism spectrum disorder (ASD): A Systematic Review and Meta-Analysis. Nutrients. 2020;13(1):86. 10.3390/nu13010086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J, Huang H, Liu C, Zhang Y, Wang W, Zou Z, et al. Research progress on the role of vitamin D in autism spectrum disorder. Front Behav Neurosci. 2022;16:859151. 10.3389/fnbeh.2022.859151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siracusano M, Riccioni A, Abate R, Benvenuto A, Curatolo P, Mazzone L. Vitamin D deficiency and autism spectrum disorder. Curr Pharm Des. 2020;26(21):2460–74. 10.2174/1381612826666200415174311. [DOI] [PubMed] [Google Scholar]
- 99.Petruzzelli MG, Marzulli L, Margari F, De Giacomo A, Gabellone A, Giannico OV, et al. Vitamin D deficiency in autism spectrum disorder: a cross-sectional study. Dis Markers. 2020;2020:9292560. 10.1155/2020/9292560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guerini FR, Bolognesi E, Chiappedi M, Mensi MM, Fumagalli O, Rogantini C, et al. Vitamin D receptor polymorphisms associated with autism spectrum disorder. Autism Res. 2020;13(5):680–90. 10.1002/aur.2279. [DOI] [PubMed] [Google Scholar]
- 101.Song L, Luo X, Jiang Q, Chen Z, Zhou L, Wang D, et al. Vitamin D supplementation is beneficial for children with autism spectrum disorder: a meta-analysis. Clin Psychopharmacol Neurosci. 2020;18(2):203–13. 10.9758/cpn.2020.18.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moradi H, Sohrabi M, Taheri H, Khodashenas E, Movahedi A. Comparison of the effects of perceptual-motor exercises, vitamin D supplementation and the combination of these interventions on decreasing stereotypical behavior in children with autism disorder. Int J Dev Disabil. 2018;66(2):122–32. 10.1080/20473869.2018.1502068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Javadfar Z, Abdollahzad H, Moludi J, Rezaeian S, Amirian H, Foroughi AA, et al. Effects of vitamin D supplementation on core symptoms, serum serotonin, and interleukin-6 in children with autism spectrum disorders: a randomized clinical trial. Nutrition. 2020;79–80:110986. 10.1016/j.nut.2020.110986. [DOI] [PubMed] [Google Scholar]
- 104.De Crescenzo F, D’Alò GL, Morgano GP, Minozzi S, Mitrova Z, Saulle R, et al. Impact of polyunsaturated fatty acids on patient-important outcomes in children and adolescents with autism spectrum disorder: a systematic review. Health Qual Life Outcomes. 2020;18(1):28. 10.1186/s12955-020-01284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barón-Mendoza I, González-Arenas A. Relationship between the effect of polyunsaturated fatty acids (PUFAs) on brain plasticity and the improvement on cognition and behavior in individuals with autism spectrum disorder. Nutr Neurosci. 2022;25(2):387–410. 10.1080/1028415X.2020.1755793. [DOI] [PubMed] [Google Scholar]
- 106.Doaei S, Bourbour F, Teymoori Z, Jafari F, Kalantari N, Abbas Torki S, et al. The effect of omega-3 fatty acids supplementation on social and behavioral disorders of children with autism: a randomized clinical trial. Pediatr Endocrinol Diabetes Metab. 2021;27(1):12–8. 10.5114/pedm.2020.101806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mazahery H, Conlon CA, Beck KL, Mugridge O, Kruger MC, Stonehouse W, et al. A randomised controlled trial of vitamin D and omega-3 long chain polyunsaturated fatty acids in the treatment of irritability and hyperactivity among children with autism spectrum disorder. J Steroid Biochem Mol Biol. 2019;187:9–16. 10.1016/j.jsbmb.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 108.Boone KM, Klebanoff MA, Rogers LK, Rausch J, Coury DL, Keim SA. Effects of omega-3-6-9 fatty acid supplementation on behavior and sleep in preterm toddlers with autism symptomatology: secondary analysis of a randomized clinical trial. Early Hum Dev. 2022;169:105588. 10.1016/j.earlhumdev.2022.105588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.de Andrade Wobido K, de Sá Barreto da Cunha M, Miranda SS, da Mota Santana J, da Silva DCG, Pereira M. 2022 Non-specific effect of omega-3 fatty acid supplementation on autistic spectrum disorder: systematic review and meta-analysis. Nutr Neurosci. 25(9):1995–2007. 10.1080/1028415X.2021.1913950 [DOI] [PubMed]
