Abstract
Brain aging is associated with altered Ca2+regulation. However, many Ca2+ signal transduction mechanisms have not been explored in the aged brain. Here, we report that cytosolic expression and activity of the Ca2+-dependent protein phosphatase calcineurin (CaN) increases in the hippocampus during aging. CaN changes were paralleled by increased activation, but not expression, of CaN-regulated protein phosphatase 1 and a reduction in the phosphorylation state of CaN substrates involved in cell survival (i.e., Bcl-2-associated death protein and cAMP response element-binding protein). The age-related increase in CaN activity was not attributable to the inability of CaN to translocate to the membrane and was reduced by blocking L-type Ca2+ channels. Finally, increased CaN activity correlated with memory function as measured with the Morris water escape task. The results suggest that altered regulation of CaN is one of the processes that could link Ca2+dyshomeostasis to age-related changes in neural function and cognition.
Keywords: aging, apoptosis, calcineurin, calcium, hippocampus, memory, phosphatase, PP2B, PP1, synaptic plasticity, CREB
An influential hypothesis of brain aging states that age-related changes in neuronal function, viability, and ultimately cognition are attributable, in part, to the gradual dysregulation of neuronal Ca2+ (Disterhoft et al., 1994; Khachaturian, 1994; Landfield, 1994; Foster and Norris, 1997; Thibault et al., 1998; Verkhratsky and Toescu, 1998). This hypothesis has received considerable support from a number of studies, including those that have shown age-dependent alterations in Ca2+-dependent synaptic plasticity (Landfield et al., 1986; Norris et al., 1996, 1998b; Shankar et al., 1998), and others that have found an age-related increase in vulnerability to Ca2+-dependent neurotoxicity and neurodegeneration (Landfield et al., 1992; Mattson, 1992). Whereas possible sources for Ca2+dysregulation have been identified and studied extensively in aged brain tissue (for review, see Thibault et al., 1998; Verkhratsky and Toescu, 1998), relatively less research has focused on the signaling pathways that translate changes in Ca2+ regulation into altered neuronal function and cognition.
In recent years, it has been recognized with increasing frequency that the Ca2+/calmodulin-dependent protein phosphatase calcineurin (CaN) provides a critical link between Ca2+ regulation, synaptic plasticity, cell survival, and cognition (Perrino and Soderling, 1998). For example, overexpression of CaN in young adult animals leads to altered synaptic function and memory retention deficits (Mansuy et al., 1998; Winder et al., 1998; Zhuo et al., 1999) remarkably similar to that observed in normal aged rodents (Barnes and McNaughton, 1985; Foster, 1999). Furthermore, excess stimulation of CaN, by raising intracellular Ca2+ levels, releases CaN into the cytosol in which it may cause neuronal apoptosis via dephosphorylation of key cytosolic components, such as the Bcl family member Bcl-2-associated death protein (BAD) (Wang et al., 1999) and the cAMP response element-binding protein (CREB) (Bito et al., 1996; Bonni et al., 1999; Riccio et al., 1999; Walton et al., 1999). Together, the evidence raises the possibility that age-related impairments in neural function and cognition may involve increased CaN activity as a result of Ca2+dyshomeostasis. However, it remains to be determined whether CaN properties are altered with advanced age.
To establish a more definitive link between brain aging and CaN activity, we characterized the expression and localization of CaN in the hippocampus of young adult and aged rats. CaN activity levels were also determined by examining the phosphorylation state of downstream targets (i.e., BAD and CREB) involved in cell survival and synaptic plasticity. Moreover, we explored whether blockade of L-type Ca2+ channels, which can ameliorate age-related neuronal and cognitive deficits (Foster, 1999), is effective at altering the activity of CaN in aged hippocampal tissue. Finally, we examined the relationship between the age-related increase in CaN activity and memory function. The results provide the first characterization of CaN expression and activity in normal aged brain tissue and may have major implications for the Ca2+ hypothesis of brain aging and cognitive decline.
MATERIALS AND METHODS
Subjects. Young adult (6–12 months) and aged (20–24 months) male Fischer 344 rats were used for all studies.
Tissue preparation. Rats were anesthetized with CO2 and decapitated, and hippocampi were removed. Hippocampi were weighed and placed in separate homogenization tubes with 3 ml of homogenization buffer, pH 7.0, containing 10 mmNaH2PO4, 100 mm NaCl, 10 mmNa4P2O7, 50 mm NaF, 1 mmNa3VO4, 5 mm EGTA, and 10 U/ml aprotinin. The tissue was homogenized for 30 sec using a Teflon pestle. Homogenates were centrifuged at 100,000 × g for 1 hr at 4° C. The supernatant fraction (750 μl) from each tube was placed into separate spin columns (Promega, Madison, WI) and spun at 600 ×g for 5 min at 4° C to remove the endogenous phosphates. The flow through was collected, and the total protein concentration for enzyme assays and Western blots (see below) was determined using the Bradford method (Bio-Rad, Hercules, CA).
Enzyme assay. Enzyme activity was examined under three conditions: basal, activation, and inhibition. For basal activity, flow through containing 5 μg of total protein, 5 μl of the phosphopeptide substrate (Promega), and 40 μl of buffer was added to each well of a standard 96-well plate. To block the activity of other phosphatases, okadaic acid (5 μm) was added to the buffer for assays of CaN.
For activation of CaN and protein phosphatase 1 (PP1), 2 mm Ca2+ and 20 U/ml calmodulin was added to the buffer. Under conditions of enzyme inhibition, FK506 (50 μm) or okadaic acid (5 μm) (Calbiochem, San Diego, CA) was added to the buffer to suppress CaN and PP1, respectively. After a 5 min incubation period (22–25°C), 50 μl of stop solution was added to each well. After an additional 15 min development time, absorbance was read at 620 nm on a plate reader (Anthos 2001; Anthos, Salzburg, Austria).
Western blot analysis. Whole-tissue homogenate and supernatant cytosolic fractions (40 μg of total protein) were resolved on 12% gels using SDS-PAGE and then transferred to nitrocellulose. The nitrocellulose sections were probed with anti-CaN, anti-PP1 polyclonal antibodies, or phospho-specific and phosphorylation state-independent polyclonal antibodies against BAD and CREB (Upstate Biotechnology, Lake Placid, NY), followed by incubation with the appropriate secondary antibodies conjugated with horseradish peroxidase (1:2000; Bio-Rad, Hercules, CA). The immunoreactive bands were visualized by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, NJ) and captured on autoradiography film (Amersham Pharmacia Biotech Hyperfilm ECL). Densitometric scans of the autoradiographs were digitized on a ScanJet 6200C (Hewlett Packard, Palo Alto, CA) with Precision Scan Pro software (Hewlett Packard) and quantified using Image Quant 5.0 (Molecular Dynamics, Sunnyvale, CA) image analysis software.
Surgery. For some studies, rats were anesthetized with ketamine–xylazine (20 and 2 mg/ml, respectively; 1 ml/kg body weight), and an incision was made to expose the skull. Holes were drilled bilaterally over the dorsal hippocampus (relative to bregma; 3.8 mm posterior; 2.1 mm lateral) for the administration of nimodipine or vehicle. The drug injection–electrophysiology procedure was similar to that of Foster and Deadwyler (1992). A glass recording pipette (<5 MΩ) was filled with nimodipine (20 μm) in artificial CSF (ACSF) and was lowered into the brain. A recording pipette filled with vehicle alone was lowered into the contralateral side. Electrophysiological recordings of cell discharge activity were used to localize the electrodes to just below the CA1 layer of the hippocampus (∼3–4 mm V), and the drug or vehicle alone was injected (∼5–10 μl) over a 10 min period. After injections, electrophysiological recording was monitored for signs of seizure activity. Twenty minutes after injections, animals were killed, and the dorsal hippocampus was collected for enzyme assays.
