Abstract
The role of c-Jun N-terminal kinase (JNK) signaling in cancer is enigmatic, and both tumor-promoting and tumor-suppressing functions have been ascribed to JNK pathway components. We have used the Drosophila eye to investigate the function of the JNK pathway in three different tumor models of increasing malignancy. Benign lesions caused by loss of the neoplastic tumor suppressor gene scribble can efficiently be eliminated by JNK-induced apoptosis. In such a scenario, the eye reverts to a wild-type phenotype, indicating that the JNK pathway prevents tumor formation. The situation changes in the case of aggressive tissue overgrowth, which can be induced by oncogenic activation of the Ras/Raf pathway in the eye, or in malignant invasive tumors resulting when Raf activation is combined with loss of scribble. The growth of these more aggressive tumor types is significantly, yet incompletely, suppressed by JNK-mediated apoptosis. Remarkably, oncogenic Raf and JNK cooperate in these tumors, to induce massive hyperplasia in adjacent wild-type tissue. Thus, depending on the genetic context, JNK signaling can eradicate tumors by removing premalignant cells, or promote aberrant overgrowth in tissues surrounding primary lesions.
Keywords: oncogene, neoplastic tumor suppressor, apoptosis, cancer
The c-Jun N-terminal kinase (JNK) signaling pathway is best known as a regulator of stress defense, cell and tissue morphogenesis, and immune cell functions (1–5). Aberrant JNK activity has been implicated in a number of pathologies, most notably in inflammatory diseases, metabolic dysfunctions, and cancer (6–9). The molecular and cellular consequences of JNK activation in the context of these pathologies are a matter of intense ongoing research. The role of the JNK pathway in cancer is particularly complex, as suggested by a host of apparently conflicting reports in the literature.
JNK has been proposed to support tumorigenesis based on a number of experimental observations. For example, c-Jun, one of the principal effectors of JNK signaling, is required for transformation of fibroblasts by Ras (10) and for the chemical induction of liver cancer in mice (11). The function of c-Jun in a transgenic skin cancer model is, at least in part, dependent on the integrity of its JNK phosphorylation sites (12), suggesting that JNK supports cell transformation in this setting.
Although these findings imply a positive role for JNK or its downstream effector Jun in tumor formation, other data lead to the opposite conclusion, namely a tumor-suppressing function of the JNK pathway (reviewed in ref. 8). For example, JNK3 (13) and MKK4 (a JNK kinase) have been found mutated in cancer cell lines and human tumors (14–19). Furthermore, experiments with JNK-deficient fibroblasts have shown that JNK function can suppress Ras-mediated tumorigenesis in vivo (20). Similarly, JNK-1 mutant mice are more susceptible than wild-type animals to phorbol 12-myristate 13-acetate (PMA)-induced skin tumor development (21). Several processes that are regulated by JNK, including cell movements, cytoskeletal remodeling, and apoptosis could conceivably be relevant in a tumor context. With regard to the latter, JNK has been characterized as a required component in many instances of signal-dependent cell death (9, 22). Tumor cells have commonly developed mechanisms to evade apoptosis, and loss of proapoptotic JNK signaling function has been connected with the transformed phenotype.
Tumorigenesis is a complex process that is brought about by the combined effect of multiple oncogenic lesions. Several genetic changes, typically gain-of-function mutations in protooncogenes and loss-of-function mutations in tumor suppressor loci have to cooperate in order for tumors to form (23). This complexity has hampered a genetic analysis of tumor development in vivo, and the study of oncogene cooperation has mostly been restricted to cell culture models. Recently however, Drosophila systems have been established to recreate and study tumor progression and oncogene cooperation. By using targeted somatic gain- and loss-of-function mutations, tumors of varying malignancy can reproducibly be induced in developing eye tissue. The loss of epithelial polarity genes such as scribble (scrib), lethal giant larvae (lgl), or discs large (dlg) in groups of cells of the eye imaginal disc epithelium results in the formation of nondifferentiating, disorganized, and mildly overproliferating tissue, leading to the designation of these genes as neoplastic tumor suppressors (24). Homologues of the Drosophila dlg, lgl and scrib genes have been identified in mammals, and several lines of indirect evidence implicate these proteins in tumor suppression as well. For example, dlg expression is consistently lost in gastric cancers, an event that is associated with increased tumor invasiveness. Furthermore, dlg and scrib are targeted for degradation by viral oncoproteins upon infection with high-risk human papillomaviruses (HPV) and retroviruses such as human T-lymphotrophic virus 1 (HTLV-1), suggesting a role of these proteins as suppressors of virally induced transformation (24, 25). The absence of scribble function from clones of developing Drosophila eye imaginal disc epithelium gives rise to localized benign, premalignant lesions in the emerging adult eye (26, 27) (The terms “benign” and “malignant” are used in a broad sense as introduced in refs. 24 and 25 to reflect the phenomenological similarities between clinical pathologies and Drosophila phenotypes.) More aggressive overgrowth can be observed upon expression of activated alleles of Raf or Ras, in the eye imaginal disk. The malformations resulting from such oncogenic activation of the Ras pathway remain, however, restricted to the adult eye (28). Yet more malignant tumors arise when Ras activation and mutations causing loss of tissue integrity are combined in the same cells. These genetic lesions cooperate to induce invasive, malignant, and, ultimately, lethal tumors (26, 27).