- 110.Green M, Arora K, Prakash S. Microbial medicine: prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int J Mol Sci. 2020;21(8):2890. 10.3390/ijms21082890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Song W, Zhang M, Teng L, Wang Y, Zhu L. 2022 Prebiotics and probiotics for autism spectrum disorder: a systematic review and meta-analysis of controlled clinical trials. J Med Microbiol. 71(4):10.1099/jmm.0.001510. 10.1099/jmm.0.001510 [DOI] [PubMed]
- 112.Grossi E, Melli S, Dunca D, Terruzzi V. Unexpected improvement in core autism spectrum disorder symptoms after long-term treatment with probiotics. SAGE Open Med Case Rep. 2016;4:2050313X16666231. 10.1177/2050313X16666231 [DOI] [PMC free article] [PubMed]
- 113.Grimaldi R, Gibson GR, Vulevic J, Giallourou N, Castro-Mejía JL, Hansen LH, et al. A prebiotic intervention study in children with autism spectrum disorders (ASDs). Microbiome. 2018;6(1):133. 10.1186/s40168-018-0523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Arnold LE, Luna RA, Williams K, Chan J, Parker RA, Wu Q, et al. Probiotics for gastrointestinal symptoms and quality of life in autism: a placebo-controlled pilot trial. J Child Adolesc Psychopharmacol. 2019;29(9):659–69. 10.1089/cap.2018.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li YQ, Sun YH, Liang YP, Zhou F, Yang J, Jin SL. Effect of probiotics combined with applied behavior analysis in the treatment of children with autism spectrum disorder: a prospective randomized controlled trial. Zhongguo Dang Dai Er Ke Za Zhi. 2021;23(11):1103–10. 10.7499/j.issn.1008-8830.2108085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sanctuary MR, Kain JN, Chen SY, Kalanetra K, Lemay DG, Rose DR, et al. Pilot study of probiotic/colostrum supplementation on gut function in children with autism and gastrointestinal symptoms. PLoS ONE. 2019;14(1):e0210064. 10.1371/journal.pone.0210064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Y, Li N, Yang JJ, Zhao DM, Chen B, Zhang GQ, et al. Probiotics and fructo-oligosaccharide intervention modulate the microbiota-gut brain axis to improve autism spectrum reducing also the hyper-serotonergic state and the dopamine metabolism disorder. Pharmacol Res. 2020;157:104784. 10.1016/j.phrs.2020.104784. [DOI] [PubMed] [Google Scholar]
- 118.Kumar APN, Kumar M, Jose A, Tomer V, Oz E, Proestos C, et al. Major phytochemicals: recent advances in health benefits and extraction method. Molecules. 2023;28(2):887. 10.3390/molecules28020887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Serra D, Almeida LM, Dinis TCP. Polyphenols as food bioactive compounds in the context of autism spectrum disorders: a critical mini-review. Neurosci Biobehav Rev. 2019;102:290–8. 10.1016/j.neubiorev.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 120.Bent S, Lawton B, Warren T, Widjaja F, Dang K, Fahey JW, et al. Identification of urinary metabolites that correlate with clinical improvements in children with autism treated with sulforaphane from broccoli. Mol Autism. 2018;9:35. 10.1186/s13229-018-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zimmerman AW, Singh K, Connors SL, Liu H, Panjwani AA, Lee LC, et al. Randomized controlled trial of sulforaphane and metabolite discovery in children with autism spectrum disorder. Mol Autism. 2021;12(1):38. 10.1186/s13229-021-00447-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lynch R, Diggins EL, Connors SL, Zimmerman AW, Singh K, Liu H, et al. 2017 Sulforaphane from broccoli reduces symptoms of autism: a follow-up case series from a randomized double-blind study. Glob Adv Health Med. 6:2164957X17735826. 10.1177/2164957X17735826 [DOI] [PMC free article] [PubMed]
- 123.Subedi L, Gaire BP. Neuroprotective effects of curcumin in cerebral ıschemia: cellular and molecular mechanisms. ACS Chem Neurosci. 2021;12(14):2562–72. 10.1021/acschemneuro.1c00153. [DOI] [PubMed] [Google Scholar]
- 124.Zhong H, Xiao R, Ruan R, Liu H, Li X, Cai Y, et al. Neonatal curcumin treatment restores hippocampal neurogenesis and improves autism-related behaviors in a mouse model of autism. Psychopharmacology. 2020;237(12):3539–52. 10.1007/s00213-020-05634-5. [DOI] [PubMed] [Google Scholar]
