Abstract
Na-H exchanger NHE3 and Cl-anion exchanger CFEX (SLC26A6, PAT1) play principal roles in the reabsorption of Na and Cl in the proximal tubule of the mammalian kidney. The mechanisms by which NHE3 and CFEX are localized to and maintained in the brush border of the proximal tubule are largely unknown. To investigate the possible interaction of NHE3 and CFEX with the PDZ-domain-containing scaffolding protein PDZK1, we performed a series of in vitro interaction assays with GST-fusion proteins and native brush border membrane proteins. These studies demonstrated that, not only were NHE3 and CFEX capable of directly interacting with PDZK1, but that this interaction was mediated through their C-terminal PDZ-interaction sites. To determine whether PDZK1 interaction is essential for brush border localization of NHE3 and CFEX in vivo, we examined the expression of NHE3 and CFEX in kidneys of wild-type and PDZK1-null mutant mice by both Western analysis and immunocytochemistry. These studies indicated that, although brush border expression of NHE3 was unaffected by the loss of PDZK1, the expression of CFEX was markedly reduced. Finally, we assayed CFEX functional activity as Cl–oxalate exchange in brush border membrane vesicles and oxalate-stimulated volume absorption in microperfused proximal tubules. Consistent with the observed decrease in CFEX protein expression, both measures of CFEX functional activity were dramatically reduced in PDZK1-null animals. In conclusion, the scaffolding protein PDZK1 is essential for the normal expression and function of Cl-anion exchanger CFEX in the proximal tubule of the mammalian kidney.
Keywords: NHE3, proximal tubule, SLC26A6
The majority of the NaCl filtered by the mammalian kidney is reabsorbed in the proximal tubule. Evidence from a variety of experimental approaches over the past three decades has indicated that ion exchange mechanisms expressed on the apical or brush border membrane of proximal tubule cells play a principal role in the reabsorption of Na+ and Cl- in this nephron segment (1–3). Although an important fraction of the NaCl reabsorption that occurs in the proximal tubule is passive and paracellular, evidence indicates that there is also substantial transcellular NaCl reabsorption (2). The transcellular component of the NaCl reabsorption occurs predominantly through the combined activities of an apical chloride-base exchange mechanism(s) and either a sodium-proton (NHE3) exchangeor sodium-sulfate (NaSi) cotransport-driven base recycling process (2, 3). Recent studies suggest that CFEX (SLC26A6, PAT1), a Cl-–anion exchanger located in the brush border of the proximal tubule, mediates the principal component of the Cl--base exchange that is observed in this segment of the nephron (4–8).
Although NHE3 and CFEX are emerging as the key brush border ion transporters involved in the reabsorption of Na+ and Cl- in the proximal tubule, the mechanisms responsible for localizing and retaining these important transporters in the apical membrane of proximal tubule cells are largely unknown. Sequence analysis of NHE3 and CFEX reveals that each possesses a classic type 1 PDZ-interaction motif at its extreme C terminus, raising the possibility that retention at the apical membrane could be mediated through interaction with a PDZ-domain-containing protein residing in the brush border. Gisler et al. (9) have proposed that PDZK1, in particular, may act as a general molecular scaffold for a large variety of transport and regulatory proteins in the brush border of the mammalian renal proximal tubule.
PDZK1 possesses four well defined PDZ-binding domains and has been identified in kidney, pancreas, liver, gastrointestinal tract, and adrenal cortex (10). Within the kidney, PDZK1 is localized exclusively in the brush border of the proximal tubule. Studies using various in vitro binding assays have suggested that PDZK1 may be capable of interaction with numerous renal proteins including MAP17, MRP2, CFTR, NaPi-I, NaPi-IIa, NHE3, CFEX, UR AT1, OCTN1, Oatp-5, NHERF-1, NHERF-2, and d-AKAP2 (9–12). To date, only interactions with CFTR, URAT1, and NaPi-IIa have been confirmed by in vivo interaction assays with native proteins (12–14).
The principal aim of the present study was to address the possibility that PDZK1 may act as a molecular scaffold to facilitate the localization of CFEX and NHE3 in the brush border of the renal proximal tubule. The ability of PDZK1 to specifically interact with NHE3 and CFEX was confirmed, and both NHE3 and CFEX were shown to be capable of direct interaction with PDZK1 via their C-terminal type 1 PDZ-interaction motifs. Protein expression profiles of NHE3 and CFEX in kidneys of PDZK1-null mutant mice indicated that, although expression levels of NHE3 are unaffected by loss of PDZK1, the expression of CFEX is significantly reduced. Functional studies with brush border membrane vesicles and microperfused proximal tubules confirmed that Cl--base exchange is dramatically reduced in the proximal tubules of kidneys from PDZK1-null mice. Our findings clearly demonstrate that PDZK1 is essential for the normal apical expression and function of CFEX in the proximal tubule in vivo. However, it appears unlikely that PDZK1-mediated scaffolding plays a direct role in either the targeting or retention of NHE3 in the brush border.