To study the complex functions of JNK in the establishment of cancer in an intact organism and to investigate context-specific contributions of JNK to tumor formation, we examined the effects of manipulating JNK activity in Drosophila tumor models of increasing malignancy.
Materials and Methods
Fly Stocks. All fly culture and crosses were carried out at 25°C. For generation of eye mosaics, the following mutant and transgenic fly strains were used: (i) eyFLP1; Act>y+>Gal4, UAS-GFP; FRT82B Tub-Gal80 (a gift of T. Xu and T. Igaki; ref. 27); (ii) w;FRT82B UAS-Rafgof, (Rafact); (iii) w; FRT82B scrib1/TM6B (or scrib2); (iv) w; FRT82B scrib1 UAS-Rafgof/TM6B (a gift from A. M. Brumby; ref. 26), (v) w; FRT82B; (vi) w; UAS-Hepact; FRT82B; (vii) w; UAS-Hepact; FRT82B scrib2/TM6B; (viii) w; UAS-Hepact; FRT82B scrib1 UAS-Rafgof/TM6B; (ix) w; FRT82B scrib1 UAS-bskDN/TM6. scrib1 and scrib2 alleles displayed similar phenotypes as reported (26, 29).
Mosaic Analysis. Positively marked GFP clones of the desired genotype were generated in eye discs by using the MARCM system (mosaic analysis with a repressible cell marker) (30) by crossing eyFLP1; Act>y+>Gal4, UAS-GFP; FRT82B Tub-Gal80 (27) females with males of the genotypes listed above. eyeless promoter-driven FLP recombinase (eyFLP) catalyzes mitotic recombination between FRT sites, resulting in loss of Gal80 expression in clones and subsequent derepression of Gal4-dependent transcription. In this manner, only clones in which both recombination events occur become positively marked with GFP (to distinguish them from surrounding tissue) and express other UAS transgenes.
Immunohistochemistry. Eye imaginal discs were dissected from third instar larvae in PBS and fixed with 4% paraformaldehyde in PBST (0.1% Triton X-100) for 30 min. The following antibodies were used: rabbit anti-active caspase 3 (1:100) (Cell Signaling Technology, Beverly, MA); rat anti-Elav (1:200) (Developmental Studies Hybridoma Bank at the University of Iowa). Rhodamine (TRITC)-conjugated or CY5-conjugated secondary antibodies (Jackson ImmunoResearch) were used. Samples were analyzed by confocal microscopy (Leica SP2). Images represent either single confocal sections or projections of several sections as indicated in the figure legends.
Flow Cytometry. Thirty to 40 pairs of eye-antennal imaginal discs were dissected from wandering third instar larvae in PBS. The tissue was dissociated by mild trypsin treatment, and FACS analyses were performed as described in ref. 31. Flow cytometry was performed on a Becton Dickinson FACSVantage by using cellquest software (Becton Dickinson). Every experiment was conducted at least twice for each genotype.
