Abstract
Background
Type 2 diabetes (T2DM) affects brain structure and function, and is associated with an increased risk of dementia and mild cognitive impairment. It is known that exercise training has a beneficial effect on cognition and brain structure and function, at least in healthy people, but the impact of exercise training on these aspects remains to be fully elucidated in patients with T2DM.
Objective
To determine the impact of exercise training on cognition and brain structure and function in T2DM, and identify the involved physiological mediators.
Methods
This paper systematically reviews studies that evaluate the effect of exercise training on cognition in T2DM, and aims to indicate the most beneficial exercise modality for improving or preserving cognition in this patient group. In addition, the possible physiological mediators and targets involved in these improvements are narratively described in the second part of this review. Papers published up until the 14th of January 2025 were searched by means of the electronic databases PubMed, Embase, and Web of Science. Studies directly investigating the effect of any kind of exercise training on the brain or cognition in patients with T2DM, or animal models thereof, were included, with the exception of human studies assessing cognition only at one time point, and studies combining exercise training with other interventions (e.g. dietary changes, cognitive training, etc.). Study quality was assessed by means of the TESTEX tool for human studies, and the CAMARADES tool for animal studies.
Results
For the systematic part of the review, 22 papers were found to be eligible. 18 out of 22 papers (81.8%) showed a significant positive effect of exercise training on cognition in T2DM, of which two studies only showed significant improvements in the minority of the cognitive tests. Four papers (18.2%) could not find a significant effect of exercise on cognition in T2DM. Resistance and endurance exercise were found to be equally effective for achieving cognitive improvement. Machine-based power training is seemingly more effective than resistance training with body weight and elastic bands to reach cognitive improvement. In addition, BDNF, lactate, leptin, adiponectin, GSK3β, GLP-1, the AMPK/SIRT1 pathway, and the PI3K/Akt pathway were identified as plausible mediators directly from studies investigating the effect of exercise training on brain structure and function in T2DM. Via these mediators, exercise training induces multiple beneficial brain changes, such as increased neuroplasticity, increased insulin sensitivity, and decreased inflammation.
Conclusion
Overall, exercise training beneficially affects cognition and brain structure and function in T2DM, with resistance and endurance exercise having similar effects. However, there is a need for additional studies, and more methodological consistency between different studies in order to define an exercise program optimal for improving cognition in T2DM. Furthermore, we were able to define several mediators involved in the effect of exercise training on cognition in T2DM, but further research is necessary to unravel the entire process.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40798-025-00836-7.
Key points
The current body of literature demonstrates a positive effect of exercise training on the brain in T2DM, but simultaneously emphasizes the need for additional studies on this topic.
BDNF, lactate, leptin, adiponectin, GSK3β, GLP-1, the AMPK/SIRT1 pathway, and the PI3K/Akt pathway have been identified as factors mediating the effect of exercise on the brain in T2DM.
There is a need for a standardised cognitive test battery to investigate the effect of exercise on cognition in T2DM.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40798-025-00836-7.
Introduction
Cognitive Decline in Aging
Cognitive decline is inherent to aging. As we age, synaptic plasticity reduces, neural mitochondrial function declines, epigenetic changes occur, etc., all contributing to decreased cognitive abilities [1–5]. Especially fluid intelligence, defined as the ability to solve problems, process new information, and learn new things, declines with aging. Fluid intelligence consists of several domains, including executive function, psychomotor ability, memory, and processing speed [6, 7].
Aging is associated with a decline in both grey and white matter volume, accompanied by ventricular enlargement and cortical thinning, as well as a decline in brain function [7, 8]. Amyloid β plaques are not only found in Alzheimer’s disease (AD) patients, but also in 20–30% of healthy adults, and are speculated to contribute to this neuronal loss [6, 9]. Also neuronal volume and the number of neuronal connections decreases due to a decline in the complexity of dendrite arborization, decreased neuritic spines, and reduced dendrite length [6, 10]. In addition, demyelination and decreased white matter integrity contribute to functional impairment of the brain [8].
