Abstract
This study reports the efficacy of a heterologous prime-boost vaccination using DNA and vaccinia viruses (Western Reserve [WR] virus and modified [attenuated] vaccinia virus Ankara [MVA]) expressing the LACK antigen (Leishmania homologue of receptors for activated C kinase) and an intradermal murine infection model employing Leishmania infantum. At 1 month postinfection, vaccinated mice showed high levels of protection in the draining lymph node (240-fold reduction in parasite burden) coupled with significant levels of gamma interferon (20 to 200 ng/ml) and tumor necrosis factor alpha/lymphotoxin (8 to 134 pg/ml). Significant but lower levels of protection (6- to 30-fold) were observed in the spleen and liver. Comparable levels of protection were found for mice boosted with either LACK-WR or LACK-MVA, supporting the use of an attenuated vaccinia virus-based vaccine against human visceral leishmaniasis.
Visceral leishmaniasis (VL) is a protozoan parasitic disease, fatal in the absence of treatment. Although drug treatment exists for VL (17, 42), alternative approaches for the control of this disease (vector control, immunotherapeutic, chemotherapeutic, and vaccine) are still needed (8, 45). Vaccine studies of VL have been less extensive (6, 11-13, 22, 36), and the level of protection found is generally poorer than those found for murine cutaneous leishmaniasis. However, studies utilizing a murine intradermal infection model of VL indicate that this is, in part, due to the animal model employed (1). Immunological studies of the mechanisms of pathogenesis as well as immunotherapeutic studies of VL indicate tissue site-specific mechanisms (for the spleen, liver, and lymph node) (9, 10, 41). Consequently, one of the challenges in the development of a vaccine against VL is the induction of protection at multiple and distinct tissue sites.
The LACK antigen (Leishmania analogue of the receptors of activated C kinase) (36 kDa) is highly conserved among Leishmania species and expressed by both the promastigote and amastigote forms of the parasite (25). Studies indicate that DNA coding for the LACK antigen provides protection against Leishmania major. However, a LACK DNA vaccine failed to protect against L. mexicana (7). Further, a LACK DNA vaccine, although highly immunogenic, failed to protect against murine VL in either intradermal or intravenous infection (23), suggesting that LACK may not be a useful antigen for a general DNA-based vaccine against leishmaniasis.
However, the antigen delivery system can be a critical component in determining antigenic efficacy. Vaccinia virus vectors have been shown to be a good antigen delivery system for the control of infectious diseases in animal model studies (24). A heterologous prime-boost regimen using DNA and vaccinia viruses expressing the LACK antigen has been shown to be highly immunogenic and protective against murine L. major infection (15, 20, 44). A heterologous prime-boost regimen using DNA and the replication-competent Western Reserve (WR) strain of vaccinia virus expressing the LACK antigen was recently explored in canine VL (37). However, the immune response in the canine model is known to significantly differ from those in the murine and human hosts of leishmaniae in terms of their regulation by interleukin-13 (IL-13), IL-12, and IL-10 (34, 35, 38, 39). Previous leishmaniasis vaccine studies, however, have demonstrated that the murine model can be predictive for vaccine outcomes in nonhuman-primate models (3, 5, 21). Therefore, in the current study, the potential of a prime-boost regimen using DNA-vaccinia virus was further explored using the murine intradermal model for VL. In order to assess the potential use of this vaccination regimen against murine VL, the efficacies of priming were examined using an L. infantum DNA-LACK construct (previously employed for vaccine studies against cutaneous leishmaniasis caused by L. major [44]) and the highly attenuated modified vaccinia virus Ankara (MVA) strain as well as the replication-competent WR strain, given the abilities of these viruses to induce both strong Th1 and CD8+ T-cell responses (2, 14, 43).