In vitro preparation. Methods for preparation of hippocampal slices have been described in detail previously (Norris et al., 1998b). Briefly, hippocampi were dissected out, and slices (400 μm) were cut parallel to the alvear fibers using a tissue chopper. Slices were then transferred to a standard recording chamber and perfused (1 ml/min, 30–32°C) with oxygenated recording medium containing (in mm): 124 NaCl, 2 KCl, 1.25 KH2PO4, 2 MgSO4, 2 CaCl2, 26 NaHCO3, and 10 glucose. Humidified air (95% O2, 5% CO2) was blown over the slices. Extracellular synaptic field potentials from CA3–CA1 synaptic contacts were recorded with glass micropipettes (4–6 MΩ) filled with recording medium. A stimulating electrode was positioned on either side of a recording electrode localized to the middle of stratum radiatum, and single pulses were delivered at 0.033 Hz. The stimulation intensity was adjusted to elicit similar EPSP responses (∼1–2 mV) across treatment groups.
Behavioral testing. Methods for using the Morris swim task to access sensorimotor and memory deficits have been published previously (Foster et al., 1991; Mabry et al., 1996; Fugger et al., 1997; Norris and Foster, 1999). Animals were trained in a black tank, 1.7 m in diameter, positioned in a well lit room that was painted flat black. Water temperature was maintained at 27 ± 2° C and at a level ∼8 cm below the rim of the tank. Behavioral data, including cumulative path length and latency to escape to the platform (29 cm in diameter) during training trials was acquired with a Columbus Instruments (Columbus, OH) tracking system. For probe trials in which the platform was removed, differential quadrant search time and platform crossings were recorded. These measures are more sensitive to effects on memory than using latency alone (Norris and Foster, 1999).
Cue discrimination. Animals were tested in groups of six to eight animals of mixed ages. Animals were initially habituated to the pool by allotting a 30 sec free swim and three trials to climb onto a platform from three different directions. A white flag was attached to the platform, and the platform was extended 1 cm above the water level. Training consisted of five blocks of three trials with all training massed into 1 d. Intertrial intervals were 20 sec, and interblock intervals were ∼15 min. At the beginning of each trial, the rat was placed in the water from one of four equally spaced start locations (north, south, east, and west). Subjects were allowed 60 sec to escape during each trial; if they did not escape within the allotted time, they were gently guided to the platform. Rats remained on the platform between trials and in home cages under heat lamps after each block. Platform and start locations were randomized across trials. Rats that failed to learn the cue task (not reaching the platform within the 60 sec on four trials of the last two blocks) were removed from the study.
Spatial discrimination. Spatial discrimination training began 3 d after cue training. For spatial discrimination, an assortment of cues was provided (e.g., wall posters). The escape platform was hidden ∼1.5 cm beneath the water level and remained in the same location relative to the distal cues in the room. Methods for training in the spatial version of the task were the same as for the cue version of the task. Training consisted of five blocks of three trials with all training massed into 1 d. Intertrial intervals and interblock intervals were the same as cue training. Fifteen minutes after the end of training, a free-swim probe trial was administered to examine acquisition of a spatial search strategy, and the probe trial was followed with a refresher training block in which the platform was reintroduced to the goal quadrant. Retention for platform location was tested 24 hr later using a second free-swim probe trial. The probe trials consisted of placing the animal in the tank for 1 min without the platform, and the time spent in each quadrant was recorded. Three days after completion of the retention probe trial, animals were killed for enzyme assays.
Statistical analyses. Unless otherwise stated, all data were analyzed using ANOVA with significance set at p< 0.05. Scheffe's post hoc tests were used to localize main effect interactions.
RESULTS
Cytosolic CaN activity and expression increase during aging
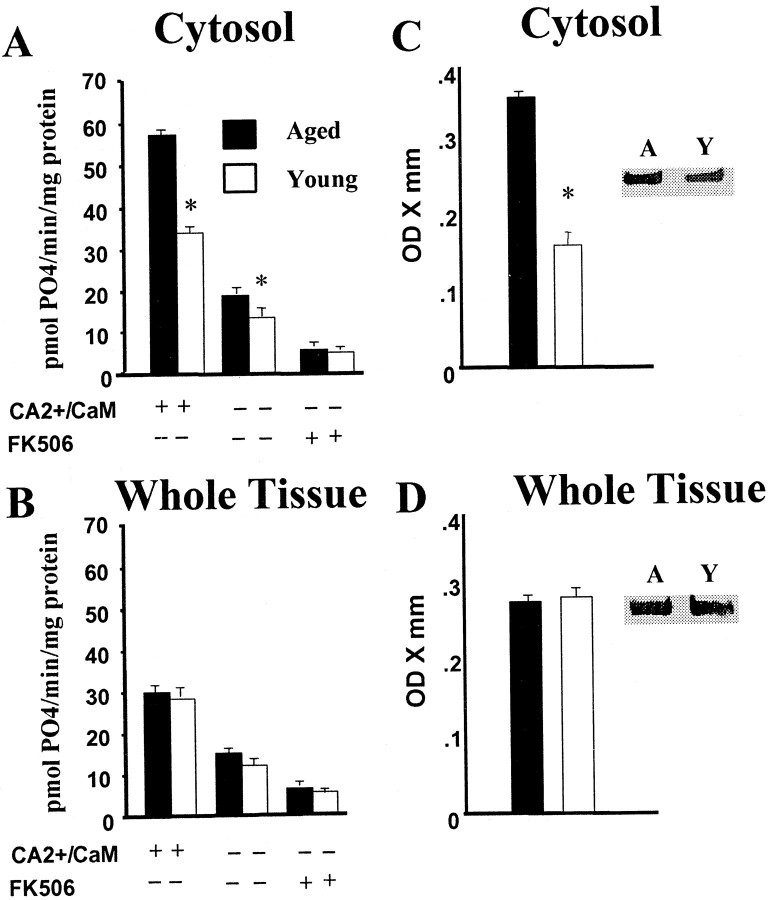
Tissue from the same animals were used for both CaN and PP1 assays. For enzyme analyses in whole-tissue homogenate samples, each age group contained six rats, whereas cytosolic fraction assays had five rats per group. Figure 1 illustrates CaN activity measured in the hippocampus of young adult (6 months) and aged rats (22 months), under three different treatment conditions (activation, basal, and inhibition) in whole-tissue homogenate and cytosolic fractions. Regardless of age, the addition of Ca2+/CaM to the reaction buffer stimulated CaN activity, and this activity was suppressed by FK506 for both the whole-tissue homogenate (p < 0.0001) and the cytosolic fraction (p < 0.0001). Furthermore, an interaction of treatment and age was observed for the cytosolic fraction (p < 0.0001) and not in the whole homogenate (Fig. 1A,B). Post hoc comparisons indicated that, for the cytosolic fraction, CaN activity was increased for aged rats under both basal and activation treatments.
Fig. 1.
Cytosolic CaN activity is increased in the hippocampus of aged (filled bars) relative to young adult (open bars) rats. Measures of phosphate released by CaN activity in the cytosolic fraction (A) and in the whole-tissue homogenate (B) from young adult and aged rats under the three conditions of activation, basal, and inhibition. The + and − signs indicate the presence or absence, respectively, of added activators (CA2+/CaM) or the CaN inhibitor FK506. Protein levels of the catalytic subunit of CaN in the cytosolic fraction (C) and in the whole-tissue homogenate (D) from young adult and aged rats.Insets show representative immunoblots of the two groups (A, aged; Y, young adult). The results suggest that increased cytosolic CaN activity in the aged group is attributable to an age-related increase in CaN protein levels in the cytosol. In this and the following figures, asterisksindicate a significant (p < 0.05) age difference, and error bars illustrate group means ± SEM.