Cognitive Dysfunction in T2DM
T2DM patients are prone to more severe cognitive decline than what occurs during normal aging. A large fraction (45%) of T2DM patients experience cognitive dysfunction and have an increased risk of dementia [11–16]. Subjects with diabetes have a relative risk of 1.46 for AD, and a relative risk of 1.51 for any form of dementia [17]. Especially executive functioning, memory, attention, and information processing speed have been shown to be affected in T2DM [14, 18, 19]. This cognitive impairment is related to the affected brain structure and metabolism in T2DM. Decreased regional grey matter volume as well as altered intrinsic activity in the default mode network have been demonstrated in these patients [20–22]. Multiple pathophysiologies have been suggested as the cause of this, including cardiovascular complications, chronic low-grade inflammation, and hyperglycemia [14, 15, 18].
Several studies have demonstrated a link between T2DM and AD, also called type 3 diabetes. These studies focus on the fact that insulin signalling also plays an important role in the brain, and that T2DM and AD seem to share a number of pathophysiological processes such as amyloid β plaques, disturbed cerebral glucose metabolism, tau hyperphosphorylation, and inflammation [18, 23–26]. Insulin and insulin-like-growth-factor (IGF)-1 are important for neuronal survival and brain function. Numerous insulin receptors (IR) and IGF-1 receptors (IGF-1R) can be found in the brain, with a large amount being present in the hippocampus. One of the major downstream pathways of the IR is the PI3K/Akt pathway, which plays an important role in the regulation of brain function, and in the inactivation of GSK3β, which is an enzyme able to phosphorylate tau at pathological tau epitopes [23, 27, 28]. Reduced cerebral IR activation and insulin levels have been demonstrated in AD [25, 29], highlighting similar processes in AD and T2DM. Moreover, insulin-degrading enzyme is capable of clearing amyloid β peptides that aggregate into amyloid plaques, a key pathological hallmark of AD. In T2DM, competitive binding of high insulin concentrations and amyloid β peptides with this enzyme could be involved in decreased amyloid β clearance, thereby potentially contributing to increased accumulation of amyloid β peptides, and formation of amyloid β plaques [15, 30, 31].
Positive Effect of Exercise on Cognitive Function
In healthy people, exercise has a positive effect on cognitive function [32–34]. This effect is mediated by multiple processes. Muscle contraction during exercise releases myokines, which stimulate the production of neurotrophic factors such as brain-derived neurotrophic factor (BDNF), promoting neurogenesis. Additionally, the exercise-induced release of anti-inflammatory factors contributes to balancing the brain’s redox status, counteracting many pathological processes [35–38]. Research shows that exercise reduces the age-related decrease in hippocampal volume [39–41], which, in combination with increased levels of neurotrophic factors, contributes to the maintenance of memory and neuroplasticity [36, 42, 43]. Moreover, there is a positive association between cardiorespiratory fitness levels and whole-brain and white matter volume in patients with early-stage AD [44]. In this way, exercise reduces the risk of dementia and neurodegenerative diseases [36, 45–47].
Lactate has been identified as an important contributor to the positive effect of exercise on cognitive function. During anaerobic exercise, lactate is released from contracting muscles, eventually leading to increased lactate levels in the brain after crossing the blood–brain-barrier (BBB). This results in an increased expression of brain plasticity genes such as BDNF, Arc, and c-fos, contributing to neurogenesis [48–50]. In addition, lactate also binds to hydroxycarboxylic acid receptor 1 (HCAR1), which leads to an increase in vascular endothelial growth factor (VEGF), stimulating angiogenesis and thereby contributing to increased cognitive function [50–52]. Also the myokine irisin has been shown to positively influence cognition [53]. Synaptic plasticity and memory in amyloid pathology mimicking AD mouse models can be rescued by boosting brain levels of FNDC5/irisin, and peripheral overexpression of FNDC5/irisin rescues memory impairment [54]. Cathepsin b is another myokine which has been associated with improved cognition. It is known to play a role in both neurogenesis and angiogenesis [55, 56]. Cathepsin b levels increase in mouse and human plasma in response to exercise, which is positively correlated with memory, while cathepsin b-knockout mice do not show any cognitive improvements following running exercise [57]. This suggests that cathepsin b is mandatory for the positive effect of exercise on cognition.