Materials and Methods
Animals and Tissue Preparation. Rabbit renal brush border membrane vesicles (BBMV) were isolated and stored as described (15). Mouse BBMV and whole kidney microsomal membrane fractions were isolated from kidneys of age-matched adult wild-type and PDZK1-null mutant mice (16). Mouse BBMV were isolated from pooled renal cortices of 23–25 mice as described by Karniski et al. (17). Renal BBMV preparations from wild-type and PDZK1-null mice used in this study were similarly enriched (13.3- and 14.1-fold, respectively) in specific activity of the brush border marker enzyme γ-glutamyltranspeptidase relative to kidney homogenates. Mouse microsomal membranes were prepared by differential centrifugation of whole kidney homogenates from individual mice. The homogenates were centrifuged at 2,000 × g for 15 min at 4°C. The supernatants were removed and subjected to a further 1 h of centrifugation at 100,000 × g at 4°C to pellet the microsomal fractions. The supernatants were discarded, and the microsomes were resuspended in fresh PBS containing protease inhibitors and stored at -70°C.
Antibodies. Two different mouse monoclonal antibodies directed against NHE3 were used in this study. Antibody 2B9 (Chemicon) (18) directed against the C-terminal 131 aa of rabbit NHE3 was used at a dilution of 1:500 for Western analysis and 1:50 for immunocytochemistry. Antibody 3H3 (a generous gift from Daniel Biemesderfer, Yale University) (19) generated against opossum NHE3 was used at a dilution of 1:4,000 for Western analysis. An anti-CFEX antibody directed against an N-terminal portion of human CFEX (PAT1) (20) was used at a dilution of 1:20 for immunocytochemistry and 1:400 for Western analysis. The chicken anti-human PDZK1 antibody developed by Kocher et al. (10) was used at a dilution of 1:4,000 for Western analysis.
The anti-opossum PDZK1 antibody, OK-66, is an affinity-purified rabbit anti-peptide antibody that was developed specifically for this study. We used a degenerate PCR approach based on the known nucleotide and amino acid sequences of human, mouse, and rat PDZK1 to isolate the first PDZ-domain of opossum (Didelphis virginiana) PDZK1 (GenBank accession no. AY665789). A 29-aa peptide (CEKDTEGHLVRVVEQGSPAEKAGLKDGDR) corresponding to a highly conserved region of this domain was coupled to KLH (Pierce Imject Maleimide Activated Immunogen Conjugation kit) and used to immunize three rabbits (Pocono Rabbit Farm, Canadensis, PA). The resulting immunosera from all three rabbits recognized human, mouse, rat, rabbit, and opossum PDZK1 by Western analysis, but only sera from a single rabbit was used for the present study. The sera (OK-66) was affinity purified against the peptide antigen coupled to BSA and used at a dilution of 1:5,000 for Western analysis and 1:1,000 for immunocytochemistry. Specificity of OK-66 was confirmed by peptide blocking and comparison with the previously characterized chicken anti-human PDZK1 antibody listed above (see Fig. 7, which is published as supporting information on the PNAS web site).
HRP-conjugated anti-mouse, anti-rabbit, and anti-chicken antibodies (Jackson ImmunoResearch) were used at a dilution of 1:20,000 for Western analysis.
Immunocytochemistry. Adult mice were anesthetized by i.p. injection of sodium pentobarbital. The kidneys were cleared (PBS) and fixed (PLP: 2% paraformaldehyde/750 mM lysine/10 mM sodium periodate in phosphate buffer, pH 7.4) by cardiac perfusion. Kidneys were excised, cut into 2- to 4-mm blocks, and postfixed in PLP for an additional 4 h. Tissue blocks were embedded in Epon 812 and sectioned on a Reichert Ultracut E ultramicrotome. One-micrometer sections were etched, subjected to antigen retrieval, and incubated with the appropriate primary and fluorochrome-labeled secondary antibodies (Alexa-Fluor 488 and 594 donkey anti-mouse IgG and donkey anti-rabbit IgG; Molecular Probes) as outlined (21).
GST-Fusion Protein Constructs. Two C-terminal NHE3 fusion protein constructs (nt131 and nt131-T) were generated by PCR from a preexisting rabbit NHE3 cDNA clone (18). The nt131 cDNA PCR product encodes the C-terminal 131 aa of rabbit NHE3 (nt131 forward primer, ACGAATTCTACTGGACAGCCCTGCCTAC; nt131 reverse primer, CGCTCGAGTTTCACATGTGTGTGGACTC). The nt131-T cDNA PCR product encodes the same C-terminal region of rabbit NHE3 minus the last nine nucleotides that encode the putative PDZ-binding motif (nt131-T forward primer, ACGAATTCTACTGGACAGCCCTGCCTAC; nt131-T reverse primer, TACTCGAGAATCAGGACTCGGGGTGTTC). Both sets of PCR primers introduced flanking restriction sites (EcoR1 and XhoI) to facilitate in-frame incorporation into the pGEX-6P-2 GST fusion vector (Amersham Pharmacia).