Western blots were performed to determine whether the increase in cytosolic CaN activity reflects an increase in the cytosolic expression of CaN. Although no age difference in CaN levels was observed in the whole-tissue homogenate, the cytosolic fraction collected from aged animals exhibited a marked increase in CaN expression (p < 0.001) (Fig. 1C,D), suggesting that increased CaN activity in the cytosol of aged rats is at least partially attributable to a specific increase in cytosolic CaN levels.
PP1 phosphatase activity is increased during aging
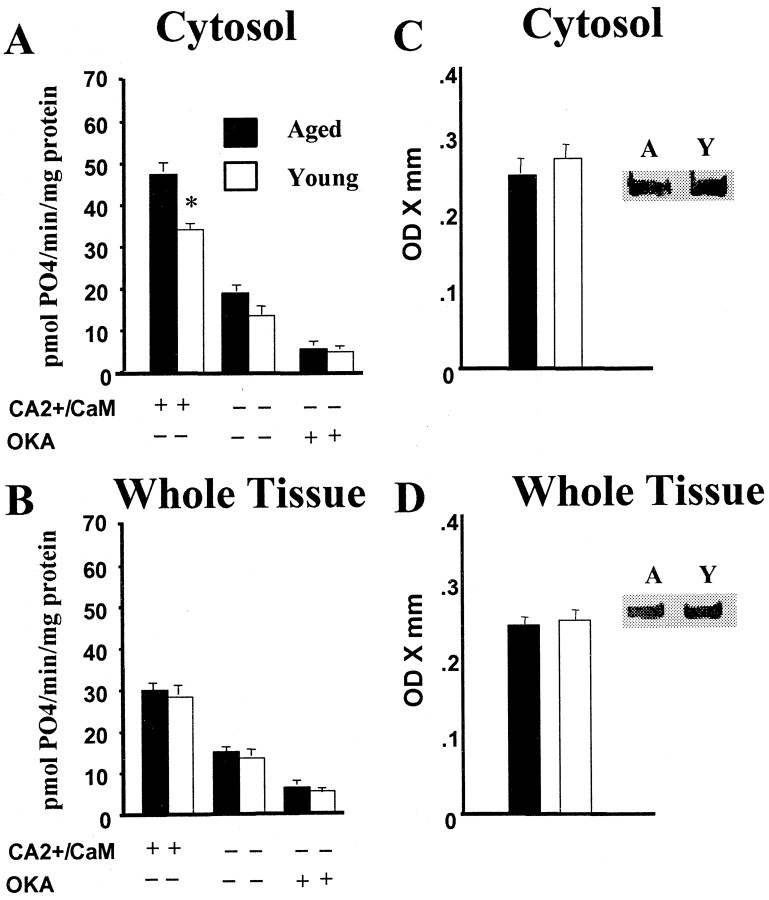
PP1 activity was influenced by the experimental conditions for both the whole-tissue homogenate (p < 0.0001) and cytosolic fraction (p < 0.0001). For both preparations, dephosphorylation of the phosphopeptide substrate under activation conditions was increased over both basal and inhibition conditions, whereas dephosphorylation under basal conditions was increased relative to inhibition conditions. Similar to CaN studies, no effect of age was observed for the whole homogenate, and the cytosolic fraction exhibited an age × treatment interaction (p < 0.0005) attributable to an increase in activity for aged animals under the activated treatment condition (Fig.2A,B). In contrast to the increased CaN expression in the cytosol, however, Western blots indicated no difference in PP1 levels in the whole homogenate or the cytosolic fraction (Fig. 2C,D). Moreover, the level of the cytoskeletal-associated protein microtubule-associated protein 2 was not different between age groups for both the whole-tissue homogenate (young adult, 0.323 ± 0.018, mean ± SEM, optical density × millimeter; aged, 0.315 ± 0.020) and the cytosolic fraction (young adult, 0.295 ± 0.015; aged, 0.298 ± 0.023), suggesting that age-related changes in CaN expression are relatively selective (data not shown). Given that PP1 can be indirectly regulated by CaN (Lisman, 1989), the results suggest that an age-related increase in PP1 activity may be driven by the increase in CaN activity.
Fig. 2.
Similar to CaN activity, cytosolic PP1 activity is increased in the hippocampus of aged (filled bars) relative to young adult (open bars) rats. Measures of phosphate released by PP1 activity in the cytosolic fraction (A) and in the whole-tissue homogenate (B) from young adult and aged rats. The + and − signs indicate the presence or absence, respectively, of added activators (CA2+/CaM) or the PP1 inhibitor okadaic acid (OKA). Protein levels of the catalytic subunit of PP1 in the cytosolic fraction (C) and in the whole-tissue homogenate (D) from young adult and aged rats.Insets show representative immunoblots of the two groups (A, aged; Y, young adult). Unlike CaN, the age-related increase in cytosolic PP1 activity is not associated with an increase in cytosolic PP1 protein levels.
Distribution of CaN is regulated by Ca2+and involves L-channel activity
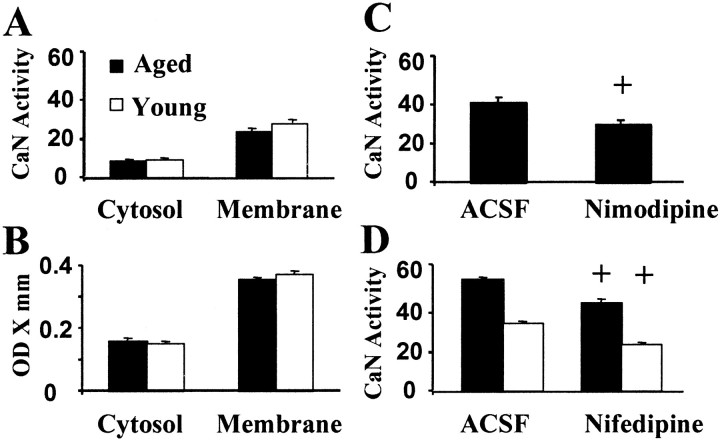
The results indicate that the increase in CaN activity during aging is likely attributable to a redistribution of CaN to the cytoplasm. To test the possibility that age-related differences in the redistribution of CaN are Ca2+ dependent, whole homogenates were incubated for 3 hr in Ca2+ free buffer. The membrane and cytosolic fractions were separated by centrifugation, and the membrane fraction was reconstituted. CaN activity was nearly undetectable in the cytosol after incubation in Ca2+-free media, indicating a loss of CaN from the cytosol in both age groups (Fig. 3A). Moreover, no age-related difference in CaN activity or expression level was observed for the cytosolic or membrane fractions (Fig. 3B). The results are consistent with the idea that age-related differences are not attributable to the inability of CaN to translocate or associate with the plasma membrane.
Fig. 3.
Increased CaN activity is a function of Ca2+ regulation and not an inability to translocate in response to Ca2+ levels. Measures of phosphate released by CaN activity (A) and protein levels of the catalytic subunit of CaN (B) in the cytosolic fraction and in the whole-tissue homogenate of hippocampus tissue of young adult (open bars) and aged (filled bars) rats after a 3 hr incubation in no Ca2+ medium. Note that when Ca2+is removed from the incubation buffer, age differences in CaN activity and expression are ameliorated. C, Measures of phosphate released by CaN activity in cytosolic fractions after the in vivo injection (0.5–1 μl/min) of nimodipine (20 μm) or vehicle (ACSF) into the hippocampus of aged rats anesthetized with ketamine–xylazine. D, Measures of phosphate released by CaN activity in cytosolic fractions from hippocampal slices bathed with ACSF or 10 μm of nifedipine.Plus signs indicate a significant difference (p < 0.05) from ACSF condition.