The Association of Physical Activity with Cognitive Function in T2DM
Because of the above-mentioned positive effects of exercise on cognitive function, and to get more insight into whether this translates to T2DM, some studies have explored the association between physical activity (PA) and cognition in T2DM. The effect of exercise training on the brain in T2DM could be different than in other populations due to the reduced insulin sensitivity in this patient group. Exercise increases insulin sensitivity, and could thus have a more pronounced effect in insulin resistant patients [58, 59]. Moreover, the existing cognitive decline in T2DM [11, 12] could affect the extent of the effect of exercise on cognition in patients with T2DM. In addition, cardiovascular complications such as atherosclerosis can increase the risk of vascular dementia in T2DM [60, 61], and are responsive to exercise training [62, 63].
For example, one study found significantly higher cognitive scores in an active group of T2DM patients compared to a sedentary group of T2DM patients [64]. They also found a significant negative correlation (r = − 0.2, p = 0.03) between cognition and BMI in the sedentary group, and a significant positive correlation (r = 0.55, p = 0.01) between cognition and minutes of weekly exercise in the active group. Another study found that active elderly T2DM patients had a significantly slower rate of cognitive decline compared to non-active/sedentary elderly T2DM patients, more specifically in the global cognition (p = 0.005), executive functioning (p = 0.014), and attention/working memory (p = 0.01) domains [65]. These findings were confirmed by multiple other studies [66–68].
In a study investigating the influence of the BDNF Val66Met polymorphism on the association between cognition and PA in diabetic patients, it was found that carriers of the Met-allele showed significantly higher scores of words recall (p < 0.001), mental status (p = 0.004), and total cognition, (p = 0.04), and had a significantly higher education level. Overall, PA was associated with better total cognition, words recall, and mental status, no matter the intensity. This association was strongest in the Met/Met carrier group, and stronger for females than for males within this group. They also found that light to moderate PA showed greater association with cognitive domains than moderate to vigorous PA. These findings thus suggest that female Met/Met diabetic carriers cognitively benefit the most from PA, and that light to moderate PA has the most influence on the brain [68].
Another study examined the individual and joint influence of diabetes status, apolipoprotein E (APOE) ε4, and PA on the risk of dementia and cognitive impairment without dementia (CIND) in cognitively normal older adults. The risk of dementia and CIND was higher in diabetic patients and APOE ε4 carriers who reported low levels of moderate to vigorous PA. The risk was even found to be nearly tenfold higher for physically inactive diabetic APOE ε4 carriers. Higher levels of PA were associated with lower dementia/CIND risk [67].
Overall, these findings suggest that higher levels of PA are associated with better cognitive function and less cognitive decline in T2DM. However, based on these studies alone, one cannot assume causality. In addition, there is a possibility of reverse causality, where reduced cognitive function contributes to lower levels of PA. Therefore, several studies have investigated the acute effect of exercise on cognitive function in T2DM.
The Acute Effect of Exercise on Cognitive Function in T2DM
The acute effect of exercise on cognition in T2DM has mainly been investigated in the domain of executive function.
One study found that both endurance and resistance training acutely improved inhibitory control and response time in T2DM patients [69]. In addition, moderate-intensity integrated concurrent exercise (ICE), consisting of both endurance and resistance training, acutely improved all three aspects of executive function (inhibition, conversion and refresh function) in cognitively normal hospitalised T2DM patients, while resistance exercise improved inhibition, and endurance exercise only caused significant improvements in the refresh function. The ICE-induced improvements in executive function were accompanied by a simultaneous increase in cerebral blood flow in the dorsolateral prefrontal cortex (DLPFC), the frontal pursuit area (FPA), and the orbitofrontal cortex (OFC), while resistance exercise showed a corresponding activation of DLPFC and FPA, and endurance exercise increased cerebral perfusion in the FPA, OFC and Broca region [70]. These results suggest that an exercise-induced increase in cerebral blood flow in certain areas can elicit specific cognitive improvements. This knowledge can be used to customise exercise programs in T2DM patients in order to obtain the desired effect.