Two C-terminal CFEX fusion protein constructs (nt185 and nt185-T) were generated by PCR from a preexisting mouse CFEX cDNA clone (4). The nt185 clone encodes the last 185 aa of mouse CFEX (nt185 forward primer, GCGAATTCTAATCACCCAGAAGAAAAAACGAATC; nt185 reverse primer, TACTCGAGAATCAGAGTTTGGTGGCCAAAAC). As with the truncated NHE3 construct, nt185-T is missing the last nine nucleotides that encode the putative PDZ-binding motif (nt185-T forward primer, GCGAATTCTAATCACCCAGAAGAAAAAACGAATC; nt185-T reverse primer, TACTCGAGAATCAGGCCAAAACAGGGCTCTT). Both sets of PCR primers also introduced EcoR1 and XhoI restriction sites to facilitate ligation into the pGEX-6P-2 GST-fusion vector.
The generation of the full-length human PDZK1 GST fusion protein construct was described by Kocher et al. (11).
Pull-Down Assays. A total of 1.25 mg of rabbit BBMV were incubated in 5 ml of ice-cold TBS solubilization buffer (50 mM Tris·HCl/100 mM NaCl/1% Triton TX-100/1 μM pepstatin/1 μM leupeptin/230 μM PMSF, pH 7.2) for 1 h at 4°C. The detergent insoluble fraction was pelleted by centrifugation at 20,000 × g for 20 min at 4°C. The supernatant was removed, combined with 15 μg of GST-fusion protein, and incubated overnight at 4°C. The next morning, the BBMV/fusion protein mixture was added to 50 μl of prewashed glutathione-Sepharose beads (Amersham Pharmacia) and incubated for an additional 2 h at 4°C. The supernatant was removed and the beads were washed with cold solubilization buffer. Captured protein was eluted by incubation with SDS/PAGE sample buffer (31.25 mM Tris·HCl/1% SDS/5% glycerol/50 mM DTT, pH 6.8) for 10 min at room temperature. The eluate was collected, subjected to SDS/PAGE, and then transferred to a poly(vinylidene difluoride) (PVDF) membrane (Immobilon-P, Millipore) for Western analysis.
Overlays. Five micrograms of each of the respective GST-fusion proteins were loaded on a 10–20% polyacrylamide gradient gel, subjected to SDS/PAGE, and transferred to a PVDF membrane. The membrane was blocked by incubation with blotto (5% nonfat dried milk in TBS/0.1% Tween-20/1 μM pepstatin/1 μM leupeptin/230 μM PMSF, pH 7.2) for 4 h at 4°C. Meanwhile, 1.25 mg of rabbit BBMV was solubilized in 5 ml of TBS solubilization buffer as above. The supernatant containing the detergent-solubilized BBMV was combined with 15 ml of blotto and incubated with the preblocked membrane overnight at 4°C. The next morning, the membrane was washed with cold blotto (six times; 10 min per wash) for 1 h. The membrane was then incubated with the anti-PDZK1 antibody OK-66 diluted 1:5,000 in blotto for 1 h at room temperature. The membrane was again washed with cold blotto (six times; 10 min per wash) for 1 h. The membrane was then incubated with an horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody (Jackson ImmunoResearch) diluted 1:20,000 in blotto for 30 min at room temperature. After the 30-min incubation period, the membrane was washed with cold blotto (six times, 10 min per wash) for 1 h, and then processed with ECL Western Blotting Detection Reagent (Amersham Pharmacia).
Measurement of Cl-–Oxalate Exchange in Mouse BBMV. Chloride-stimulated oxalate uptake was assessed by rapid filtration as described (22). Briefly, vesicles were washed twice and then preloaded with chloride by preincubation in a pH 8.2 buffer containing 120 mM potassium gluconate, 30 mM potassium chloride, and 15 mM Hepes for 2 h at 20°C. Timed 30-s uptake of 13 μM [14C]oxalate performed in quintuplicate was assayed after 1:20 dilution of the vesicles into either 120 mM potassium gluconate, 30 mM potassium chloride, and 15 mM Hepes, pH 8.2 (Cli = Clo) or 150 mM potassium gluconate and 15 mM Hepes, pH 8.2 (Cli > Clo).
In Situ Microperfusion of Mouse Kidney Proximal Tubules. Oxalate-stimulated fluid absorption in proximal tubules of age-matched adult PDZK1-null and wild-type mice was assessed by in situ microperfusion as described (23). Briefly, superficial proximal convoluted tubules were perfused at a rate of 15 nl/min with a solution containing 140 mM NaCl, 5 mM NaHCO3, 4 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 2 mM NaPO4, and 20 μCi/ml low-Na+ [3H]methoxyinulin (pH 6.7) (1 Ci = 37 GBq). The rate of net fluid absorption (JV) is based on changes in the concentration of [3H]inulin and is expressed per millimeter of tubule length. Oxalate stimulation of JV was assessed by addition of 1 μM oxalate to the luminal perfusate.
Results
Our approach to address the possibility that PDZK1 may function as a molecular scaffold to facilitate the brush border localization of NHE3 and CFEX in the proximal tubule was to confirm that NHE3 and CFEX were capable of interacting with PDZK1 in vitro and then evaluate the effect of PDZK1 removal on the expression of native NHE3 and CFEX in an in vivo model system.