Previous research indicates that aging is associated with Ca2+ dysregulation, attributable in part to an increase in Ca2+ influx through L-type voltage-dependent Ca2+ channels (L-VDCCs) (Campbell et al., 1996; Thibault and Landfield, 1996). To explore whether L-VDCCs could contribute to the age-related increase in cytosolic CaN, aged rats (n = 4) were anesthetized with ketamine–xylazine and received an intrahippocampal injection of the L-VDCC antagonist nimodipine and a contralateral intrahippocampal injection of vehicle alone. In general, cytosolic CaN activity was reduced compared with rats anesthetized with CO2(compare Figs. 1, 3C), possibly attributable to anesthetic effects on NMDA receptor function (MacDonald et al., 1987). However, compared with their contralateral, vehicle-treated counterparts, hippocampi injected with nimodipine exhibited a significant decrease in cytosolic CaN activity (Fig. 3C) (paired t test;p < 0.05).
To further examine the effects of L-channel blockade, CaN activity was investigated in hippocampal slices. Previous research indicates that nifedipine can rapidly block L-channels in hippocampal slices and modify Ca2+-dependent synaptic plasticity (Norris et al., 1998b). Slice health was confirmed by recording CA3–CA1 evoked extracellular synaptic field potentials of at least 2 mV. Once it was determined that at least six slices were viable, three slices were harvested from the recording chamber and combined for enzyme assays, and nifedipine (10 μm) was applied to the remaining slices in the recording chamber. Approximately 15–20 min after nifedipine application, slice viability was reconfirmed, and three slices were harvested from the chamber and combined for enzyme assays. Figure 3D shows the results of L-channel blockade on phosphatase activity in hippocampal slices from young adult (6 months;n = 4) and aged (22 months; n = 4) rats. In contrast to the decrease in CaN activity for rats anesthetized with ketamine–xylazine, CaN activity in hippocampal slices was comparable with that observed for rats anesthetized with CO2 (compare Figs. 1,3D). An ANOVA indicated a significant effect of age (p < 0.0001) and treatment (p < 0.0001) in the absence of an age × treatment interaction. Thus, although CaN activity in aged animals was reduced by L-channel blockade, the percentage decrease in CaN activity was similar across the two groups.
Phosphorylation state of CaN substrates is reduced during aging
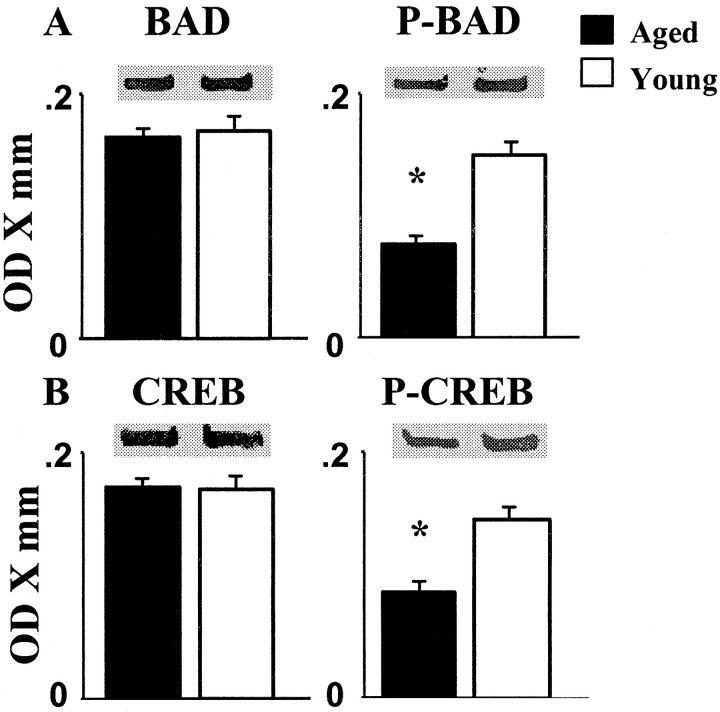
Phosphorylation of the proapoptotic factor BAD and CREB is controlled, in part, by CaN phosphatase activity. As such, these phosphoproteins may link CaN activation to processes such as apoptosis and long-lasting synaptic modification (Wang et al., 1999; Walton and Dragunow, 2000). We tested whether the phosphorylation levels of these proteins change as a function of advanced age. Western blots for cytosolic BAD and CREB were run using antibodies that were either phospho-specific or phosphorylation state independent. The results indicated that the levels of the two phospho-independent substrates are not altered with aging (Fig. 4). In contrast, the phosphorylated forms of both substrates were significantly reduced in aged animals (phospho-CREB, p< 0.001; phospho-BAD, p < 0.0005) (Fig. 4), consistent with an aging-related increase in hippocampal CaN activity.
Fig. 4.
The CaN substrates BAD and CREB exhibit decreased phosphorylation in the hippocampus of aged (filled bars) relative to young adult (open bars) rats.A, Measures of the cytosolic protein levels of phosphorylation state-independent BAD (BAD) and phospho-BAD (p-BAD). B, Measures of the cytosolic levels of phosphorylation state-independent CREB (CREB) and phospho-CREB (p-CREB). In contrast to their phosphorylation-independent counterparts, phospho-CREB and phospho-BAD are decreased in aged animals, consistent with an age-related increase in CaN activity. Insetsillustrate representative immunoblots.
Cytosolic CaN activity correlates with spatial memory decline during aging
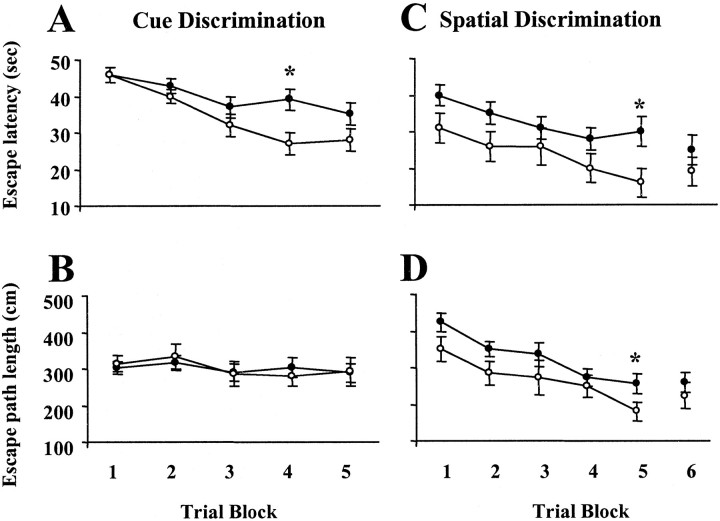
For these studies, young adult (n = 16) and aged (n = 29) rats were first trained on cue discrimination in the Morris water escape task, followed 3 d later by training on the spatial discrimination version of the task. Six of the aged animals were removed from the study because of sensorimotor deficits that prevented them from finding the platform in the cue or spatial tasks. Previous work indicates that young adult and aged animals can learn water escape tasks, observed as a decrease in escape latency and path length. However, differences can emerge because of reduced swim speed and impaired motor performance in aged animals (Foster et al., 1991;Frick et al., 1995). Repeated-measures ANOVAs across training blocks during the cue task confirmed a significant effect of training (p < 0.0001) and age (p< 0.05) for the escape latency in the absence of a training or age effect on path length to escape (Fig. 5). The absence of a training effect on path length is a consequence of animals that either floated or pawed at a limited segment of the pool wall during the initial training blocks, resulting in long latencies and short path lengths. ANOVAs within each age group across training blocks indicated that the latency to find the platform decreased over training (p < 0.05) for both groups, and ANOVAs across age groups within individual blocks indicated an age difference in latency only for training block 4.