Results and Discussion
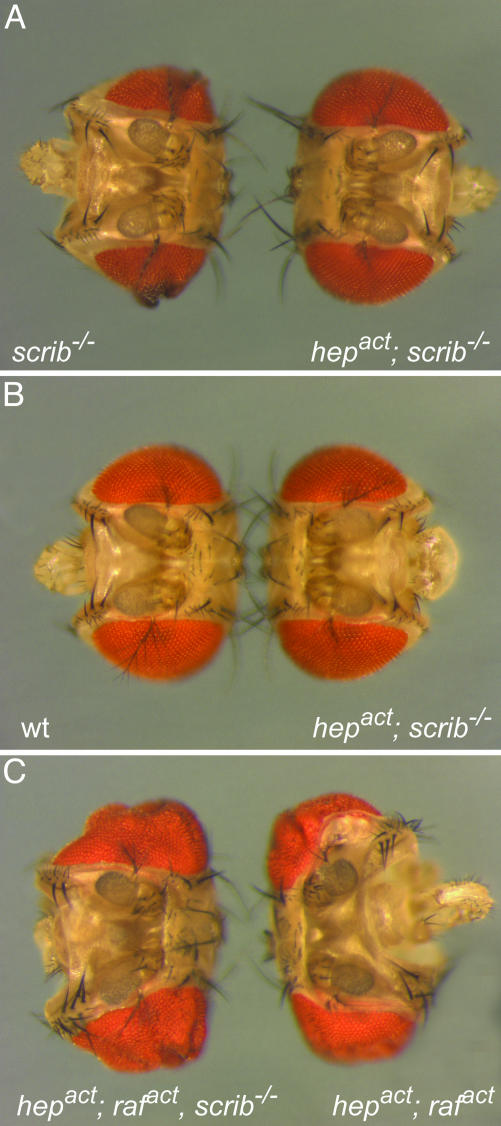
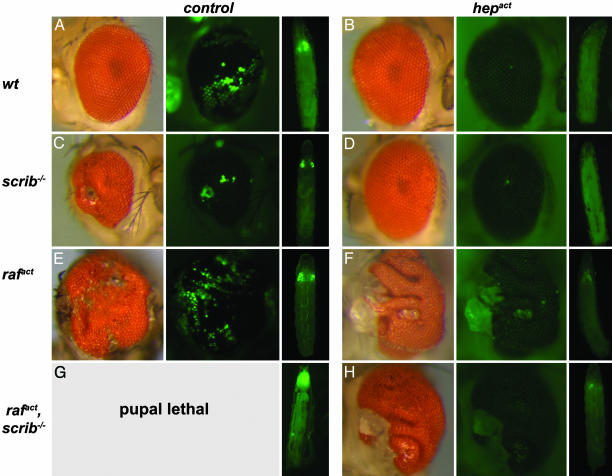
To investigate the possible contribution of JNK signaling to cell transformation in an intact tissue we used the Drosophila eye as a model. First, we induced clones of GFP-labeled scrib-deficient cells in larval eye imaginal discs by using the MARCM (mosaic analysis with a repressible cell marker) system (30) in combination with eye-specific expression of FLP recombinase (32). Consistent with previous observations (26), such scrib–/– clones form benign lesions in the adult eye. The GFP-labeled clonal areas in such eyes seem undifferentiated and disorganized (Figs. 1A and 2C). To examine the influence of JNK at such a premalignant stage of tumorigenesis, we generated scrib mutant clones in which JNK signaling was increased by the expression of an activated version of the JNK kinase, Hemipterous [Hepact, (33)]. Adult eyes of this genotype reverted to an essentially wild-type appearance and never showed the marked lesions resulting from scrib-deficient tissue (Figs. 1 A and 2D). Significantly, these phenotypically rescued eyes did not contain any GFP-positive cells (Fig. 2D), suggesting that JNK activity counteracts the outgrowth of scrib–/– tissue or causes the removal of the mutant cells.
Fig. 1.
JNK cooperates with Raf to induce massive overgrowth in clones of eye tissue. Shown is a dorsal view of fly heads in which clones of the following genotypes were induced during larval stages of eye development: scrib–/– and scrib–/–, UAS-hepact (A), wild-type and scrib–/–, UAS-hepact (B), and scrib–/–, UAS-rafact, UAS-hepact and UAS-rafact, UAS-hepact (C). Note that the scrib–/–, UAS-hepact eyes revert to wild-type size and pattern.
Fig. 2.