These studies already give some insight into the effect of exercise on cognition in T2DM. However, with the aim to incorporate exercise in T2DM treatment for cognitive improvement, it is necessary to know the effect of long-term exercise training on the brain, which can be different from the acute effect. Therefore, this review will focus on pre-post intervention studies. This review only describes RCTs and clinical trials investigating the effect of exercise training on cognition and brain structure in T2DM and animal models thereof, with special emphasis on the mediators and targets involved in this. So far, this is the first review of this kind. The knowledge provided by this review could more reliably provide insights in the mechanisms of cognitive decline and cognitive improvement in T2DM, and could be used to design more specific exercise programs to target the brain in T2DM patients.
Methods
This review consists of two parts, the first of which is a systematic review of the papers investigating the effect of exercise training on cognition in T2DM. The second part narratively describes studies that show beneficial brain changes or brain changes in combination with cognitive improvement in T2DM as a result of exercise training, and aims to identify the involved mediators by pooling studies discussing similar pathways or targets. The addition of this second part distinguishes this review from previous similar reviews [71–73], and deepens the understanding of how exercise improves cognition, and identifies pathways that could be pharmacologically targeted to mimic the effect of exercise on cognition in individuals unable to exercise.
Eligibility
The PICO (population, intervention, comparison, outcome) method was used to determine whether articles were eligible for inclusion in this review. In order to be included in the systematic part of this review, the articles had to be written in English, and had to investigate the effect of any kind of exercise training on cognition in T2DM patients or T2DM animal models. Studies investigating the effect of a single exercise bout or physical activity during daily living were not included. Exercise training in T2DM was compared to usual care, or a different kind (intensity/modality/…) of exercise training in T2DM. Studies without a T2DM control group were not included. Articles discussing only type 1 diabetes were excluded, as well as articles only investigating depression, anxiety, or other mental disorders in T2DM. Also studies investigating the effect of exercise training in combination with another intervention (e.g. dietary changes, cognitive training, etc.) were excluded. Studies in which cognitive function was only assessed at one time point in human subjects were excluded since these make it impossible to accurately assess the effect of exercise training on cognition (pre- and post-intervention assessments mandatory). The narrative part of this review includes studies from the systematic section that also examined certain cerebral pathways, mediators, or targets. Additionally, to further explain the findings, we also reference other studies that explore the effects of exercise on these cerebral pathways, mediators, or targets in T2DM, even if they do not directly assess cognition.
Study Selection
The papers described in the systematic part of this review were found by entering the following search string into PubMed, Embase and Web of Science: (exercise OR "physical activity" OR "aerobic exercise" OR "aerobic training" OR "resistance exercise" OR "resistance training" OR "combined exercise" OR "combined training" OR “endurance exercise” OR “endurance training”) AND (T2DM OR diabetes OR "type 2 diabetes") AND (memory OR "executive function*" OR "processing speed" OR attention OR brain OR cognition OR "cognitive function"). The filters “randomised controlled trial” and “clinical trial” were applied. Relevant related articles suggested by PubMed when accessing articles found by entering the aforementioned search string were also considered. Reference lists of reviews in line with the present one [71–79] were examined as well. The current review discusses articles published up until the 14th of January 2025. Duplicate articles (identical PMID) and studies not meeting the TESTEX or CAMARADES criteria were removed (Fig. 1). The following information was extracted from the studies: author(s), year of publication, age of the subjects, type of subjects (population), number of subjects in the intervention group, type of control group, number of subjects in the control group, type, duration, frequency, and intensity of the exercise intervention, duration of each exercise session, outcome measures (cognitive tests), and main cognitive results. Effect sizes were extracted, or calculated if not mentioned. For the narrative part of the review, only studies directly investigating the effect of exercise on the brain in T2DM were considered to identify plausible mediators and pathways.
Fig. 1.
Prisma flow chart of study selection procedure. Reason 1: Does not discuss the effect of exercise training on cognition in T2DM; Reason 2: Article not available in English; Reason 3: Article only discusses the protocol. Study is not conducted yet. Reason 4: No T2DM control group. T1DM = type 1 diabetes. From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71
Quality Control
Quality control of the included human studies was conducted by means of the Tool for the assEssment of Study qualiTy and reporting in EXercise (TESTEX), which is designed specifically for exercise training studies [80]. With this tool, a maximal score of 15 can be reached, with 5 points assigned to study quality, and 10 points to reporting. A score of ≥ 7/15 was required for inclusion (Table 1). Quality of the animal studies was assessed by means of the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) checklist [81]. Animal studies with a score of < 6/10 were excluded (Table 2). If nothing was mentioned about the criterion, it was indicated as not fulfilled.