Fig. 5.
Behavioral measures for aged (filled circles) and young adults (open circles) during behavioral training. Mean latency (A) and mean path length (B) to escape during cue discrimination training. Mean latency (C) and mean path length (D) to escape during spatial discrimination training. Each block consisted of three training trials, and training on each task was massed into a single day with 3 d between tasks. The break in the x-axis between blocks 5 and 6 indicates the time point at which a probe trial was administered to measure acquisition (see Fig. 6).Asterisks indicate a significant difference between the two age groups. Error bars indicate SEM.
Figure 5 also illustrates the escape latency and path length for the spatial discrimination version of the water escape task. Repeated-measures ANOVAs across training blocks indicated a significant effect of age (p < 0.05) and training (p < 0.0001) for both escape latency and path length. ANOVAs within each age group indicated that the latency to find the platform (p < 0.005) and the escape distance (p < 0.0005) decreased over training for both age groups, suggesting that the animals acquired a successful escape strategy (Fig. 5). Aged animals generally exhibit longer escape latency and path length, and examination of individual training blocks indicated an age difference in latency and path length for training block 5.
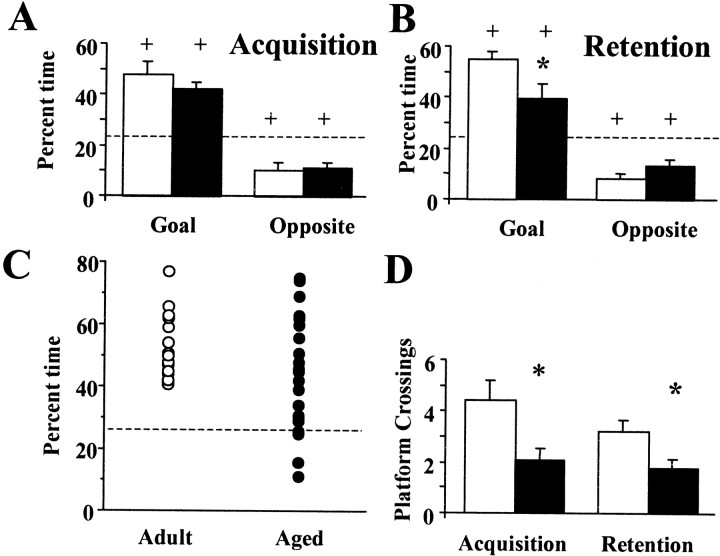
After completion of five training blocks, acquisition of a spatial search strategy was determined using a free-swim probe trial, which provides a more valid measure of spatial discrimination learning (Foster et al., 1991; Fugger et al., 1997; Norris and Foster, 1999). No age-related differences were observed for time spent in the goal quadrant or the quadrant opposite the goal (Fig.6A). Furthermore, for both age groups, the percentage of time spent in the goal and opposite quadrants was significantly above and below chance, respectively, indicating that animals were using a spatial search strategy focused on the goal quadrant. Despite a similar pattern of quadrant search times, focused on the goal quadrant, there was an age-related decrease in the number of platform crossings (p < 0.01), which may relate to the decline in motor performance with age (Frick et al., 1995).
Fig. 6.
Searching behavior during testing of acquisition and retention of spatial discrimination for aged (filled bars) and young adult (open bars) rats.A, After block 5 of training on the spatial version of the task, a probe trial was administered to measure acquisition. The mean percentage of time spent searching the goal quadrant (Goal) and the quadrant opposite the goal (Opposite) is illustrated for both age groups.B, An age-related decrease in percentage of time searching the goal quadrant was observed during the retention probe trial administered 24 hr after the acquisition probe trial.C, Relative to young adult animals, the aged groups exhibited larger variability in goal quadrant search time during the retention probe trial. D, An age-related decrease in platform crossings was observed during both the acquisition and retention probe trials. Asterisks indicate significant differences across age groups. Plus signs indicate significant difference from chance (dashed lines). Error bars indicate SEM.
Twenty-four hours later, a second probe trial was administered to determine retention. Although both age groups exhibited search behavior that was different from chance, the percentage of time searching the goal quadrant was reduced for aged animals (p < 0.05) relative to young adults (Fig. 6B). Also, the number of platform crossings (p < 0.05) continued to be reduced in the aged group (Fig. 6D). Furthermore, a characteristic increase in variability across individuals was observed for the aged group (Fig. 6C). Some aged animals appeared to randomly search the pool, spending ∼25% of the time searching each quadrant. For other aged animals, spatial discriminative search behavior was similar to that of young adult animals, with most of the time spent searching the goal quadrant.
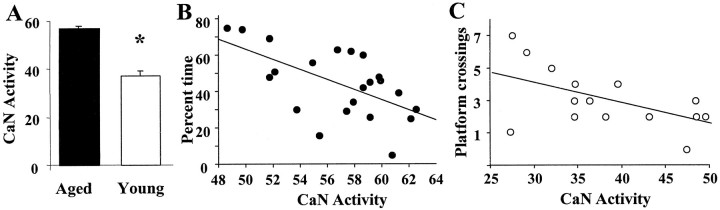
Examination of cytosolic CaN activity for these behaviorally characterized animals confirmed that CaN activity was reduced in young adults (p < 0.0001) relative to aged animals (Fig. 7A). Regression analysis was run on CaN activity and the probe trial data within each age group. No correlation was observed between CaN activity and quadrant search or platform crossings for the acquisition probe trial. In contrast, CaN activity was negatively correlated with the percentage of time searching the goal quadrant during retention testing of aged animals (R2 = 0.35; p< 0.005) (Fig. 7B). Quadrant search time did not correlate with CaN activity in young animals. The absence of a correlation for young adults was attributable to asymptotic performance by this age group, with all young adult animals exhibiting search times greater than two SDs above chance (Fig. 6). In contrast, the number of platform crossings during retention testing was highly variable for young adult rats (range of zero to seven crossings) and was negatively correlated with CaN activity (R2 = 0.28; p< 0.05) (Fig. 7C). For aged animals, platform crossing (range of zero to five crossings) was not correlated with CaN activity because of a floor effect in which seven of the 23 aged animals failed to cross the platform at least once.
Fig. 7.
Increased cytosolic CaN activity is associated with retention deficits examined by the water escape task.A, Mean cytosolic CaN activity for young adults (open bars) and aged animals (filled bars) that were behaviorally characterized on the water escape task. B, Correlation between cytosolic CaN activity and percentage of time in the goal quadrant during the retention probe trial for aged rats. C, Correlation between cytosolic CaN activity and number of platform crossings during the retention probe trial for young adult rats. Asterisks indicate a significant difference between young adult and aged groups (p < 0.05).
DISCUSSION
Age differences in CaN activity and Ca2+ dyshomeostasis
The main conclusion of this study is that the cytosolic activity and expression level of the protein phosphatase CaN increases with age in the hippocampus, and higher CaN activity levels were associated with poorer memory. The age-related change in CaN activity was not attributable to an increase in overall CaN expression, because similar protein levels were observed in the whole-tissue homogenate. Furthermore, because a similar level of CaN translocation to cellular membranes could be demonstrated in aged and young adult hippocampal tissue when Ca2+ was removed from the assay buffer (Fig. 3), an age-related inability to translocate or a loss in anchoring proteins cannot fully account for differences in CaN activity (Pascale et al., 1998; Jicha et al., 1999).