Activation of the JNK-signaling pathway by expression of hepact in scrib–/–, in rafact, and in scrib–/–, rafact clones causes elimination of mutant tissue from the adult eyes. Eyes carrying mutant clones of the indicated genotypes are shown in normal illumination (Left image of each panel) or by using epifluorescence to visualize GFP marked clonal areas (Center image). The image on the Right of each panel illustrates the growth of GFP-labeled clonal tissue of the respective genotypes during larval development. Eye imaginal discs of the scrib–/–, rafact larvae fuse with the brain and cannot be distinguished from other organs. Eighty percent of the scrib–/–, rafact animals cannot pupate and die as giant larvae. Note that the tissue of scrib–/– tumors (C) and of the Rafact-expressing tumors (E) are all labeled by GFP, whereas the overgrowth in animals in which rafact, hepact (F) or rafact, scrib–/–, hepact (H) clones had been induced is GFP-negative and thus is not clonal in origin.
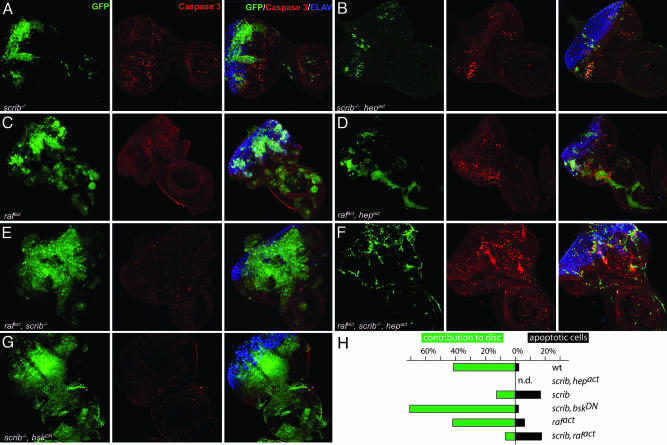
To elucidate the cellular basis for the observed phenotypes, we examined the behavior of scrib–/– as well as scrib–/–, hepact cells in larval eye imaginal discs. Consistent with previous reports (26, 34), the scrib–/– cells in the eye imaginal disc grow into sizable clones of disorganized appearance and do not express neuronal markers, indicating their failure to differentiate (Fig. 3A). In contrast, scrib–/– clones that also express Hepact do not grow appreciably. In the small clones that can occasionally be detected, expression of apoptotic markers, such as activated caspase 3, is prevalent (Fig. 3B; for higher magnification images, see Fig. 5, which is published as supporting information on the PNAS web site). This finding indicates that JNK signaling efficiently eliminates scrib-deficient tissue by an apoptotic mechanism. Consistent with this interpretation, we find that down-regulation of JNK signaling in clones of scrib mutant tissue by overexpression of the dominant negative form of Drosophila JNK, Basket (BskDN) suppresses apoptosis and, presumably as a consequence of that, increases the overgrowth of this tissue (Fig. 3G and ref. 26).
Fig. 3.
Activation of JNK-signaling pathway induces apoptosis in scrib–/– and rafact clones as well as in scrib–/–, rafact clones. Eye-antennal discs were dissected from third instar larvae and analyzed by immunostaining and confocal microscopy. All images were taken at the same magnification (×40) and represent projections of multiple sections. Mutant clones of the indicated genotypes are marked by GFP fluorescence (Left, green). Introduction of an activated allele of JNKK (hepact)(B, D, and F) significantly suppressed the expansion of benign scrib–/– (A) and hyperproliferative rafact (C), or malignant scrib–/–, rafact (F) clones. Many cells within the clones underwent apoptosis as shown by staining with anti-active caspase 3 antibody (Center, red). Moderately elevated levels of apoptosis, which are not restricted to the GFP-marked mutant areas, are consistently observed in eye-antennal discs containing scrib–/– clones (A). Developing photoreceptors are shown by Elav staining (Right, blue). Neuronal differentiation is severely impaired in scrib-null clones in all genotypes. Note that micrographs are not representative of the size of the different imaginal disc because the preparation for confocal microscopy may distort the dimensions of the organ.
To assess the effect of JNK signaling on apoptosis in scrib mutant tissue quantitatively, we dissected third instar eye-antennal imaginal discs bearing clones of the relevant genotypes, separated them into cell suspensions, and analyzed them by flow cytometry. Control discs in which GFP-expressing (but otherwise wild-type) clones were induced contained ≈41.2% of clonal GFP-positive cells. Very few (2%) of these normal eye disc cells underwent apoptosis as measured by the fraction of cells that display a DNA content lower than 2n, registering in the subG1 region of the FACS profile. The analysis of cells from scrib mutant clones revealed that their contribution to the eye-antennal disc was reduced to 12.4%. Consistently, the number of apoptotic cells in the clonal fraction was significantly increased (16.6%, Fig. 3H). Apoptosis of scrib mutant cells is mediated by JNK, because coexpression of BskDN in scrib mutant clones reduced the proportion of apoptotic cells (2%) and resulted in a significant growth advantage of the mutant tissue, even compared with wild-type cells. Strikingly, when JNK activity is thus suppressed, scrib mutant clones can contribute >70% of the tissue in third instar eye imaginal discs. Conversely, expression of Hepact in scrib mutant tissue reduces the fraction of GFP-labeled mutant cells to undetectable levels (Fig. 3 B and H).