Table 1.
TESTEX characteristics of included human studies
| 1 | 2 | 3 | 4 | 5 | 6 | 6a | 6b | 6c | 7 | 8 | 8a | 8b | 9 | 10 | 11 | 12 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leischik et al. [82] | √ | √ | √ | √ | X | X | / | / | / | X | √ | √ | √ | √ | X | X | X | 7/15 |
| Ploydang et al. [83] | √ | √ | √ | √ | X | √ | √ | √ | X | √ | √ | √ | √ | √ | X | X | √ | 11/15 |
| Wang et al. [84] | √ | √ | √ | √ | √ | X | / | / | / | X | √ | √ | √ | √ | √ | X | X | 9/15 |
| Zhao et al. [85] | √ | √ | √ | X | √ | √ | X | √ | √ | √ | √ | √ | √ | √ | X | √ | √ | 12/15 |
| Yamamoto et al. [86] | √ | √ | X | √ | X | √ | √ | √ | √ | X | √ | √ | √ | √ | X | X | X | 9/15 |
| Teixeira et al. [87] | √ | √ | X | √ | X | X | / | / | / | √ | √ | √ | √ | √ | √ | √ | √ | 10/15 |
| Silveira-Rodrigues et al. [88] | √ | X | X | √ | X | √ | X | X | √ | √ | √ | √ | √ | √ | √ | √ | √ | 10/15 |
| Espeland et al. [89] | √ | √ | X | √ | √ | √ | X | √ | √ | X | √ | √ | √ | √ | X | X | X | 9/15 |
| Silveira-Rodrigues et al. [90] | √ | X | X | √ | X | X | / | / | / | X | √ | √ | √ | √ | X | √ | √ | 7/15 |
| Ghodrati et al. [91] | √ | √ | X | √ | X | √ | √ | X | X | X | √ | √ | √ | √ | X | √ | √ | 9/15 |
| Martinez-Velilla et al. [92] | √ | √ | √ | √ | √ | √ | √ | √ | X | √ | √ | √ | √ | √ | √ | X | X | 12/15 |
| Callisaya et al. [93] | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | X | X | X | 12/15 |
| Ghahfarrokhi et al. [94] | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | X | X | √ | 13/15 |
| Chen et al. [95] | √ | √ | X | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | X | X | X | 11/15 |
| Cai et al. [96] | √ | X | X | √ | X | √ | √ | √ | X | X | √ | √ | √ | √ | X | X | X | 7/15 |
| Liu et al. [97] | √ | √ | √ | √ | √ | X | / | / | / | X | √ | √ | X | √ | X | X | √ | 8/15 |
√ = fulfilled, X = not fulfilled, 1 = Eligibility criteria specified, 2 = Randomisation specified, 3 = Allocation concealed, 4 = Groups similar at baseline, 5 = Blinding of assessor, 6 = Outcome measures assessed in 85% of patients, 6a = adherence > 85%, 6b = adverse events reported, 6c = exercise attendance reported, 7 = Intention-to-treat analysis, 8 = Between-group statistical comparisons reported, 8a = between-group statistical comparisons reported for primary outcome measure of interest, 8b = between-group statistical comparisons reported for at least one secondary outcome measure, 9 = Point measures and measures of variability for all reported outcome measures, 10 = Activity monitoring in control groups, 11 = Relative exercise intensity remained constant, 12 = Exercise volume and energy expenditure
Table 2.