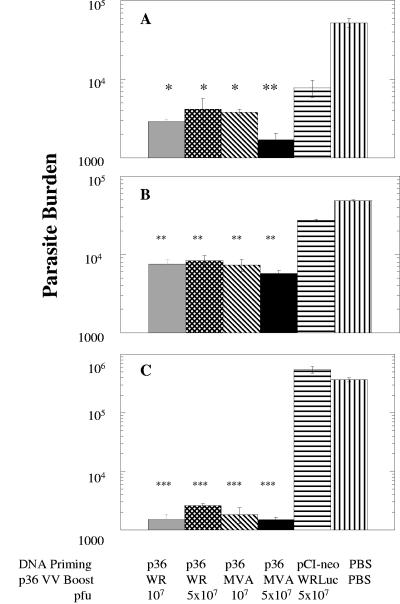
BALB/c mice (4 to 6 weeks of age) were vaccinated intradermally with 100 μg of DNA encoding the LACK antigen (DNAp36) and then boosted 2 weeks later intraperitoneally with 1 × 107 or 5 × 107 PFU of either recombinant Western Reserve-wild-type (WR-LACK or WRp36) or Ankara-MVA (MVA-LACK or MVAp36) vaccinia viruses expressing the LACK antigen. Three and one-half weeks after boosting, mice were infected intradermally in the ear pinnae using 107 metacyclic promastigotes of L. infantum, as previously described (1). One month after infection, the parasite burdens were evaluated by limiting dilution analysis in vaccinated and control groups of mice (1). This evaluation of protection in the spleen, the liver, and the draining lymph node demonstrated that the mice receiving a prime-boost vaccination using the LACK (p36) antigen were significantly protected against infection (Fig. 1). The levels of protection at each tissue site were comparable among the various vaccinated groups of mice and did not statistically differ between mice receiving the WRp36 or the MVAp36 virus. However, the level of protection did vary with the target organ site, with the highest levels of protection achieved in the draining lymph node (Fig. 1C). The level of protection in the draining lymph node was evidenced by a 144- to 244-fold reduction in the parasite burdens in comparison to those of control mice. Lower levels of protection were achieved when the parasite burdens were evaluated in the spleen and the liver. These results ranged from 6- to 9-fold reductions in parasite burdens in the liver and 9- to 30-fold reductions in the spleen. In the spleen, a slight protective effect was also observed for the mice receiving control DNA and vaccinia virus (WR-Luc), which may be due to the low gamma interferon (IFN-γ) response observed for these mice (Fig. 2). However, in other vaccine experiments employing WR-Luc (107 PFU) (44; data not shown) an IFN-γ response and a reduction of the splenic parasite burden were not consistently observed, nor were reductions in parasite burdens observed in the livers and lymph nodes of the WR-Luc-vaccinated mice (Fig. 1). However, the differences between the parasite burdens observed for the WR-Luc-vaccinated mice and those for mice receiving WR-LACK or MVA-LACK were significant (P < 0.02 to 0.05) (Fig. 1A), demonstrating a LACK antigen-specific effect.
FIG. 1.
Protection against visceral leishmaniasis in LACK DNA-LACK vaccinia virus-vaccinated mice. Shown are the results of parasite burden analyses of BALB/c mice vaccinated with a prime-boost regimen (DNA genes and recombinant vaccinia virus [VV]) using the LACK antigen and then infected intradermally with L. infantum promastigotes. Parasite burdens were determined using limiting dilution analyses and represent the averaged values for at least four mice/group. (A) Spleen; (B) liver; (C) lymph node. Statistical analyses were performed using Student's t test comparing vaccine groups to a vector control group (pCI-neo-WRLuc). ***, P < 0.001; **, P < 0.01; *, P < 0.05. PBS, phosphate-buffered saline.
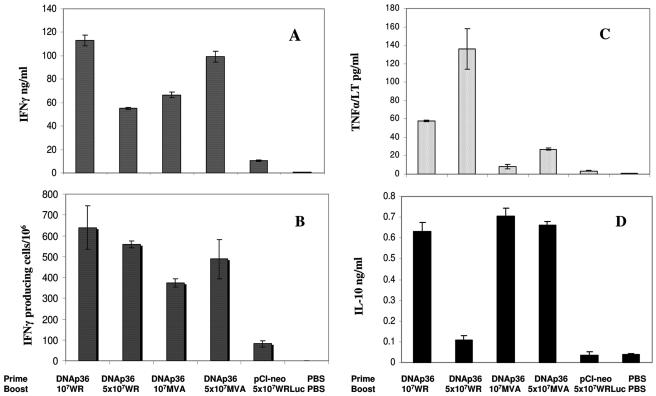
FIG. 2.