- 125.Jayaprakash P, Isaev D, Shabbir W, Lorke DE, Sadek B, Oz M. Curcumin potentiates α7 nicotinic acetylcholine receptors and alleviates autistic-like social deficits and brain oxidative stress status in mice. Int J Mol Sci. 2021;22(14):7251. 10.3390/ijms22147251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Viana CE, Bortolotto VC, Araujo SM, Dahleh MMM, Machado FR, de Souza PA, et al. Lutein-loaded nanoparticles reverse oxidative stress, apoptosis, and autism spectrum disorder-like behaviors induced by prenatal valproic acid exposure in female rats. Neurotoxicology. 2023;94:223–34. 10.1016/j.neuro.2022.12.006. [DOI] [PubMed] [Google Scholar]
- 127.Avraham Y, Mankuta D, Lipsker L, Vorobiev L, Patael S, Hassid G, et al. Beta-carotene derivatives as novel therapy for the prevention and treatment of autistic symptoms. Bioorg Chem. 2021;115:105224. 10.1016/j.bioorg.2021.105224. [DOI] [PubMed] [Google Scholar]
- 128.Bhandari R, Paliwal J, Kuhad A. Naringenin and its nanocarriers as potential phytotherapy for autism spectrum disorders. J Funct Foods. 2018;47:361–75. 10.1016/j.jff.2018.05.065. [Google Scholar]
- 129.Alvarez-Arellano L, Salazar-García M, Corona JC. Neuroprotective effects of quercetin in pediatric neurological diseases. Molecules. 2020;25(23):5597. 10.3390/molecules25235597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Moghaddam AH, Eslami A, Jelodar SK, Ranjbar M, Hasantabar V. Preventive effect of quercetin-loaded nanophytosome against autistic-like damage in maternal separation model: The possible role of Caspase-3, Bax/Bcl-2 and Nrf2. Behav Brain Res. 2023;441:114300. 10.1016/j.bbr.2023.114300. [DOI] [PubMed] [Google Scholar]
- 131.de Mattos BDS, Soares MSP, Spohr L, Pedra NS, Teixeira FC, de Souza AA, et al. Quercetin prevents alterations of behavioral parameters, delta-aminolevulinic dehydratase activity, and oxidative damage in brain of rats in a prenatal model of autism. Int J Dev Neurosci. 2020;80(4):287–302. 10.1002/jdn.10025. [DOI] [PubMed] [Google Scholar]
- 132.Urdaneta KE, Castillo MA, Montiel N, Semprún-Hernández N, Antonucci N, Siniscalco D. Autism spectrum disorders: potential neuro-psychopharmacotherapeutic plant-based drugs. Assay Drug Dev Technol. 2018;16(8):433–44. 10.1089/adt.2018.848. [DOI] [PubMed] [Google Scholar]
- 133.Malaguarnera M, Khan H, Cauli O. Resveratrol in autism spectrum disorders: behavioral and molecular effects. Antioxidants (Basel). 2020;9(3):188. 10.3390/antiox9030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marchezan J, Deckmann I, da Fonseca GC, Margis R, Riesgo R, Gottfried C. Resveratrol treatment of autism spectrum disorder-a pilot study. Clin Neuropharmacol. 2022;45(5):122–7. 10.1097/wnf.0000000000000516. [DOI] [PubMed] [Google Scholar]
- 135.Hendouei F, Sanjari Moghaddam H, Mohammadi MR, Taslimi N, Rezaei F, et al. Resveratrol as adjunctive therapy in treatment of irritability in children with autism: a double-blind and placebo-controlled randomized trial. J Clin Pharm Ther. 2020;45(2):324–34. 10.1111/jcpt.13076. [DOI] [PubMed] [Google Scholar]
- 136.Bhandari R, Kuhad A. Resveratrol suppresses neuroinflammation in the experimental paradigm of autism spectrum disorders. Neurochem Int. 2017;103:8–23. 10.1016/j.neuint.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 137.Smith J, Rho JM, Teskey GC. Ketogenic diet restores aberrant cortical motor maps and excitation-to-inhibition imbalance in the BTBR mouse model of autism spectrum disorder. Behav Brain Res. 2016;304:67–70. 10.1016/j.bbr.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 138.El-Rashidy O, El-Baz F, El-Gendy Y, Khalaf R, Reda D, Saad K. Ketogenic diet versus gluten free casein free diet in autistic children: a case-control study. Metab Brain Dis. 2017;32(6):1935–41. 10.1007/s11011-017-0088-z. [DOI] [PubMed] [Google Scholar]
- 139.Newell C, Bomhof MR, Reimer RA, Hittel DS, Rho JM, Shearer J. Ketogenic diet modifies the gut microbiota in a murine model of autism spectrum disorder. Mol Autism. 2016;7(1):37. 10.1186/s13229-016-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ghalichi F, Ghaemmaghami J, Malek A, Ostadrahimi A. Effect of gluten free diet on gastrointestinal and behavioral indices for children with autism spectrum disorders: a randomized clinical trial. World J Pediatr. 2016;12(4):436–42. 10.1007/s12519-016-0040-z. [DOI] [PubMed] [Google Scholar]