We conclude that loss of scribble from developing epithelia has two opposing effects: it promotes cell proliferation, but at the same time induces JNK-dependent apoptosis. Our results suggest that the balance between growth and death can be shifted by increasing or suppressing JNK signaling. When the JNK pathway is suppressed, overgrowth prevails so that scribble mutant clones persist and overgrow, ultimately causing pupal death (data not shown and ref. 26). Conversely, strong activation of JNK as delivered by Hepact expression shifts the balance toward apoptosis, and the mutant cells are eliminated.
Interestingly, eyes in which the growth of scrib–/– clones is thus impeded by JNK activation display the same size as those of wild-type flies (Figs. 1B and 2 A and D). This result suggests the existence of a corrective mechanism to maintain normal eye size even when significant fractions of the eye anlage are removed by JNK-dependent apoptosis. Similar processes have been proposed to readjust organ size after cellular injury caused by ionizing irradiation (35–37) or when cells whose growth is compromised, for example, by minute mutations are replaced by their wild-type neighbors (38). Recent studies by Ryoo et al. (39) have suggested that, in imaginal disc cells that are committed to apoptosis, JNK signals the release of a transient mitogenic signal to their neighbors. The ensuing growth would serve to replace the dying cells and restore the affected tissue to its original size and form.
The data described above show that JNK signaling can suppress a benign, premalignant tumor state by apoptotically removing the affected cells. Next, we asked how JNK activation might influence the behavior of more aggressive tumors. Clones of eye imaginal disc cells that express activated Ras or Raf autonomously develop into vigorously proliferating tissue during larval stages and ultimately overgrow much of the adult eye (Fig. 2E). Such Raf-induced tissue overgrowth remains restricted to the tissues derived from the eye-antennal disc and, consistent with previous findings (Pagliarini and Xu, ref. 27), is apparently not invasive. The animals survive until pharate adult stages, with rare adult escapers (Fig. 2E). If, however, the activated Raf allele is combined with a loss-of-function condition for scribble, tumors become invasive and lethal to the affected animals. When GFP-labeled rafact, scrib–/– clones are generated in the eye disc, massive and invasive overgrowth occurs in larval stages, which kills the animal before pupation, presumably because the transformed cells functionally impair tissues other than the eye (Fig. 2G). These results confirm similar observations by Brumby and Richardson (26) and by Pagliarini and Xu (27).
We investigated whether aggressive rafact or rafact, scrib–/–-induced tumors might be cured by JNK signaling, as seen in the case of the benign scrib–/– lesions. In rafact, hepact, as well as rafact, scrib–/–, hepact clones, aggressive expansion is suppressed, and clones of this genotype, although easily detectable (as opposed to scrib–/–, hepact clones, see Fig. 3), remain much smaller than clones in which Hepact is not coexpressed. This size reduction is likely a consequence of apoptosis induced by Hepact. Although the overgrowing clones of the rafact, scrib–/– and rafact cells show little or no signs of apoptosis, JNK activation in such genetic backgrounds elicits prominent caspase 3 activation (Fig. 3 D and F). This conclusion is further supported by FACS analyses of the respective eye-antennal imaginal discs. These data indicate that Hepact expression can elevate the occurrence of apoptosis among Rafact (activated version of the Raf oncoprotein)-expressing cells (from 6.0% to 17.3%). However, unlike scrib mutant clones, which are virtually eliminated by Hepact expression, a significant fraction of the rafact, hepact cells (6.4%) survive (Fig. 3H).