Antigen-specific IFN-γ, TNF-α, and IL-10 responses in prime-boost vaccinated mice prior to infection. Shown are the IFN-γ, TNF-α, and IL-10 responses found for the vaccinated and control groups of mice (as indicated) prior to infection. The results of (A) ELISAs of IFN-γ from culture supernatants of splenic cells in response to recombinant LACK antigen, (B) corresponding enzyme-linked immunospot analyses of IFN-γ, and ELISAs of (C) TNF-α/LT and (D) IL-10 are shown. PBS, phosphate-buffered saline.
IFN-γ and tumor necrosis factor alpha/lymphotoxin (TNF-α/LT) have been found to be involved in resistance to infection in murine VL (27, 29, 30, 46), while IL-10 correlates with susceptibility (26, 33). The levels of IFN-γ, IL-10, and TNF-α/LT produced by spleen cells of vaccinated and nonvaccinated mice in response to LACK antigen were evaluated before infection and at 1 month after infection (Fig. 2). Before infection, mice receiving 107 WRp36 or 5 × 107 MVAp36 PFU appeared to produce somewhat higher levels of IFN-γ (100 to 113 ng/ml) than mice boosted with either 5 × 107 WRp36 or 107 MVAp36 PFU (55 to 67 ng/ml) (Fig. 2A). As shown in Fig. 2B, enzyme-linked immunospot analyses (44) indicated that the number of IFN-γ-secreting cells correlated with the levels of IFN-γ found by enzyme-linked immunosorbent assay (ELISA), with the frequency of IFN-γ-producing cells ranging from 380 to 640/106 spleen cells. In addition, significant levels of TNF-α/LT (58 and 134 pg/ml) were observed for mice boosted with recombinant wild-type WRp36, while lower levels of TNF-α/LT were produced in response to LACK antigen by mice receiving MVAp36 (27 pg/ml and 8 pg/ml, respectively). These differences in the levels of induction of TNF-α may reflect, in part, the different abilities of WR and attenuated MVA vaccinia viruses to induce an inflammatory response and NF-κB activation, which result in distinct cytokine profiles. MVA has been shown to enhance NF-κB activation, while WR appears to inhibit it (18, 32, 40).
The amounts of LACK-specific IL-10 produced by splenocytes before challenge varied from 0.1 ng/ml in mice boosted with 5 × 107 WRp36 PFU to 0.7 ng/ml in those receiving 107 WRp36 or MVAp36 PFU (Fig. 2D).
The cytokine responses at 1 month postinfection paralleled but were somewhat higher than those found prior to infection. IFN-γ levels ranged from 20 ng/ml in mice receiving 5 × 107 WRp36 PFU to 204 ng/ml in those boosted with 5 × 107 MVAp36 PFU. The levels of TNF-α/LT in response to LACK antigen stimulation in vaccinated mice ranged from 64 pg/ml in mice boosted with 107 MVAp36 PFU to 120 pg/ml in the group boosted with 5 × 107 WRp36 PFU. Significant levels of IL-10 (0.04 ng/ml to 0.54 ng/ml) were also produced in response to LACK antigen at 1 month postinfection. Both the level of IFN-γ and the IFN-γ/IL-10 ratio found at 1 month postinfection appeared to correlate with the protection levels found (Table 1).
TABLE 1.