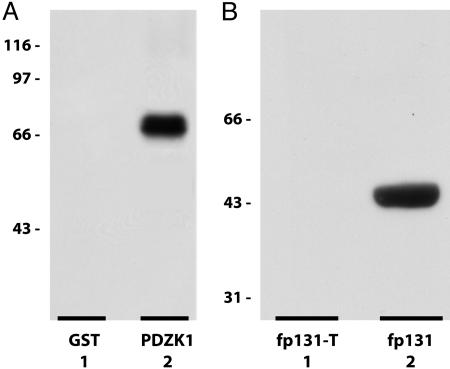
NHE3 Is Capable of Interaction with PDZK1 Through a Classic C-Terminal Type-1 PDZ-Interaction Motif. Preliminary yeast two-hybrid studies suggested that NHE3 may be capable of interaction with PDZK1 (9, 24). To confirm this observation, we performed a series of pull-down assays from detergent solubilized rabbit BBMV with either a full-length human GST-PDZK1 fusion protein or GST alone. Western analysis of the pull-down eluates with the anti-NHE3 antibody, 3H3, indicated that the GST-PDZK1 fusion protein was capable of specifically interacting with native NHE3 (Fig. 1A).
Fig. 1.
In vitro interaction of NHE3 with PDZK1. (A) Western analysis of GST-PDZK1 pull-down of NHE3 from rabbit renal BBMV. Lane 1, GST-pull-down; lane 2, GST-PDZK1 pull-down. Lanes 1 and 2 were probed with the anti-NHE3 antibody, 3H3. Pull-down of GST and GST-PDZK1 was confirmed by Ponceau protein stain. (B) Western analysis of GST-PDZK1 overlay of intact and truncated C-terminal GST-NHE3 fusion proteins. Lane 1, fp131-T, truncated C-terminal 131 aa of rabbit NHE3; lane 2, fp131, intact C-terminal 131 aa of rabbit NHE3. Lanes 1 and 2 were probed with the anti-PDZK1 antibody, OK-66.
To determine whether this interaction was directly mediated through the putative PDZ-interaction motif located at the extreme C terminus of NHE3, the GST-PDZK1 fusion protein was overlaid onto the C-terminal NHE3-GST fusion proteins fp131 and fp131-T that had been subjected to SDS/PAGE and transferred to a PVDF membrane. The fusion protein fp131 contains the intact C-terminal 131 aa of rabbit NHE3. The fusion protein fp131-T is similar, but lacks the C-terminal 3-aa putative PDZ-interaction motif. Western analysis of the overlay with the anti-PDZK1 antibody, OK-66, clearly demonstrated that the putative NHE3 C-terminal PDZ-interaction motif is required for the direct interaction between NHE3 and PDZK1 (Fig. 1B).
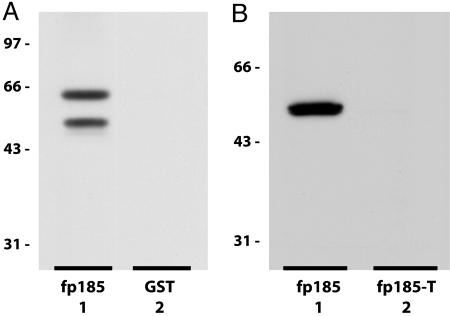
The Interaction of CFEX with PDZK1 Is Also Mediated Through a Classic C-Terminal Type-1 PDZ-Interaction Motif. Gisler et al. (9) reported that a C-terminal CFEX fusion protein was capable of direct interaction with a full-length mouse PDZK1 fusion protein. To corroborate this observation and to confirm that it could occur with a native protein binding partner, we performed a series of pull-down assays from detergent-solubilized rabbit BBMV with either the GST-CFEX fusion protein fp185 or GST alone. The fusion protein fp185 contains the intact C-terminal 185 aa of mouse CFEX. Western analysis of pull-down assays performed with fp185 demonstrated that, despite extensive proteolytic degradation, fp185 was capable of specific interaction with native PDZK1 (Fig. 2A).
Fig. 2.
In vitro interaction of CFEX with PDZK1. (A) Western analysis of GST-CFEX pull-down of PDZK1 from rabbit renal BBMV. Lane 1, GST-CFEX fp185 pull-down; lane 2, GST pull-down. Lanes 1 and 2 were probed with the anti-PDZK1 antibody, OK-66. Pull-down of GST and GST-CFEX fp185 was confirmed by Ponceau protein stain. (B) Western analysis of GST-PDZK1 overlay of intact and truncated C-terminal GST-CFEX fusion proteins. Lane 1, fp185, intact C-terminal 185 aa of mouse CFEX; lane 2, fp185-T, truncated C-terminal 185 aa of mouse CFEX. Lanes 1 and 2 were probed with the anti-PDZK1 antibody, OK-66.
To determine whether this interaction was mediated through the putative PDZ-interaction motif located at the C terminus of CFEX, we overlaid the GST-PDZK1 fusion protein on fp185 and fp185-T GST-CFEX fusion proteins that had been subjected to SDS/PAGE and transferred to a PVDF membrane. The fp185-T fusion protein is similar to fp185, but lacks the C-terminal 3-aa putative PDZ-interaction motif. Western analysis of the overlay indicated that PDZK1 is capable of interacting directly with CFEX and that this interaction requires the presence of the PDZ-interaction motif at the C terminus of CFEX (Fig. 2B).