CAMARADES characteristics of included animal studies
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parsa et al. [98] | √ | √ | √ | X | √ | √ | √ | X | √ | √ | 8/10 |
| Shekarchian et al. [99] | √ | √ | √ | X | X | √ | √ | X | X | √ | 6/10 |
| Jesmin et al. [100] | √ | √ | √ | X | X | √ | √ | X | X | √ | 6/10 |
| Lang et al. [101] | √ | √ | √ | √ | √ | √ | √ | X | √ | √ | 9/10 |
| Shima et al. [102] | √ | √ | √ | X | X | √ | √ | X | √ | √ | 7/10 |
| Shima et al. [103] | √ | √ | X | X | X | √ | √ | X | √ | √ | 6/10 |
√ = fulfilled, X = not fulfilled, 1 = Published in peer-reviewed journal, 2 = Control of temperature, 3 = Randomisation, 4 = Allocation concealed, 5 = Blinding of assessors, 6 = No anaesthetics with marked intrinsic properties, 7 = Use of animals with diabetes, 8 = Sample size calculation, 9 = Compliance with regulatory requirements, 10 = Statement regarding conflict of interest
Results
Study Selection
See Fig.1.
Quality Control
See Table 1.
Systematic Review on the Effect of Exercise Training on Cognition in T2DM
Endurance Exercise Training
Human Studies
Endurance exercise as simple as walking can already significantly improve cognition in T2DM patients. In a study by Leischik et al. (2021), T2DM patients were randomised into a walking group, pedometer group or control group. The walking group had to walk for 40 min, 3 times a week. The pedometer group had to reach 10,000 steps per day. Both the walking group and the pedometer group, the latter of which managed to reach an average of 8700 steps per day, showed significant improvements in attention and non-verbal memory. Verbal memory also significantly improved in the walking group [82]. In contrast, a pilot RCT on the effect of 3 months of walking at moderate intensity on cognitive function in T2DM failed to find significant improvements in executive function, episodic memory, working memory, and processing speed [97]. Besides normal walking, also aquatic Nordic walking performed for 60 min 3 times a week for 12 weeks, has proven to cause significantly increased Montreal Cognitive Assessment (MoCA) scores in T2DM patients. However, no significant improvements were seen in the MMSE, trail making test part B, or Stroop colour and word test [83].
Wang et al. (2023) tested the effect of outdoor aerobic dancing on hippocampal volume and cognition in 82 T2DM patients. The patients were divided into a control group and a training group. After one year of endurance training, they found significantly increased MMSE and MoCA scores in the training group compared to the control group, suggesting that endurance exercise can improve cognitive function in T2DM [84].
Animal Studies
Also animal studies show a predominantly positive effect of endurance exercise training on cognition. Shekarchian et al. (2023) found that streptozotocin- and high fat diet-induced diabetic C57BL/6 J mice that received 4 weeks of swimming training scored significantly better on tests for working, spatial, and recognition memory compared to non-exercising diabetic mice [99]. Another study showed that swimming training for 12 weeks in streptozotocin-nicotinamide-induced type 2 diabetic rats improved exploratory behaviour, locomotor activity, passive avoidance memory, and non-spatial cognitive memory compared to their sedentary counterparts. However, this improvement was found to be insignificant [98].
The Morris water maze test has oftentimes been used to demonstrate the effect of endurance exercise on cognition in animal models of T2DM. In this test, the animal is placed in a circular swimming pool where a platform is hidden somewhere under the water surface. After a few practice rounds, the time it takes the animal to find the platform, and the length of the path that is followed, are measured. These measures give an indication of spatial learning and memory [104]. For example, one study looked into the effect of 4 months of both light and moderate intensity treadmill running on memory in pre-symptomatic Otsuka-Long-Evans-Tokushima fatty (OLETF) rats. Before the exercise intervention, the OLETF rats spent considerably less time in the quadrant area where the platform was located during the learning phase, compared to control Long-Evans Tokushima (LETO) rats. After the intervention, both exercise intensities showed to have improved memory function in the OLETF rats, since both escape latency and swim length were shortened [100]. Lang et al. (2020) also used the Morris water maze test to test the effect of 8 weeks of moderate-intensity treadmill exercise on memory in T2DM mice. The exercising T2DM mice showed a significantly reduced escape latency on day 4–5 compared to the non-exercising T2DM mice, and their number of platform crossings significantly increased over time [101]. Another similar study was performed where the effect of 4 weeks of moderate-intensity treadmill running in OLETF rats was investigated. They found that both swim path length and escape latency of the exercising OLETF rats were reduced at trials on days 2–4, and that their time spent in the platform area significantly improved after the exercise intervention [102]. Another study by Shima et al. (2023) assessed the effect of 4 weeks of light-intensity running on a forced exercise wheel bed in obese-hyperglycemic (ob/ob) mice. However, here they did not find an effect of exercise on the swim distance, escape latency, or speed. They did find that the times of crossing the target platform during the probe test in the exercised ob/ob mice did not differ significantly from that in the control C57BL/6 mice, and that it was greater than in the sedentary ob/ob mice, but not significantly [103].