Age-related changes in Ca2+ regulation provide another possible explanation for differences in CaN activity. Previous research indicates that aging is associated with increased Ca2+ influx through L-VDCCs (Campbell et al., 1996; Thibault and Landfield, 1996), which are thought to underlie a number of electrophysiological markers of brain aging (Disterhoft et al., 1994; Landfield, 1994; Norris et al., 1998b). In the current study, L-VDCC blockade was associated with a rapid decrease in cytosolic CaN activity, indicating that VDCC activity is crucial for the cellular localization and activation of CaN in aged animals (Faux and Scott, 1997; Graef et al., 1999). However, in hippocampal slices, nifedipine decreased CaN activity to a similar extent in young adult and aged animals. It is possible that the absence of an age difference was attributable to the short time interval between treatment and measurement (i.e., 20 min). Alternatively, age-related differences in CaN activity may depend on multiple Ca2+regulation mechanisms (Foster and Norris, 1997). Indeed, ketamine treatment, which would be expected to decrease Ca2+ influx from NMDA receptors (MacDonald et al., 1987), was associated with decreased CaN activity in aged animals. Thus, it will be informative for future studies to examine the interaction of different Ca2+ sources in mediating age-related differences in Ca2+-dependent processes.
Alterations in CaN and biological markers of brain aging
An increase in CaN activity provides an important element for the hypothesized link between altered Ca2+homeostasis and a number of physiological changes that are characteristic of aged neurons (Fig. 8). For example, increased CaN activity could mediate the decrease in synaptic strength that occurs with advanced age (Barnes, 1994; Foster and Norris, 1997), either directly (Wang and Kelly, 1997) or through activation of PP1 (Mulkey et al., 1994; Norris et al., 1998a; Yan et al., 1999; Banke et al., 2000). Indeed, inhibition of PP1 in hippocampal slices results in a selective increase in synaptic function for aged relative to young adult rats (Norris et al., 1998a). In addition, CaN may contribute to altered synaptic plasticity that favors decreased synaptic strength in aged animals (Foster, 1999).
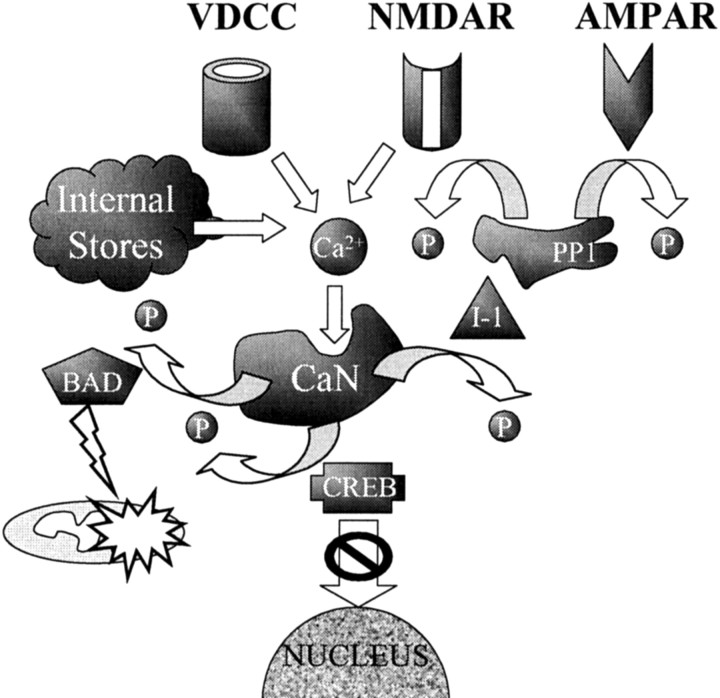
Fig. 8.
Model illustrating how, during aging, an increase in intracellular Ca2+ activates CaN, causing it to move into the cytoplasm. In turn, CaN induces the translocation of BAD from the cytosol to the mitochondrial membrane, a step involved in Ca2+-mediated apoptosis. Furthermore, CaN-mediated dephosphorylation of the CREB inhibits the passage of CREB into the nucleus and is associated with decreased cell viability. Finally, by dephosphorylating inhibitor-1 (I-1), CaN increases the activity of PP1, leading to dephosphorylation of glutamate receptors (NMDAR, AMPAR), resulting in altered synaptic transmission and plasticity.
Another characteristic of brain aging is increased vulnerability to factors, such as stress hormones and glutamate, which appear to challenge neuronal viability by elevating cytosolic Ca2+ (Sapolsky et al., 1986; Landfield, 1987). A rise in the intracellular Ca2+load has been suggested to initiate or hasten neurodegeneration and cell death through a number of mechanisms, some of which require the activation of CaN (Morioka et al., 1999). Indeed, CaN inhibitors are often shown to exert neuroprotection (Dawson et al., 1993; Ankarcrona et al., 1996; Asai et al., 1999; Moore et al., 1999; Wang et al., 1999), possibly by impeding dephosphorylation of several proteins implicated in cell survival. For example, dephosphorylation of the Bcl family member BAD induces the translocation of BAD from the cytosol to the mitochondrial membrane, a step involved in Ca2+-mediated apoptosis (Wang et al., 1999; Walton and Dragunow, 2000). Furthermore, dephosphorylation of CREB inhibits the passage of CREB into the nucleus and is associated with decreased cell viability (Walton and Dragunow, 2000). Finally, by dephosphorylating CREB, CaN is thought to influence the maintenance of long-term potentiation (LTP) and memory (Impey et al., 1998; Silva et al., 1998; Mayford and Kandel, 1999), providing another means by which increased CaN activity during aging may influence neural function and cognition.
Our results indicate that the phosphorylation states of BAD and CREB are significantly reduced in the hippocampus during aging, although the phosphorylation-independent levels of these proteins are unchanged. However, the observed changes in CaN may not be entirely responsible for the age-related differences in electrophysiology and susceptibility to neurotoxicity. In fact, age-related changes in protein kinase pathways have also been reported (Norris et al., 1998a; Pascale et al., 1998). Unfortunately, characterization of age-related changes in kinase pathways that specifically regulate phosphorylation of these cell survival proteins in the hippocampus is sparse (Datta et al., 1997;Dudek et al., 1997; Soderling, 1999). Regardless, the results are consistent with the idea that the balance of protein phosphatase–kinase activity is shifted in favor of decreased phosphorylation with aging (Norris et al., 1998a; Pascale et al., 1998), and this shift is at least partly attributable to an increase in CaN activity. Figure 8 schematically illustrates how increased CaN activity could provide a means by which Ca2+ dyshomeostasis leads to the diverse electrophysiological and behavioral markers of brain aging.
CaN activity and memory
Cognitive decline during aging is associated with a rapid decay of LTP (Barnes and McNaughton, 1985) and an increase in susceptibility to synaptic long-term depression (LTD) and LTP reversal (Norris et al., 1996). Moreover, altered Ca2+ regulation during aging is thought to shift the balance of Ca2+-dependent phosphatase–kinase activity, which determines the threshold for induction of synaptic plasticity during aging, impairing LTP and facilitating induction of LTD (Foster, 1999). Thus, increased CaN activity could link Ca2+ dyshomeostasis with memory impairments through mechanisms controlling synaptic modification. In parallel, overexpression of CaN in younger animals produces remarkably similar alterations in these synaptic plasticity processes (Mayford and Kandel, 1999). Finally, the hypothesis that increased CaN activity is involved in age-related memory impairments is supported by research demonstrating a similar increase in forgetting–impaired consolidation in young adults that overexpress CaN (Mayford and Kandel, 1999).