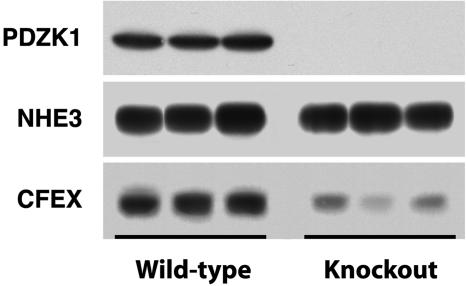
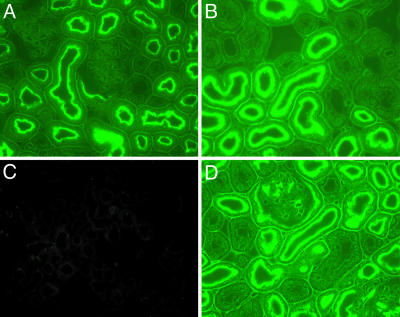
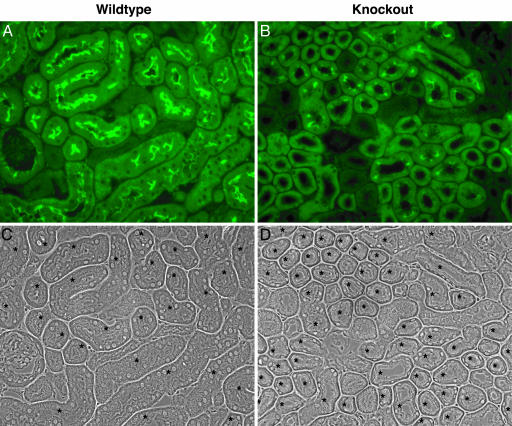
PDZK1 Is Essential for the Normal Apical Expression and Function of CFEX in the Proximal Tubule in Vivo. It is clear that NHE3 and CFEX are capable of strong interactions with PDZK1 when the binding partners are intentionally brought together under contrived conditions. To determine whether PDZK1 coupling is actually a prerequisite for brush border localization of either NHE3 or CFEX in vivo, we assessed the expression of NHE3 and CFEX in kidneys from three PDZK1-null mutant mice and three age-matched wild-type controls. Western analysis of renal microsomal membranes isolated from the PDZK1-null and wild-type kidneys indicated that, although levels of NHE3 were unchanged, the expression of CFEX was dramatically reduced in the kidneys from the PDZK1-null animals (Fig. 3). To address the possibility that PDZK1 loss could induce changes in membrane localization of NHE3 without concomitant changes in levels of expression, and to corroborate the Western CFEX observation, we performed an immunohistochemical analysis of kidneys from both PDZK1-null and wild-type mice. As predicted by the Western analysis, loss of PDZK1 had no discernable effect on the membrane localization of NHE3 (Fig. 4). However, PDZK1 loss did have a profound effect on the level of CFEX expression in the bush border of the mouse proximal tubule (Fig. 5). There was no indication of a redistribution of CFEX expression in the kidneys from the PDZK1-null animals, but there was a significant degree of heterogeneity in the level of expression observed between proximal tubules. The heterogeneity did not clearly correlate with proximal tubule segment (e.g., S1 or S2), and in all cases the expression levels were much lower than those observed in kidneys from wild-type animals.
Fig. 3.
Western analysis of renal microsomes from PDZK1-null and wild-type mice. Microsomes were prepared from three wild-type and three knockout adult animals. (Top) Probed with the anti-PDZK1 antibody, OK-66. (Middle) Probed with the anti-NHE3 antibody, 3H3. (Lower) Probed with anti-CFEX antibody.
Fig. 4.
Immunolocalization of NHE3 and PDZK1 in PDZK1-null and wild-type mouse kidney cortex. (A) Wild-type kidney probed with the anti-PDZK1 antibody, OK66. (B) Wild-type kidney probed with the anti-NHE3 antibody, 2B9. (C) PDZK1-null kidney probed with the anti-PDZK1 antibody, OK66. (D) PDZK1-null kidney probed with the anti-NHE3 antibody, 2B9.
Fig. 5.
Immunolocalization of CFEX in PDZK1-null and wild-type mouse kidney cortex. (A) Wild-type kidney probed with anti-CFEX antibody. (B) PDZK1-null kidney probed with anti-CFEX antibody. (C and D) Phase contrast images of A and B, respectively. Asterisks indicate proximal tubules.