Consistent with the reduction in clonal growth, flies in which Hepact has been introduced into the tumorigenic genotypes overcome lethality and in most cases yield viable adults. Strikingly, however, the eyes of such flies display massive overgrowth. The heads are significantly bigger (Fig. 1C) and the retina of these mutants is dramatically larger than that of a wild-type eye. In many cases, the retina is folded and bunched to accommodate the surfeit of tissue (Fig. 2 F and H). In contrast to scrib–/– lesions or tumors induced by rafact alone, these severely hyperplastic eye structures are well patterned and show a distinctive ommatidial organization (Fig. 2 F and H). Remarkably, inspection of GFP fluorescence shows that the overgrown tissue was not derived from clonal cells (compare Fig. 2 E, F, and H). Evidently, the tumorous overgrowth was induced cell nonautonomously in the phenotypically wild-type cells surrounding the clones of Rafact- and Hepact-expressing cells.
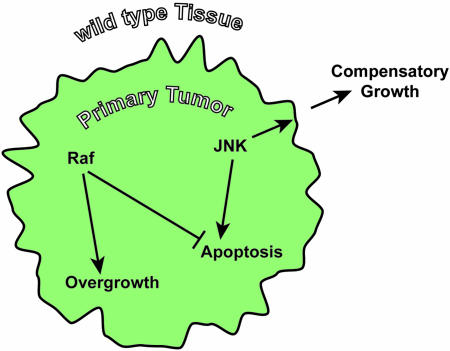
How can the extraordinary overgrowth of wild-type tissue in f lies carrying clones of scrib mutant and Rafact/Hepact-expressing cells be explained? Pérez-Garijo et al. (40) have recently demonstrated a proapoptotic function of the JNK pathway in wing imaginal disc cells exposed to cytotoxic stress. Interestingly, JNK signaling not only triggers apoptosis in the stressed cells, it also causes adjacent cells to undergo compensatory proliferation. By this mechanism, neighboring cells restore tissue size after the apoptotic removal of damaged cells. The signal for compensatory growth (in the wing disc delivered by Wingless and Dpp) is normally transient in nature, because the signal-producing cells are slated to die shortly after JNK signaling engages. If, however, apoptosis is artificially abrogated, for example by the expression of p35 or by inhibition of hid/rpr/grim expression, cells that are prevented from dying (“undead” cells) continue to induce cell proliferation in their surroundings. Such a situation can induce significant nonautonomous tissue overgrowth. Based on these reports and our findings, we propose the following mechanism to explain the observed effects of JNK activation in the Drosophila tumor progression model (Fig. 4). In wild-type or scribble mutant cells, a strong JNK signal, as it is delivered by Hepact, will initiate apoptosis. Consistently, clones in which Hepact is expressed in a wild-type or scrib–/– background do not survive larval development and do not contribute to the adult eye. In dying, the Hepact-expressing cells prompt adjacent wild-type cells to undergo compensatory growth, which restores the adult eye to a wild-type appearance. Thus, JNK signaling acts as a suppressor of a premalignant state. In more aggressive and invasive tumors of the rafact or rafact, scrib–/– genotypes, however, JNK signaling changes its role from an antagonist of cell transformation to a promoter of tumor growth. Hepact promotes apoptosis in the clones, which significantly reduces the expansion of primary tumors. At the same time, however, the apoptotic effect of JNK is attenuated by activated Raf, and cell death is delayed. It has been reported that Ras/Raf signaling can act anti-apoptotically in imaginal disc cells. This effect may occur by the inhibition of the death protein Hid through direct ERK-mediated phosphorylation (41). This effect of Rafact gives surviving cells more time to produce mitogenic factors. So, although Raf and Hepact antagonize each other with regard to the growth of primary tumors, they cooperate in stimulating overgrowth in a cell non-autonomous manner.
Fig. 4.
Model for the cooperation between Raf and JNK in the non-cell-autonomous induction of cell growth. Raf induces the formation of hyperplastic tumors by promoting cell growth and proliferation and suppressing apoptosis. JNK acts proapoptotically and at the same time induces a mitogenic signal to neighboring cells. When Raf attenuates the apoptotic response to JNK signaling, this mitogenic signal is emitted for an extended period, causing overgrowth of genotypically wild-type tissue adjacent to the tumor.
A combination of hyperactive JNK and Raf as we created it in the clones of Drosophila eye imaginal disc might conceivably occur frequently in clinically relevant settings of Ras-driven tumorigenesis. Many circumstances may activate the JNK pathway in the course of tumor progression. In addition to biological signals resulting from the neoplasia, such as cytokine secretion or hypoxia, external stimuli in the form of chemotherapeutic drugs can potently activate the JNK pathway. It is thus important to explore potential beneficial or adverse effects of JNK in cancer. The results described here indicate that JNK can act both as an antagonist of cell transformation, or as an oncogenic factor that can cooperate with components of the Ras pathway in tumor formation. The specific role of JNK signaling with regard to tumor formation thus seems to be highly dependent on the cellular and genetic context. This multifaceted function of the JNK pathway in cell transformation has to be considered in evaluating the system as a target for therapeutic intervention.