- 141.Hyman SL, Stewart PA, Foley J, Cain U, Peck R, Morris DD, et al. The gluten-free/casein-free diet: a double-blind challenge trial in children with autism. J Autism Dev Disord. 2016;46(1):205–20. 10.1007/s10803-015-2564-9. [DOI] [PubMed] [Google Scholar]
- 142.Bashir S, Al-Ayadhi LY. Effect of camel milk on thymus and activation-regulated chemokine in autistic children: double-blind study. Pediatr Res. 2014;75(4):559–63. 10.1038/pr.2013.248. [DOI] [PubMed] [Google Scholar]
- 143.Saad K, Abdel-Rahman AA, Elserogy YM, Al-Atram AA, Cannell JJ, Bjørklund G, et al. Vitamin D status in autism spectrum disorders and the efficacy of vitamin D supplementation in autistic children. Nutr Neurosci. 2016;19(8):346–51. 10.1179/1476830515Y.0000000019. [DOI] [PubMed] [Google Scholar]
- 144.Ooi YP, Weng SJ, Jang LY, Low L, Seah J, Teo S, et al. Omega-3 fatty acids in the management of autism spectrum disorders: findings from an open-label pilot study in Singapore. Eur J Clin Nutr. 2015;69(8):969–71. 10.1038/ejcn.2015.28. [DOI] [PubMed] [Google Scholar]
- 145.Mazahery H, Conlon CA, Beck KL, Mugridge O, Kruger MC, Stonehouse W, et al. Inflammation (IL-1β) modifies the effect of vitamin D and omega-3 long chain polyunsaturated fatty acids on core symptoms of autism spectrum disorder-an exploratory pilot study. Nutrients. 2020;12(3):661. 10.3390/nu12030661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Inoue R, Sakaue Y, Kawada Y, Tamaki R, Yasukawa Z, Ozeki M, et al. Dietary supplementation with partially hydrolyzed guar gum helps improve constipation and gut dysbiosis symptoms and behavioral irritability in children with autism spectrum disorder. J Clin Biochem Nutr. 2019;64(3):217–23. 10.3164/jcbn.18-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shaaban SY, El Gendy YG, Mehanna NS, El-Senousy WM, El-Feki HSA, Saad K, et al. The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr Neurosci. 2018;21(9):676–81. 10.1080/1028415X.2017.1347746. [DOI] [PubMed] [Google Scholar]
- 148.Santocchi E, Guiducci L, Prosperi M, Calderoni S, Gaggini M, Apicella F, et al. Effects of probiotic supplementation on gastrointestinal, sensory and core symptoms in autism spectrum disorders: a randomized controlled trial. Front Psychiatry. 2020;11:550593. 10.3389/fpsyt.2020.550593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bhandari R, Kuhad A. Neuropsychopharmacotherapeutic efficacy of curcumin in experimental paradigm of autism spectrum disorders. Life Sci. 2015;141:156–69. 10.1016/j.lfs.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 150.Momtazmanesh S, Amirimoghaddam-Yazdi Z, Moghaddam HS, Mohammadi MR, Akhondzadeh S. Sulforaphane as an adjunctive treatment for irritability in children with autism spectrum disorder: A randomized, double-blind, placebo-controlled clinical trial. Psychiatry Clin Neurosci. 2020;74(7):398–405. 10.1111/pcn.13016. [DOI] [PubMed] [Google Scholar]
- 151.Tsilioni I, Taliou A, Francis K, Theoharides TC. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl Psychiatry. 2015;5(9):e647. 10.1038/tp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bambini-Junior V, Zanatta G, Della Flora Nunes G, Mueller de Melo G, Michels M, Fontes-Dutra M, et al. Resveratrol prevents social deficits in animal model of autism induced by valproic acid. Neurosci Lett. 2014;583:176–81. 10.1016/j.neulet.2014.09.039. [DOI] [PubMed] [Google Scholar]
- 153.Babinska K, Celusakova H, Belica I, Szapuova Z, Waczulikova I, Nemcsicsova D, et al. Gastrointestinal symptoms and feeding problems and their associations with dietary ınterventions, food supplement use, and behavioral characteristics in a sample of children and adolescents with autism spectrum disorders. Int J Environ Res Public Health. 2020;17(17):6372. 10.3390/ijerph17176372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in this article are included in the article. Additional data related to this article may be requested from the authors.