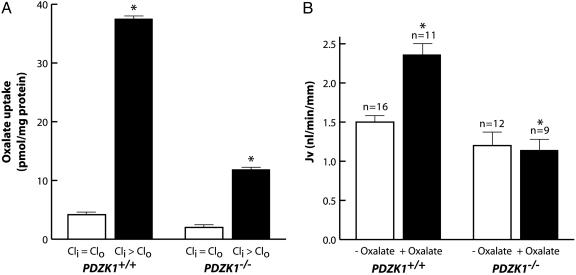
CFEX has been shown to be capable of mediating multiple anion exchange modes in vitro, including Cl-–oxalate, Cl-–HCO3-,Cl-–formate, and Cl-–OH- exchange (5–7), but recent studies with CFEX-null mice suggest that Cl-–oxalate exchange is the principal Cl-–anion exchange mode assumed by CFEX in the proximal tubule and that CFEX mediates all of the Cl--oxalate exchange that occurs in this segment (8). Therefore, we assessed Cl-–oxalate exchange activity in isolated BBMV to confirm that the reduction in CFEX protein expression in PDZK1-null mice was associated with a corresponding reduction in CFEX transport activity. Densitometric quantification of the Western data presented in Fig. 3 suggests that mean CFEX expression levels were reduced by ≈79% in renal microsomes isolated from PDZK1-null mice relative to levels observed in microsomes isolated from wild-type mice. In close agreement with the protein expression data, the level of Cl--stimulated oxalate uptake observed in BBMV from PDZK1-null kidneys was reduced by ≈70% compared to that observed in BBMV isolated from wild-type kidneys (Fig. 6A).
Fig. 6.
Renal proximal tubule Cl-–oxalate exchange activity in PDZK1-null and wild-type mice. (A) Chloride-stimulated oxalate uptake in BBMV isolated from PDZK1-null (PDZK1-/-) and wild-type (PDZK1+/+) mouse kidneys. Cli > Clo and Cli = Clo, respectively indicate the presence or absence of an outwardly directed 30 mM chloride gradient. Values are means ± SE. Asterisk indicates significant difference by Student's t test, P < 0.05. (B) Oxalate-stimulated fluid absorption (JV) in microperfused proximal tubules of PDZK1-null and wild-type mouse kidneys. “+ and - Oxalate” indicates presence or absence of 1 μM oxalate in the luminal perfusate, respectively. Values are means ± SE. n, no. of perfused tubules; kidneys from three to five animals were used for each group. Asterisk indicates significant difference by Student's t test, P < 0.05.
To assess CFEX activity at the level of the intact proximal tubule in vivo, we measured the ability of oxalate to stimulate the rate of fluid volume absorption in superficial proximal tubules of PDZK1-null and wild-type mice microperfused in situ with a high Cl-, low 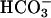 luminal perfusate (see Materials and Methods). Under these conditions, the rate of net volume absorption (JV) reflects net NaCl absorption in the proximal tubule (23). Importantly, in CFEX-null mice, the ability of oxalate to stimulate JV under these conditions is completely abolished (8), validating this measurement as an in vivo assay of CFEX activity in the proximal tubule. In the absence of oxalate, JV was slightly lower in PDZK1-null mice than in wild-type mice, but the difference was not statistically significant (Fig. 6B). Consistent with previous studies (8, 23), addition of 1 μM oxalate to the luminal perfusate induced an ≈50% increase in JV in wild-type mouse proximal tubules. However, oxalate addition had no effect on JV in proximal tubules from PDZK1-null mice, confirming that PDZK1-mediated interactions are essential for oxalate-dependent NaCl reabsorption and normal CFEX function in the proximal tubule.
luminal perfusate (see Materials and Methods). Under these conditions, the rate of net volume absorption (JV) reflects net NaCl absorption in the proximal tubule (23). Importantly, in CFEX-null mice, the ability of oxalate to stimulate JV under these conditions is completely abolished (8), validating this measurement as an in vivo assay of CFEX activity in the proximal tubule. In the absence of oxalate, JV was slightly lower in PDZK1-null mice than in wild-type mice, but the difference was not statistically significant (Fig. 6B). Consistent with previous studies (8, 23), addition of 1 μM oxalate to the luminal perfusate induced an ≈50% increase in JV in wild-type mouse proximal tubules. However, oxalate addition had no effect on JV in proximal tubules from PDZK1-null mice, confirming that PDZK1-mediated interactions are essential for oxalate-dependent NaCl reabsorption and normal CFEX function in the proximal tubule.
Discussion
NHE3 and CFEX are both clearly capable of direct interaction with PDZK1 under a wide variety of in vitro conditions. For brevity's sake, each of these interactions was depicted in a single direction only, but under our experimental conditions the reversal of bait and prey constructs had no discernable effect on either the occurrence or the strength of the observed interactions (data not shown). As reported for the interaction of CFEX with the two PDZ proteins NHERF-1 and NHERF-2 (25) and for the interaction of NHE3 with the PDZ protein NHERF-1 (26), the interactions of both CFEX and NHE3 with PDZK1 were mediated through putative PDZ-interaction motifs located at their respective extreme C termini.
Given the avidity of the in vitro interaction between NHE3 and PDZK1 it is surprising that the deletion of the PDZK1 gene has no effect on the immunolocalization of NHE3 in the mouse proximal tubule. Capuano et al. (14) and Shenolikar et al. (27), however, reported similar findings with PDZK-1 and NHERF-1-null mice, respectively. NHERF-1 is a 55-kDa protein containing two PDZ domains and a C-terminal ezrin-binding domain that has been localized to, among other regions, the brush border of the renal proximal tubule. In vitro studies strongly suggested that NHERF-1 may play a critical role in hormone induced regulation of both transport activity and membrane localization of NHE3 (28). Studies with the NHERF-1-null mouse confirmed that NHERF-1 interaction was essential for cAMP-mediated regulation of NHE3 transport activity, but indicated that like PDZK1, it was unlikely to be directly involved in either the targeting or maintenance of NHE3 in the brush border of the proximal tubule (29).