The surprising finding that cooperation between JNK and Ras signaling causes non-autonomous tumor growth emphasizes the relevance of tissue interactions in tumorigenesis. Tumor–stroma interactions are known to make relevant contributions to the pathology of cancer, for example by inducing vascularization or inflammatory responses (23). The potentially very important role of stromal tissue in cancer pathology is obviously difficult to study in cell culture, and Drosophila seems to offer a facile and genetically versatile model system in which relevant studies can be performed.
Supplementary Material
Acknowledgments
We thank T. Xu, T. Igaki, A. Brumby, and H. Richardson for generous gifts of fly stocks. H. Land, M. Noble and H. Beug are acknowledged for discussions. We thank T. Calcagni for help with FACS analyses. C. Ovitt made helpful comments on the manuscript. This work was supported by National Institutes of Health Grant R01 EY014624-01 (to D.B.).
Author contributions: M.U., H.J., and D.B. designed research; M.U. performed research; M.U. and D.B. analyzed data; and M.U. and D.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: JNK, c-Jun N-terminal kinase; scrib, scribble; Hepact, activated version of the JNK kinase Hemipterous; Rafact, activated version of the Raf oncoprotein.
References
- 1.Xia, Y. & Karin, M. (2004) Trends Cell Biol. 14, 94–101. [DOI] [PubMed] [Google Scholar]
- 2.Stronach, B. E. & Perrimon, N. (1999) Oncogene 18, 6172–6182. [DOI] [PubMed] [Google Scholar]
- 3.Kockel, L., Homsy, J. G. & Bohmann, D. (2001) Oncogene 20, 2347–2364. [DOI] [PubMed] [Google Scholar]
- 4.Ip, Y. T. & Davis, R. J. (1998) Curr. Opin. Cell Biol. 10, 205–219. [DOI] [PubMed] [Google Scholar]
- 5.Noselli, S. & Agnes, F. (1999) Curr. Opin. Genet. Dev. 9, 466–472. [DOI] [PubMed] [Google Scholar]
- 6.Waetzig, V. & Herdegen, T. (2004) Neurosci. Lett. 361, 64–67. [DOI] [PubMed] [Google Scholar]
- 7.Manning, A. M. & Davis, R. J. (2003) Nat. Rev. Drug Discov. 2, 554–565. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy, N. J. & Davis, R. J. (2003) Cell Cycle 2, 199–201. [PubMed] [Google Scholar]
- 9.Davis, R. J. (2000) Cell 103, 239–252. [DOI] [PubMed] [Google Scholar]
- 10.Lloyd, A., Yancheva, N. & Wasylyk, B. (1991) Nature 352, 635–638. [DOI] [PubMed] [Google Scholar]
- 11.Eferl, R., Ricci, R., Kenner, L., Zenz, R., David, J. P., Rath, M. & Wagner, E. F. (2003) Cell 112, 181–192. [DOI] [PubMed] [Google Scholar]
- 12.Behrens, A., Jochum, W., Sibilia, M. & Wagner, E. F. (2000) Oncogene 19, 2657–2663. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida, S., Fukino, K., Harada, H., Nagai, H., Imoto, I., Inazawa, J., Takahashi, H., Teramoto, A. & Emi, M. (2001) J. Hum. Genet. 46, 182–187. [DOI] [PubMed] [Google Scholar]
- 14.Yamada, S. D., Hickson, J. A., Hrobowski, Y., Vander Griend, D. J., Benson, D., Montag, A., Karrison, T., Huo, D., Rutgers, J., Adams, S. & Rinker-Schaeffer, C. W. (2002) Cancer Res. 62, 6717–6723. [PubMed] [Google Scholar]
- 15.Kim, H. L., Vander Griend, D. J., Yang, X., Benson, D. A., Dubauskas, Z., Yoshida, B. A., Chekmareva, M. A., Ichikawa, Y., Sokoloff, M. H., Zhan, P., et al. (2001) Cancer Res. 61, 2833–2837. [PubMed] [Google Scholar]