Spatial tasks, particularly the Morris water maze, are well established as a sensitive test of age-related decline in hippocampal-dependent memory function in rodents (Foster, 1999). However, care must be taken to avoid confounds caused by decreases in swim speed and motor coordination of older animals (Foster et al., 1991; Frick et al., 1995). In this regard, probe trial quadrant search time is relatively insensitive to swim speed and motor coordination and thus provides one of the most valid measures of cognitive function in aged animals (Foster et al., 1991; Mabry et al., 1996; Fugger et al., 1997; Norris and Foster, 1999). Furthermore, aged animals exhibit increased individual variability in probe trial performance on the water maze, which has proven useful for correlating markers of brain aging with cognitive decline (Barnes and McNaughton, 1985; Rapp et al., 1987;Colombo et al., 1997). In the current study, the decrease in goal quadrant search time during retention testing was correlated with CaN activity in aged animals, indicating that hippocampal CaN activity provides a useful marker of cognitive decline. Compared with aged animals, the low variability and asymptotic performance of young adults may render quadrant search time an inadequate parameter for correlative analysis of memory in young animals. Interestingly, manipulations to decrease CaN activity can improve memory in young adults for hippocampal-dependent tasks other than the water maze, possibly attributable to the performance level of control animals (Ikegami and Inokuchi, 2000). Together, the results suggest that CaN may provide a useful target for treatments of age-related memory impairments.
In conclusion, the current study suggests that, along with previous research demonstrating an age-related decline in protein kinase activity (Meier-Ruge et al., 1980; Fordyce and Wehner, 1993; Battaini et al., 1997; Colombo et al., 1997; Norris et al., 1998a; Pascale et al., 1998; Bach et al., 1999), alterations in protein phosphorylation may represent an important marker for age-related changes in neuronal function and cognition. Furthermore, given the importance of CaN for cell survival and the regulation of neuronal function, CaN may be a useful target for treatment of age-related memory impairments and neurodegenerative disease.
Footnotes
This work was supported by National Institutes of Heath Grants AG/NS14979 and MH59891 (to T.C.F.) and AG10836 (to C.M.N.).
Correspondence should be addressed to Dr. Thomas C. Foster, University of Kentucky, College of Medicine, Department of Molecular and Biomedical Pharmacology, Lexington, KY 40536. E-mail:tfoster@pop.uky.edu.
REFERENCES
- 1.Ankarcrona M, Dypbukt JM, Orrenius S, Nicotera P. Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Lett. 1996;394:321–324. doi: 10.1016/0014-5793(96)00959-3. [DOI] [PubMed] [Google Scholar]
- 2.Asai A, Qiu J, Narita Y, Chi S, Saito N, Shinoura N, Hamada H, Kuchino Y, Kirino T. High level calcineurin activity predisposes neuronal cells to apoptosis. J Biol Chem. 1999;274:34450–34458. doi: 10.1074/jbc.274.48.34450. [DOI] [PubMed] [Google Scholar]
- 3.Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banke TG, Bowie D, Lee H, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17:13–18. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 6.Barnes CA, McNaughton BL. An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behav Neurosci. 1985;99:1040–1048. doi: 10.1037//0735-7044.99.6.1040. [DOI] [PubMed] [Google Scholar]
- 7.Battaini F, Pascale A, Paoletti R, Govoni S. The role of anchoring protein RACK1 in PKC activation in the ageing rat brain. Trends Neurosci. 1997;20:410–415. doi: 10.1016/s0166-2236(97)01084-9. [DOI] [PubMed] [Google Scholar]
- 8.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 9.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 10.Campbell LW, Hao SY, Thibault O, Blalock EM, Landfield PW. Aging changes in voltage-gated calcium currents in hippocampal CA1 neurons. J Neurosci. 1996;16:6286–6295. doi: 10.1523/JNEUROSCI.16-19-06286.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colombo PJ, Wetsel WC, Gallagher M. Spatial memory is related to hippocampal subcellular concentrations of calcium-dependent protein kinase C isoforms in young and aged rats. Proc Natl Acad Sci USA. 1997;94:14195–14199. doi: 10.1073/pnas.94.25.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 13.Dawson TM, Steiner JP, Dawson VL, Dinerman JL, Uhl GR, Snyder SH. Immunosuppressant FK506 enhances phosphorylation of nitric oxide synthase and protects against glutamate neurotoxicity. Proc Natl Acad Sci USA. 1993;90:9808–9812. doi: 10.1073/pnas.90.21.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Disterhoft JF, Moyer JR, Jr, Thompson LT. The calcium rationale in aging and Alzheimer's disease. Evidence from an animal model of normal aging. Ann NY Acad Sci. 1994;747:382–406. doi: 10.1111/j.1749-6632.1994.tb44424.x. [DOI] [PubMed] [Google Scholar]
- 15.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 16.Faux MC, Scott JD. Regulation of the AKAP79-protein kinase C interaction by Ca2+/Calmodulin. J Biol Chem. 1997;272:17038–17044. doi: 10.1074/jbc.272.27.17038. [DOI] [PubMed] [Google Scholar]
- 17.Fordyce DE, Wehner JM. Effects of aging on spatial learning and hippocampal protein kinase C in mice. Neurobiol Aging. 1993;14:309–317. doi: 10.1016/0197-4580(93)90116-s. [DOI] [PubMed] [Google Scholar]
- 18.Foster TC. Involvement of hippocampal synaptic plasticity in age-related memory decline. Brain Res Rev. 1999;30:236–249. doi: 10.1016/s0165-0173(99)00017-x. [DOI] [PubMed] [Google Scholar]
- 19.Foster TC, Deadwyler SA. Acetylcholine modulates averaged sensory evoked responses and perforant path evoked field potentials in the rat dentate gyrus. Brain Res. 1992;587:95–101. doi: 10.1016/0006-8993(92)91432-e. [DOI] [PubMed] [Google Scholar]
- 20.Foster TC, Norris CM. Age-associated changes in Ca2+-dependent processes: relation to hippocampal synaptic plasticity. Hippocampus. 1997;7:602–612. doi: 10.1002/(SICI)1098-1063(1997)7:6<602::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 21.Foster TC, Barnes CA, Rao G, McNaughton BL. Increase in perforant path quantal size in aged F-344 rats. Neurobiol Aging. 1991;12:441–448. doi: 10.1016/0197-4580(91)90071-q. [DOI] [PubMed] [Google Scholar]
- 22.Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- 23.Fugger HN, Lichtenvoort JM, Foster TC. Entorhinal cortex lesions as a model of age-related changes in hippocampal function. Psychobiology. 1997;25:277–285. [Google Scholar]
- 24.Graef IA, Mermelstein PG, Stankunas K, Neilson JR, Deisseroth K, Tsien RW, Crabtree GR. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- 25.Ikegami S, Inokuchi K. Antisense DNA against calcineurin facilitates memory in contextual fear conditioning by lowering the threshold for hippocampal long-term potentiation induction. Neuroscience. 2000;98:637–646. doi: 10.1016/s0306-4522(00)00161-5. [DOI] [PubMed] [Google Scholar]
- 26.Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci. 1998;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- 27.Jicha GA, Weaver C, Lane E, Vianna C, Kress Y, Rockwood J, Davies P. cAMP-dependent protein kinase phosphorylations on tau in Alzheimer's disease. J Neurosci. 1999;19:7486–7494. doi: 10.1523/JNEUROSCI.19-17-07486.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khachaturian ZS. Calcium hypothesis of Alzheimer's disease and brain aging. Ann NY Acad Sci. 1994;747:1–11. doi: 10.1111/j.1749-6632.1994.tb44398.x. [DOI] [PubMed] [Google Scholar]