Although the physiological importance of the interaction between NHE3 and PDZK1 may be uncertain, it is clear that PDZK1 plays a prominent role in the localization of CFEX in the brush border of the proximal tubule. The ability of CFEX and PDZK1 fusion proteins to associate in vitro strongly suggests that the dramatic reduction in CFEX expression observed in the PDZK1-null animals is directly due to a disruption of a CFEX-PDZK1 association rather than a downstream indirect effect mediated through a PDZK1-dependent pathway. The reduced apical expression of CFEX observed in the proximal tubules of the PDZK1-null mice is consistent with a role for PDZK1 in the stabilization of CFEX in the brush border. In analogy with this interpretation, Swiatecka-Urban et al. (30) reported that deletion of the PDZ-interaction motif from the extreme C terminus of CFTR dramatically reduced the residence-time of CFTR in the apical membrane of renal epithelial cells.
Despite the heterogeneity of CFEX expression in the proximal tubules of PDZK1-null mice, a consistent minimum low level of CFEX expression appeared to be maintained in all proximal tubule profiles examined suggesting that CFEX may have other binding partners in the brush border. As alluded to above, Lohi et al. (25) reported that under in vitro conditions the human isoform of CFEX was capable of specifically interacting with the two PDZ proteins, NHERF-1 and NHERF-2. At this point, it is not clear whether there are multiple pools of CFEX in the brush border or whether the low background level of CFEX expression observed in the PDZK1-null animals merely reflects the natural half-life of CFEX residence in the brush border without a PDZK1-mediated apical membrane retention signal.
The transport studies with isolated BBMV and in situ microperfused tubules from PDZK1-null mice clearly indicate that through its effect on brush border expression of CFEX protein, PDZK1 interaction plays a crucial role in maintaining CFEX functional activity in the proximal tubule. Thus, we observed a profound defect in oxalate-stimulated NaCl absorption (JV) in the proximal tubules of PDZK1-null animals. This defect was in fact identical to that observed in CFEX-null mice (8). However, no abnormalities of plasma electrolytes have been observed in either PDZK1- or CFEX-null mice (8, 16). A defect in proximal tubule NaCl absorption might not give rise to a significant renal phenotype due to the presence of large capacity NaCl transport mechanisms in downstream nephron segments like the loop of Henle. It is possible that deficient CFEX activity in the proximal tubule may give rise to a more significant phenotype in pathophysiologic states such as NaCl depletion in which proximal tubule NaCl reabsorption may play a more essential role in electrolyte balance than under baseline conditions.
In conclusion, we have demonstrated that both NHE3 and CFEX are capable of directly interacting with PDZK1 in vitro, and that this interaction is mediated through the classic PDZ-interaction motifs located at the extreme C termini of NHE3 and CFEX, respectively. However, expression studies with PDZK1-null mice suggest that PDZK1-mediated scaffolding is unlikely to play a direct role in either the targeting or retention of NHE3 in the apical membrane of renal proximal tubule cells. In contrast, it is clear that PDZK1 plays an essential role in maintaining normal brush border expression and function of CFEX in the proximal tubule in vivo.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DK33793 and DK17433 (to P.S.A.), DK62289 (to T.W.), and DK62829 (to M.S.).
Author contributions: R.B.T., T.W., B.R.T., L.T., A.G., S.M., and P.S.A. designed research; R.B.T., T.W., B.R.T., L.T., A.G., and S.M. performed research; R.B.T., B.R.T., M.S., and O.K. contributed new reagents/analytic tools; R.B.T., T.W., B.R.T., L.T., S.M., and P.S.A. analyzed data; and R.B.T., T.W., B.R.T., and P.S.A. wrote the paper.
Abbreviations: BBMV, brush border membrane vesicles; PVDF, poly(vinylidene difluoride).
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AY665789).