Based on these studies, endurance exercise training conducted for 40–60 min, 3 times per week seems to enable cognitive improvements in T2DM patients. Especially memory shows to be sensitive to endurance exercise-induced improvement. However, two out of four human studies discussed here only found significant improvements in MoCA and/or MMSE scores. Since the MMSE and MoCA were designed as screening tools to diagnose mild cognitive impairment (MCI) [105, 106], they cannot be considered very reliable for detecting changes in cognitive function.
Resistance Exercise Training
Human Studies
The beneficial effect of resistance exercise on cognition in T2DM patients was shown by Zhao et al. (2022). They examined the effect of 12 months of power training on executive function, attention/speed, memory, and global cognition in older adults with T2DM, and aimed to determine whether there is an association between cognitive improvements and improvements in muscle strength, body composition, and/or endurance. 103 patients were divided into a power training group or a sham low-intensity group that performed the same exercises as the power training group, but without added weight. Cognition of both groups improved over time, with increased scores in the Trails A, Trails B, word list recall, and word list memory. In addition, improved memory was associated with both increased skeletal muscle mass and reduced body fat mass, and improvement in Trails B minus A in the power training group was associated with increases in knee extension strength [85]. Yamamoto et al. (2021) failed to find similar results. In their study, 60 T2DM patients aged 72.9 ± 2.4 years were divided into a control group, a resistance training group, and a resistance training group with leucine (an amino acid promoting muscle synthesis) supplementation. The training groups performed daily bodyweight resistance exercises and exercises with elastic bands every day at home, for a total period of 48 weeks. Cognitive function was assessed by means of the MMSE at baseline and after the intervention period. The MMSE score in the control group was significantly decreased to 27.5 ± 2.6 after 48 weeks, while the score in the training groups had not significantly changed. This caused the MMSE score at 48 weeks to be significantly higher in the training groups than in the control group [86].
Both studies suggest a positive effect of resistance exercise training on cognition in T2DM, with machine-based resistance exercise training being the most effective. However, a sufficient number of studies to draw a definite conclusion is lacking. Moreover, the study of Yamamoto et al. only assessed cognitive function by means of the MMSE, limiting the information on cognitive function, and thus the reliability of the results.
Endurance Versus Resistance Exercise Training
Human Studies
To determine the exercise modality most effective in improving cognition in T2DM, some studies compared the effect of endurance and resistance exercise training. Teixeira et al. (2029) investigated the effect of either endurance or resistance exercise on cognitive function in T2DM patients or patients with arterial hypertension. The patients were randomised into a resistance exercise group or an endurance exercise group. Both groups exercised at moderate intensity for 12 weeks. Cognitive function was assessed before and after the intervention by means of the mental test and training system (MTTS), consisting of the cognitrone (attention and concentration), the determination test (reaction time), and the visual pursuit test (selective attention). The group with both T2DM and hypertension, but not the group with only hypertension, showed a significantly improved performance in the cognitrone, but no significantly improved reaction time. No differences were observed between the endurance and resistance exercise group. These results show that both endurance and resistance exercise are capable of increasing attention and concentration in T2DM patients with arterial hypertension [87].
This study shows that endurance and resistance exercise training have a comparable positive effect on cognitive function in T2DM, however, additional evidence confirming these results is needed.