Cytokine levels and corresponding reductions (n-fold) in parasite burden at 1 month postinfection
| Expl group | IFN-γ/ IL-10 ratio | Amt of IFN-γ (ng/ml) | Fold reduction in parasite burden, by tissue site
|
||
|---|---|---|---|---|---|
| Spleen | Liver | Lymph node | |||
| DNAp36 + 5 × 107 MVA PFU | 4,633 | 204 | 31 | 9 | 244 |
| DNAp36 + 107 WR PFU | 720 | 153 | 18 | 6.5 | 244 |
| DNAp36 + 107 MVA PFU | 271 | 147 | 12.8 | 6.7 | 203 |
| DNAp36 + 5 × 107 WR PFU | 92 | 20 | 9.2 | 6 | 144 |
Both IFN-γ and TNF-α are implicated in the macrophage killing of intracellular L. donovani, through the up-regulation of inducible NO synthase (iNOS) and production of nitrite oxide (28). Nitric oxide has been demonstrated to be critical for the leishmanicidal activity of murine macrophages (4, 16, 19) and also has been shown to enhance, at low levels, a Th1 response (31). However, IL-10 is known to down-regulate macrophage NO production (26). To further determine the ongoing immune response dynamics in the vaccinated versus control mice, the induction of NO/nitrite at 1 month postchallenge was examined. Significant amounts of this antimicrobial agent were observed and ranged from 6 to 7 μM in the vaccinated mice, with control group NO/nitrite levels ranging from 0.6 μM to 1 μM. Therefore, the vaccinated mice, consistent with the findings for levels of IFN-γ (Fig. 2; Table 1), had higher levels of nitric oxide induction and potential leishmanicidal activity. These results were consistent with the protection found in the LACK-DNA-LACK-WR- or MVA-vaccinated mice.
In conclusion, this study demonstrated that a heterologous prime-boost regimen using DNA and vaccinia virus, both expressing the same antigen, LACK, is highly immunogenic and confers protection against murine L. infantum infection. Notably, the highly attenuated MVA and the replication-competent WR strain vaccinia viruses achieved comparable levels of protection. This heterologous prime-boost approach resulted in higher levels of IFN-γ (up to 200 ng/ml) than those reported for DNA-DNA vaccination (6 to 12 ng/ml) (23), where protection was not achieved. This observation prompts a question on the biologically effective amount of IFN-γ required to induce protection against VL. Although additional effector mechanisms may be involved, these results suggest that higher levels of IFN-γ may be required for protection against visceral disease than are needed against cutaneous leishmaniasis. Future studies will explore the use of this vaccine approach for a composite/multicomponent strategy against visceral leishmaniasis.
Acknowledgments
This work was supported through grants from NIH (AI45044 and AI27811) and grants from the EU (QLK2-CT-2002-01867) and Communidad de Madrid (GR/SAL/0862/2004). Eva Perez-Jimenez is a recipient of a predoctoral fellowship from the Ministerio de Educacion y Ciencia, Spain.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Ahmed, S., M. Colmenares, L. Soong, K. Goldsmith-Pestana, L. Munstermann, R. Molina, and D. McMahon-Pratt. 2003. Intradermal infection model for pathogenesis and vaccine studies of murine visceral leishmaniasis. Infect. Immun. 71:401-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, R. J., C. M. Hannan, S. C. Gilbert, S. M. Laidlaw, E. G. Sheu, S. Korten, R. Sinden, G. A. Butcher, M. A. Skinner, and A. V. Hill. 2004. Enhanced CD8+ T cell immune responses and protection elicited against Plasmodium berghei malaria by prime boost immunization regimens using a novel attenuated fowlpox virus. J. Immunol. 172:3094-3100. [DOI] [PubMed] [Google Scholar]