References
- 1.Alpern, R. J. (1990) Physiol. Rev. 70, 79-114. [DOI] [PubMed] [Google Scholar]
- 2.Aronson, P. S. & Giebisch, G. (1997) Am. J. Physiol. 273, F179-F192. [DOI] [PubMed] [Google Scholar]
- 3.Aronson, P. S. (2002) Cell Biochem. Biophys. 36, 147-153. [DOI] [PubMed] [Google Scholar]
- 4.Knauf, F., Yang, C. L., Thomson, R. B., Mentone, S. A., Giebisch, G. & Aronson, P. S. (2001) Proc. Natl. Acad. Sci. USA 98, 9425-9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang, Z., Grichtchenko, I. I., Boron, W. F. & Aronson, P. S. (2002) J. Biol. Chem. 277, 33963-33969. [DOI] [PubMed] [Google Scholar]
- 6.Xie, Q., Welch, R., Mercado, A., Romero, M. F. & Mount, D. B. (2002) Am. J. Physiol. 283, F826-F838. [DOI] [PubMed] [Google Scholar]
- 7.Wang, Z., Petrovic, S., Mann, E. & Soleimani, M. (2002) Am. J. Physiol. 282, G573-G579. [DOI] [PubMed] [Google Scholar]
- 8.Wang, Z., Wang, T., Petrovic, S., Tuo, B., Riederer, B., Barone, S., Lorenz, J., Seidler, U., Aronson, P. S. & Soleimani, M. (2005) Am. J. Physiol. 288, C957-C965. [DOI] [PubMed] [Google Scholar]
- 9.Gisler, S. M., Pribanic, S., Bacic, D., Forrer, P., Gantenbein, A., Sabourin, L. A., Tsuji, A., Zhao, Z., Manser, E., Biber, J. & Murer, H. (2003) Kidney Int. 64, 1733-1745. [DOI] [PubMed] [Google Scholar]
- 10.Kocher, O., Comella, N., Tognazzi, K. & Brown, L. F. (1998) Lab. Invest. 78, 117-125. [PubMed] [Google Scholar]
- 11.Kocher, O., Comella, N., Gilchrist, A., Pal, R., Tognazzi, K., Brown, F. & Knoll, J. H. M. (1999) Lab. Invest. 79, 117-125. [PubMed] [Google Scholar]
- 12.Wang, S., Yue, H., Derin, R. B., Guggino, W. B. & Li, M. (2000) Cell 103, 169-179. [DOI] [PubMed] [Google Scholar]
- 13.Anzai, N., Miyazaki, H., Noshiro, R., Khamdang, S., Chairoungdua, A., Shin, H., Enomoto, A., Sakamoto, S., Hirata, T., Tomita, K., et al. (2004) J. Biol. Chem. 279, 45942-45950. [DOI] [PubMed] [Google Scholar]
- 14.Capuano, P., Bacic, D., Stange, G., Kaissling, B., Pal, R., Kocher, O., Biber, J., Wagner, C. & Murer, H. (2005) Pflugers Arch. 449, 392-402. [DOI] [PubMed] [Google Scholar]
- 15.Aronson, P. S. (1978) J. Membr. Biol. 42, 81-98. [DOI] [PubMed] [Google Scholar]
- 16.Kocher, O., Rinku, P., Roberts, M., Cirovic, C. & Gilchrist, A. (2003) Mol. Cell. Biol. 23, 1175-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karniski, L. P., Wang, T., Everett, L. A., Green, E. D., Giebisch, G. & Aronson, P. S. (2002) Am. J. Physiol. 283, F952-F956. [DOI] [PubMed] [Google Scholar]
- 18.Biemesderfer, D., Rutherford, P. A., Nagy, T., Pizzonia, J. H., Abu-Alfa, A. K. & Aronson, P. S. (1997) Am. J. Physiol. 273, F289-F299. [DOI] [PubMed] [Google Scholar]
- 19.Goyal, S., Mentone, S. & Aronson, P. S. (2005) Am. J. Physiol. 288, F530-F538. [DOI] [PubMed] [Google Scholar]
- 20.Petrovic, S., Wang, Z., Ma, L., Seidler, U., Forte, J. G., Shull, G. E. & Soleimani, M. (2002) Am. J. Physiol. 283, G1207-G1216. [DOI] [PubMed] [Google Scholar]
- 21.Thomson, R. B., Mentone, S. A., Kim, R., Earle, K., Delpire, E., Somlo, S. & Aronson, P. S. (2003) Am. J. Physiol. 285, F870-F880. [DOI] [PubMed] [Google Scholar]
- 22.Kuo, S. & Aronson, P. S. (1996) J. Biol. Chem. 271, 15491-15497. [DOI] [PubMed] [Google Scholar]
- 23.Wang, T., Yang, C. L., Abbiati, T., Shull, G. E., Giebisch, G. & Aronson, P. S. (2001) Am. J. Physiol. 281, F288-F292. [DOI] [PubMed] [Google Scholar]
- 24.Gisler, S. M., Stagljar, I., Traebert, M., Bacic, D., Biber, J. & Murer, H. (2001) J. Biol. Chem. 276, 9206-9213. [DOI] [PubMed] [Google Scholar]
- 25.Lohi, H., Lamprecht, G., Markovich, D., Heil, A., Kujala, M., Seidler, U. & Kere, J. (2003) Am. J. Physiol. 284, C769-C779. [DOI] [PubMed] [Google Scholar]
- 26.Weinman, E. J., Wang, Y., Wang, F., Greer, C., Steplock, D. & Shenolikar, S. (2003) Biochemistry 42, 12662-12668. [DOI] [PubMed] [Google Scholar]
- 27.Shenolikar, S., Voltz, J. W., Minkoff, C. M., Wade, J. B. & Weinman, E. J. (2002) Proc. Natl. Acad. Sci. USA 99, 11470-11475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shenolikar, S. & Weinman, E. J. (2001) Am. J. Physiol. 280, F389-F395. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham, R., Steplock, D., Wang, F., Huang, H., E, X., Shenolikar, S. & Weinman, E. J. (2004) J. Biol. Chem. 279, 37815-37821. [DOI] [PubMed] [Google Scholar]
- 30.Swiatecka-Urban, A., Duhaime, M., Coutermarsh, B., Karlson, K. H., Collawn, J., Milewski, M., Cutting, G. R., Guggino, W. B., Langford, G. & Stanton, B. A. (2002) J. Biol. Chem. 277, 40099-40105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.