- 29.Landfield PW. Modulation of brain aging correlates by long-term alterations of adrenal steroids and neurally-active peptides. Prog Brain Res. 1987;72:279–300. doi: 10.1016/s0079-6123(08)60215-0. [DOI] [PubMed] [Google Scholar]
- 30.Landfield PW. Increased hippocampal Ca2+ channel activity in brain aging and dementia. Hormonal and pharmacologic modulation. Ann NY Acad Sci. 1994;747:351–364. doi: 10.1111/j.1749-6632.1994.tb44422.x. [DOI] [PubMed] [Google Scholar]
- 31.Landfield PW, Pitler TA, Applegate MD. The effects of high Mg2+-to-Ca2+ ratios on frequency potentiation in hippocampal slices of young and aged rats. J Neurophysiol. 1986;56:797–811. doi: 10.1152/jn.1986.56.3.797. [DOI] [PubMed] [Google Scholar]
- 32.Landfield PW, Thibault O, Mazzanti ML, Porter NM, Kerr DS. Mechanisms of neuronal death in brain aging and Alzheimer's disease: role of endocrine-mediated calcium dyshomeostasis. J Neurobiol. 1992;23:1247–1260. doi: 10.1002/neu.480230914. [DOI] [PubMed] [Google Scholar]
- 33.Lisman J. A mechanism for the Hebb and the anti-Hebb processes underlying learning and memory. Proc Natl Acad Sci USA. 1989;86:9574–9578. doi: 10.1073/pnas.86.23.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mabry TR, McCarty R, Gold PE, Foster TC. Age and stress history effects on spatial performance in a swim task in Fischer-344 rats. Neurobiol Learn Mem. 1996;66:1–10. doi: 10.1006/nlme.1996.0038. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald JF, Miljkovic Z, Pennefather P. Use-dependent block of excitatory amino acid currents in cultured neurons by ketamine. J Neurophysiol. 1987;58:251–266. doi: 10.1152/jn.1987.58.2.251. [DOI] [PubMed] [Google Scholar]
- 36.Mansuy IM, Mayford M, Jacob B, Kandel ER, Bach ME. Restricted and regulated overexpression reveals calcineurin as a key component in the transition from short-term to long-term memory. Cell. 1998;92:39–49. doi: 10.1016/s0092-8674(00)80897-1. [DOI] [PubMed] [Google Scholar]
- 37.Mattson MP. Calcium as sculptor and destroyer of neural circuitry. Exp Gerontol. 1992;27:29–49. doi: 10.1016/0531-5565(92)90027-w. [DOI] [PubMed] [Google Scholar]
- 38.Mayford M, Kandel ER. Genetic approaches to memory storage. Trends Genet. 1999;15:463–470. doi: 10.1016/s0168-9525(99)01846-6. [DOI] [PubMed] [Google Scholar]
- 39.Meier-Ruge W, Iwangoff P, Reichlmeier K, Sandoz P. Neurochemical findings in the aging brain. Adv Biochem Psychopharmacol. 1980;23:323–338. [PubMed] [Google Scholar]
- 40.Moore AN, Kampfl AW, Zhao X, Hayes RL, Dash PK. Sphingosine-1-phosphate induces apoptosis of cultured hippocampal neurons that requires protein phosphatases and activator protein-1 complexes. Neuroscience. 1999;94:405–415. doi: 10.1016/s0306-4522(99)00288-2. [DOI] [PubMed] [Google Scholar]
- 41.Morioka M, Hamada J, Ushio Y, Miyamoto E. Potential role of calcineurin for brain ischemia and traumatic injury. Prog Neurobiol. 1999;58:1–30. doi: 10.1016/s0301-0082(98)00073-2. [DOI] [PubMed] [Google Scholar]
- 42.Mulkey RM, Endo S, Shenolikar S, Malenka RC. Involvement of a calcineurin/inhibitor-1 phosphatase cascade in hippocampal long-term depression. Nature. 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- 43.Norris CM, Foster TC. MK-801 improves retention in aged rats: implications for altered neural plasticity in age-related memory deficits. Neurobiol Learn Mem. 1999;71:194–206. doi: 10.1006/nlme.1998.3864. [DOI] [PubMed] [Google Scholar]
- 44.Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norris CM, Halpain S, Foster TC. Alterations in the balance of protein kinase/phosphatase activities parallel reduced synaptic strength during aging. J Neurophysiol. 1998a;80:1567–1570. doi: 10.1152/jn.1998.80.3.1567. [DOI] [PubMed] [Google Scholar]
- 46.Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J Neurosci. 1998b;18:3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pascale A, Govoni S, Battaini F. Age-related alteration of PKC, a key enzyme in memory processes: physiological and pathological examples. Mol Neurobiol. 1998;16:49–62. doi: 10.1007/BF02740602. [DOI] [PubMed] [Google Scholar]
- 48.Perrino BA, Soderling TR. Biochemistry and pharmacology of calmodulin-related phosphatase calcineurin. In: Van Eldik LJ, Watterson DM, editors. Calmodulin and signal transduction. Academic; New York: 1998. pp. 170–234. [Google Scholar]
- 49.Rapp PR, Rosenberg RA, Gallagher M. An evaluation of spatial information processing in aged rats. Behav Neurosci. 1987;101:3–12. doi: 10.1037//0735-7044.101.1.3. [DOI] [PubMed] [Google Scholar]
- 50.Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286:2358–2361. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- 51.Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7:284–301. doi: 10.1210/edrv-7-3-284. [DOI] [PubMed] [Google Scholar]
- 52.Shankar S, Teyler TJ, Robbins N. Aging differentially alters forms of long-term potentiation in rat hippocampal area CA1. J Neurophysiol. 1998;79:334–341. doi: 10.1152/jn.1998.79.1.334. [DOI] [PubMed] [Google Scholar]
- 53.Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 54.Soderling TR. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci. 1999;24:232–236. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- 55.Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272:1017–1020. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- 56.Thibault O, Porter NM, Chen KC, Blalock EM, Kaminker PG, Clodfelter GV, Brewer LD, Landfield PW. Calcium dysregulation in neuronal aging and Alzheimer's disease: history and new directions. Cell Calcium. 1998;24:417–433. doi: 10.1016/s0143-4160(98)90064-1. [DOI] [PubMed] [Google Scholar]
- 57.Verkhratsky A, Toescu EC. Calcium and neuronal ageing. Trends Neurosci. 1998;21:2–7. doi: 10.1016/s0166-2236(97)01156-9. [DOI] [PubMed] [Google Scholar]
- 58.Walton M, Woodgate AM, Muravlev A, Xu R, During MJ, Dragunow M. CREB phosphorylation promotes nerve cell survival. J Neurochem. 1999;73:1836–1842. [PubMed] [Google Scholar]
- 59.Walton MR, Dragunow I. Is CREB a key to neuronal survival? Trends Neurosci. 2000;23:48–53. doi: 10.1016/s0166-2236(99)01500-3. [DOI] [PubMed] [Google Scholar]
- 60.Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- 61.Wang JH, Kelly PT. Postsynaptic calcineurin activity downregulates synaptic transmission by weakening intracellular Ca2+ signaling mechanisms in hippocampal CA1 neurons. J Neurosci. 1997;17:4600–4611. doi: 10.1523/JNEUROSCI.17-12-04600.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Winder DG, Mansuy IM, Osman M, Moallem TM, Kandel ER. Genetic and pharmacological evidence for a novel, intermediate phase of long-term potentiation suppressed by calcineurin. Cell. 1998;92:25–37. doi: 10.1016/s0092-8674(00)80896-x. [DOI] [PubMed] [Google Scholar]
- 63.Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P. Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat Neurosci. 1999;2:13–17. doi: 10.1038/4516. [DOI] [PubMed] [Google Scholar]
- 64.Zhuo M, Zhang W, Son H, Mansuy I, Sobel RA, Seidman J, Kandel ER. A selective role of calcineurin Aα in synaptic depotentiation in hippocampus. Proc Natl Acad Sci USA. 1999;96:4650–4655. doi: 10.1073/pnas.96.8.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]