- 3.Belli, S., A. Formenton, T. Noll, A. Ivens, R. Jacquet, C. Desponds, D. Hofer, and N. Fasel. 1999. Leishmania major: histone H1 gene expression from the sw3 locus. Exp. Parasitol. 91:151-160. [DOI] [PubMed] [Google Scholar]
- 4.Bhakuni, V., U. K. Singha, G. P. Dutta, H. B. Levy, and R. K. Maheshwari. 1996. Killing of Leishmania donovani amastigotes by poly ICLC in hamsters. J. Interferon Cytokine Res. 16:321-325. [DOI] [PubMed] [Google Scholar]
- 5.Campos-Neto, A., R. Porrozzi, K. Greeson, R. N. Coler, J. R. Webb, Y. A. W. Seiky, S. G. Reed, and G. Grimaldi, Jr. 2001. Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infect. Immun. 69:4103-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coelho, E. A. F., C. A. P. Tavares, F. A. A. Carvalho, K. F. Chaves, K. N. Teixeira, R. C. Rodrigues, H. Charest, G. Matlashewski, R. T. Gazzinelli, and A. P. Fernandes. 2003. Immune responses induced by the Leishmania (Leishmania) donovani A2 antigen, but not by the LACK antigen, are protective against experimental Leishmania (Leishmania) amazonensis infection. Infect. Immun. 71:3988-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumonteil, E., R. S. Maria Jesus, E. O. Javier, and G. M. Maria del Rosario. 2003. DNA vaccines induce partial protection against Leishmania mexicana. Vaccine 21:2161-2168. [DOI] [PubMed] [Google Scholar]
- 8.Dye, C. 1996. The logic of visceral leishmaniasis control. Am. J. Trop. Med. Hyg. 55:125-130. [DOI] [PubMed] [Google Scholar]
- 9.Engwerda, C. R., and P. M. Kaye. 2000. Organ-specific immune responses associated with infectious disease. Immunol. Today 21:73-78. [DOI] [PubMed] [Google Scholar]
- 10.Engwerda, C. R., M. L. Murphy, S. E. Cotterell, S. C. Smelt, and P. M. Kaye. 1998. Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur. J. Immunol. 28:669-680. [DOI] [PubMed] [Google Scholar]
- 11.Fragaki, K., I. Suffia, B. Ferrua, D. Rousseau, Y. Le Fichoux, and J. Kubar. 2001. Immunisation with DNA encoding Leishmania infantum protein papLe22 decreases the frequency of parasitemic episodes in infected hamsters. Vaccine 19:1701-1709. [DOI] [PubMed] [Google Scholar]
- 12.Ghosh, A., S. Labrecque, and G. Matlashewski. 2001. Protection against Leishmania donovani infection by DNA vaccination: increased DNA vaccination efficiency through inhibiting the cellular p53 response. Vaccine 19:3169-3178. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh, A., W. W. Zhang, and G. Matlashewski. 2001. Immunization with A2 protein results in a mixed Th1/Th2 and a humoral response which protects mice against Leishmania donovani infections. Vaccine 20:59-66. [DOI] [PubMed] [Google Scholar]
- 14.Gomez, C. E., D. Rodriguez, J. R. Rodriguez, F. Abaitua, C. Duarte, and M. Esteban. 2001. Enhanced CD8+ T cell immune response against a V3 loop multiepitope polypeptide (TAB13) of HIV-1 Env after priming with purified fusion protein and booster with modified vaccinia virus Ankara (MVA-TAB) recombinant: a comparison of humoral and cellular immune responses with the vaccinia virus Western Reserve (WR) vector. Vaccine 20:961-971. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalo, R. M., G. del Real, J. R. Rodriguez, D. Rodriguez, R. Heljasvaara, P. Lucas, V. Larraga, and M. Esteban. 2002. A heterologous prime-boost regime using DNA and recombinant vaccinia virus expressing the Leishmania infantum P36/LACK antigen protects BALB/c mice from cutaneous leishmaniasis. Vaccine 20:1226-1231. [DOI] [PubMed] [Google Scholar]
- 16.Green, S. J., M. S. Meltzer, J. B. Hibbs, Jr., and C. A. Nacy. 1990. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J. Immunol. 144:278-283. [PubMed] [Google Scholar]