- 16.Yoshida, B. A., Dubauskas, Z., Chekmareva, M. A., Christiano, T. R., Stadler, W. M. & Rinker-Schaeffer, C. W. (1999) Cancer Res. 59, 5483–5487. [PubMed] [Google Scholar]
- 17.Teng, D. H., Perry, W. L., 3rd, Hogan, J. K., Baumgard, M., Bell, R., Berry, S., Davis, T., Frank, D., Frye, C., Hattier, T., et al. (1997) Cancer Res. 57, 4177–4182. [PubMed] [Google Scholar]
- 18.Su, G. H., Hilgers, W., Shekher, M. C., Tang, D. J., Yeo, C. J., Hruban, R. H. & Kern, S. E. (1998) Cancer Res. 58, 2339–2342. [PubMed] [Google Scholar]
- 19.Su, G. H., Song, J. J., Repasky, E. A., Schutte, M. & Kern, S. E. (2002) Hum. Mutat. 19, 81. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy, N. J., Sluss, H. K., Jones, S. N., Bar-Sagi, D., Flavell, R. A. & Davis, R. J. (2003) Genes Dev. 17, 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.She, Q. B., Chen, N., Bode, A. M., Flavell, R. A. & Dong, Z. (2002) Cancer Res. 62, 1343–1348. [PubMed] [Google Scholar]
- 22.Varfolomeev, E. E. & Ashkenazi, A. (2004) Cell 116, 491–497. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan, D. & Weinberg, R. A. (2000) Cell 100, 57–70. [DOI] [PubMed] [Google Scholar]
- 24.Bilder, D. (2004) Genes Dev. 18, 1909–1925. [DOI] [PubMed] [Google Scholar]
- 25.Humbert, P., Russell, S. & Richardson, H. (2003) BioEssays 25, 542–553. [DOI] [PubMed] [Google Scholar]
- 26.Brumby, A. M. & Richardson, H. E. (2003) EMBO J. 22, 5769–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pagliarini, R. A. & Xu, T. (2003) Science 302, 1227–1231. [DOI] [PubMed] [Google Scholar]
- 28.Karim, F. D. & Rubin, G. M. (1998) Development (Cambridge, U.K.) 125, 1–9. [DOI] [PubMed] [Google Scholar]
- 29.Zeitler, J., Hsu, C. P., Dionne, H. & Bilder, D. (2004) J. Cell Biol. 167, 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee, T. & Luo, L. (1999) Neuron 22, 451–461. [DOI] [PubMed] [Google Scholar]
- 31.Neufeld, T. P., de la Cruz, A. F., Johnston, L. A. & Edgar, B. A. (1998) Cell 93, 1183–1193. [DOI] [PubMed] [Google Scholar]
- 32.Newsome, T. P., Asling, B. & Dickson, B. J. (2000) Development (Cambridge, U.K.) 127, 851–860. [DOI] [PubMed] [Google Scholar]
- 33.Weber, U., Paricio, N. & Mlodzik, M. (2000) Development (Cambridge, U.K.) 127, 3619–3629. [DOI] [PubMed] [Google Scholar]
- 34.Dow, L. E., Brumby, A. M., Muratore, R., Coombe, M. L., Sedelies, K. A., Trapani, J. A., Russell, S. M., Richardson, H. E. & Humbert, P. O. (2003) Oncogene 22, 9225–9230. [DOI] [PubMed] [Google Scholar]
- 35.Milan, M., Campuzano, S. & Garcia-Bellido, A. (1997) Proc. Natl. Acad Sci USA 94, 5691–5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James, A. A. & Bryant, P. J. (1981) Radiat. Res. 87, 552–564. [PubMed] [Google Scholar]
- 37.Bryant, P. J. (1971) Dev. Biol. 26, 637–651. [DOI] [PubMed] [Google Scholar]
- 38.Moreno, E., Basler, K. & Morata, G. (2002) Nature 416, 755–759. [DOI] [PubMed] [Google Scholar]
- 39.Ryoo, H. D., Gorenc, T. & Steller, H. (2004) Dev. Cell 7, 491–501. [DOI] [PubMed] [Google Scholar]
- 40.Pérez-Garijo, A., Martin, F. A. & Morata, G. (2004) Development (Cambridge, U.K.) 131, 5591–5598. [DOI] [PubMed] [Google Scholar]
- 41.Bergmann, A., Agapite, J., McCall, K. & Steller, H. (1998) Cell 95, 331–341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.