- 17.Guerin, P. J., P. Olliaro, S. Sundar, M. Boelaert, S. L. Croft, P. Desjeux, M. K. Wasunna, and A. D. Bryceson. 2002. Visceral leishmaniasis: current status of control, diagnosis, and treatment, and a proposed research and development agenda. Lancet Infect. Dis. 2:494-501. [DOI] [PubMed] [Google Scholar]
- 18.Guerra, S., L. A. López-Fernández, R. Conde, A. Pascual-Montano, K. Harshman, and M. Esteban. 2004. Microarray analysis reveals characteristic changes of host cell gene expression in response to attenuated modified vaccinia virus Ankara infection of human HeLa cells. J. Virol. 78:5820-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liew, F. Y., S. Millott, C. Parkinson, R. M. Palmer, and S. Moncada. 1990. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from l-arginine. J. Immunol. 144:4794-4797. [PubMed] [Google Scholar]
- 20.Lopez-Fuertes, L., E. Perez-Jimenez, A. J. Vila-Coro, F. Sack, S. Moreno, S. A. Konig, C. Junghans, B. Wittig, M. Timon, and M. Esteban. 2002. DNA vaccination with linear minimalistic (MIDGE) vectors confers protection against Leishmania major infection in mice. Vaccine 21:247-257. [DOI] [PubMed] [Google Scholar]
- 21.Masina, S., M. M. Gicheru, S. O. Demotz, and N. J. Fasel. 2003. Protection against cutaneous leishmaniasis in outbred vervet monkeys using a recombinant histone H1 antigen. J. Infect. Dis. 188:1250-1257. [DOI] [PubMed] [Google Scholar]
- 22.Mazumdar, T., K. Anam, and N. Ali. 2004. A mixed Th1/Th2 response elicited by a liposomal formulation of Leishmania vaccine instructs Th1 responses and resistance to Leishmania donovani in susceptible BALB/c mice. Vaccine 22:1162-1171. [DOI] [PubMed] [Google Scholar]
- 23.Melby, P. C., J. Yang, W. Zhao, L. E. Perez, and J. Cheng. 2001. Leishmania donovani p36(LACK) DNA vaccine is highly immunogenic but not protective against experimental visceral leishmaniasis. Infect. Immun. 69:4719-4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moss, B. 1996. Genetically engineered poxviruses for recombinant gene expression, vaccination, and safety. Proc. Natl. Acad. Sci. USA 93:11341-11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mougneau, E., F. Altare, A. E. Wakil, S. Zheng, T. Coppola, Z. E. Wang, R. Waldmann, R. M. Locksley, and N. Glaichenhaus. 1995. Expression cloning of a protective Leishmania antigen. Science 268:563-566. [DOI] [PubMed] [Google Scholar]
- 26.Murphy, M. L., U. Wille, E. N. Villegas, C. A. Hunter, and J. P. Farrell. 2001. IL-10 mediates susceptibility to Leishmania donovani infection. Eur. J. Immunol. 31:2848-2856. [DOI] [PubMed] [Google Scholar]
- 27.Murray, H. W., A. Jungbluth, E. Ritter, C. Montelibano, and M. W. Marino. 2000. Visceral leishmaniasis in mice devoid of tumor necrosis factor and response to treatment. Infect. Immun. 68:6289-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray, H. W., and C. F. Nathan. 1999. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 189:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray, H. W., G. L. Spitalny, and C. F. Nathan. 1985. Activation of mouse peritoneal macrophages in vitro and in vivo by interferon-gamma. J. Immunol. 134:1619-1622. [PubMed] [Google Scholar]
- 30.Murray, H. W., J. J. Stern, K. Welte, B. Y. Rubin, S. M. Carriero, and C. F. Nathan. 1987. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-gamma, tissue immune reaction, and response to treatment with interleukin 2 and interferon-gamma. J. Immunol. 138:2290-2297. [PubMed] [Google Scholar]
- 31.Niedbala, W., X. Q. Wei, D. Piedrafita, D. Xu, and F. Y. Liew. 1999. Effects of nitric oxide on the induction and differentiation of Th1 cells. Eur. J. Immunol. 29:2498-2505. [DOI] [PubMed] [Google Scholar]
- 32.Oie, K. L., and D. J. Pickup. 2001. Cowpox virus and other members of the orthopoxvirus genus interfere with the regulation of NF-kappaB activation. Virology 288:175-187. [DOI] [PubMed] [Google Scholar]
- 33.Padigel, U. M., J. Alexander, and J. P. Farrell. 2003. The role of interleukin-10 in susceptibility of BALB/c mice to infection with Leishmania mexicana and Leishmania amazonensis. J. Immunol. 171:3705-3710. [DOI] [PubMed] [Google Scholar]
- 34.Pinelli, E., S. Y. van der Kaaij, R. Slappendel, C. Fragio, E. J. Ruitenberg, W. Bernadina, and V. P. Rutten. 1999. Detection of canine cytokine gene expression by reverse transcription-polymerase chain reaction. Vet. Immunol. Immunopathol. 69:121-126. [DOI] [PubMed] [Google Scholar]
- 35.Quinnell, R. J., O. Courtenay, M. A. Shaw, M. J. Day, L. M. Garcez, C. Dye, and P. M. Kaye. 2001. Tissue cytokine responses in canine visceral leishmaniasis. J. Infect. Dis. 183:1421-1424. [DOI] [PubMed] [Google Scholar]
- 36.Rachamim, N., and C. L. Jaffe. 1993. Pure protein from Leishmania donovani protects mice against both cutaneous and visceral leishmaniasis. J. Immunol. 150:2322-2331. [PubMed] [Google Scholar]
- 37.Ramiro, M. J., J. J. Zarate, T. Hanke, D. Rodriguez, J. R. Rodriguez, M. Esteban, J. Lucientes, J. A. Castillo, and V. Larraga. 2003. Protection in dogs against visceral leishmaniasis caused by Leishmania infantum is achieved by immunization with a heterologous prime-boost regime using DNA and vaccinia recombinant vectors expressing LACK. Vaccine 21:2474-2484. [DOI] [PubMed] [Google Scholar]
- 38.Santos, W. R., V. M. de Lima, E. P. de Souza, R. R. Bernardo, M. Palatnik, and C. B. de Sousa. 2002. Saponins, IL-12 and BCG adjuvant in the FML-vaccine formulation against murine visceral leishmaniasis. Vaccine 21:30-43. [DOI] [PubMed] [Google Scholar]
- 39.Santos-Gomes, G. M., R. Rosa, C. Leandro, S. Cortes, P. Romao, and H. Silveira. 2002. Cytokine expression during the outcome of canine experimental infection by Leishmania infantum. Vet. Immunol. Immunopathol. 88:21-30. [DOI] [PubMed] [Google Scholar]
- 40.Shisler, J. L., and X.-L. Jin. 2004. The vaccinia virus K1L gene product inhibits host NF-κB activation by preventing IκBα degradation. J. Virol. 78:3553-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smelt, S. C., S. E. Cotterell, C. R. Engwerda, and P. M. Kaye. 2000. B cell-deficient mice are highly resistant to Leishmania donovani infection, but develop neutrophil-mediated tissue pathology. J. Immunol. 164:3681-3688. [DOI] [PubMed] [Google Scholar]
- 42.Sundar, S., T. K. Jha, C. P. Thakur, J. Engel, H. Sindermann, C. Fischer, K. Junge, A. Bryceson, and J. Berman. 2002. Oral miltefosine for Indian visceral leishmaniasis. N. Engl. J. Med. 347:1739-1746. [DOI] [PubMed] [Google Scholar]
- 43.Sutter, G., and C. Staib. 2003. Vaccinia vectors as candidate vaccines: the development of modified vaccinia virus Ankara for antigen delivery. Curr. Drug Targets Infect. Disord. 3:263-271. [DOI] [PubMed] [Google Scholar]
- 44.Tapia, E., E. Perez-Jimenez, L. Lopez-Fuertes, R. Gonzalo, M. M. Gherardi, and M. Esteban. 2003. The combination of DNA vectors expressing IL-12 + IL-18 elicits high protective immune response against cutaneous leishmaniasis after priming with DNA-p36/LACK and the cytokines, followed by a booster with a vaccinia virus recombinant expressing p36/LACK. Microbes Infect. 5:73-84. [DOI] [PubMed] [Google Scholar]
- 45.Tesh, R. B. 1995. Control of zoonotic visceral leishmaniasis: is it time to change strategies? Am. J. Trop. Med. Hyg. 52:287-292. [DOI] [PubMed] [Google Scholar]
- 46.Tumang, M. C., C. Keogh, L. L. Moldawer, D. C. Helfgott, R. Teitelbaum, J. Hariprashad, and H. W. Murray. 1994. Role and effect of TNF-α in experimental visceral leishmaniasis. J. Immunol. 153:768-775. [PubMed] [Google Scholar]