Combined Endurance and Resistance Exercise Training
Human Studies
Several studies have explored the effect of combined exercise training, consisting of both resistance and endurance exercise, sometimes in combination with flexibility or balance exercises, on cognition in T2DM patients. One study found significantly improved inhibitory control, working memory, cognitive flexibility, and attention after 8 weeks of combined exercise training in T2DM patients, compared to a control group [88]. The same research team also examined the effect of 8 weeks of combined exercise on plasma BDNF levels, executive function and long-term memory in T2DM patients. They observed significantly improved executive function following the combined exercise, while BDNF levels were not found to be significantly changed [90]. Espeland et al. (2027) conducted an exploratory analysis of data from the Lifestyle Interventions and Independence for Elders (LIFE) trial, which was a randomised controlled clinical trial of exercise intervention consisting of walking, resistance training, and flexibility exercises in sedentary non-demented T2DM patients and healthy subjects. Cognitive function was tested at baseline and 2 years after randomisation. They found that cognitive function, more specifically global cognitive function and delayed memory, significantly improved in the diabetic participants of the intervention group only, suggesting a beneficial effect of combined training on cognition in T2DM [89]. Additionally, one study determined the effect of 12 weeks of combined exercise training, consisting of endurance, resistance, and balance exercises, on cognition in women with T2DM. Cognition was tested by means of the MoCA, the digit symbol substitution test, and the forward digit span test. After the 12-week intervention, the exercise group scored significantly higher on the MoCA compared to the control group, and, with an increase of 3.1, improved significantly compared to baseline. However, neither of the groups showed improvement in the other tests [91].
In a study by Martinez-Velilla et al. (2021), 103 acutely hospitalised elderly T2DM patients were randomised to an exercise group or a control group. Cognitive function was assessed by means of the MMSE at baseline and at discharge. The exercise intervention consisted of a combination of resistance, balance, and walking exercises. The median length of stay, and thus of the intervention, was 8 days. There was no difference in MMSE score between both groups at baseline. However, after the intervention, the exercise group scored on average 1.6 points higher than the control group (23.7 vs. 22.1), which was found to be significant [92]. In addition, a pilot study looked into the effect of 6 months of a progressive endurance- and resistance-training program on the brain and cognition in T2DM. 50 T2DM patients were randomised into an intervention group and a control group. The intervention group performed 6 months of endurance and progressive resistance training. The control group received upper and lower limb stretching of light intensity and a gentle movement program, which were performed in the same volume, frequency and setting as in the intervention group. The intervention group showed improved hippocampal and total brain volumes, improved white matter integrity, and less decline in white matter volume. They also showed a better global cognitive score, and better performance on the Digit Symbol Coding Test, Rey Complex Copy test, Stroop C-D, Trail Making Test A and B, Hopkins verbal learning test (intermediate and recognition scores), and Controlled Oral Word Association Test compared to the control group [93]. Another pilot study examined the effect of 6 weeks of high-intensity low-volume (HIFT) vs. low-intensity high-volume (LIFT) functional training on cognition in cognitively impaired elderly T2DM patients. The HIFT group exercised three times a week at 100–120% of the lactate threshold, while the LIFT group exercised five times a week at 70–75% of the lactate threshold. The MMSE was used to diagnose cognitive impairment (MMSE ≤ 23). Processing speed, learning, memory, and attention were assessed by means of the Symbol Digit Modalities Test (SDMT), California Verbal Learning Test Second Edition (CVLT-II), Brief Visuospatial Memory Test-Revised (BVMT-R), and Stroop tests respectively. After the intervention, MMSE, Stroop, SDMT, CVLT-II and BVMT-R scores had improved significantly in the HIFT group, while only MMSE and Stroop scores had improved significantly in the LIFT group. However, the only cognitive score change that was significantly different from the control group that did not receive an exercise intervention, was the change in Stroop scores in the HIFT group [94].
All included studies suggest a positive effect of combined exercise training on cognitive function in T2DM.
Other Types of Exercise
Human Studies
Besides the well-known exercise forms such as running and cycling, some studies have explored the effect of unconventional exercise training on cognition in T2DM.
For example, one study compared the effect of 36 weeks of Tai Chi Chuan, a mind–body exercise, and 36 weeks of fitness walking on global cognitive function in T2DM patients. Both interventions significantly increased MoCA scores, with a significantly larger effect of Tai Chi Chuan compared to fitness walking. At 24 weeks and 36 weeks, the Weschler memory quotient (MQ), digit symbol substitution test, and trail making test part B scores also improved significantly more in the Tai Chi Chuan group than in the control group. At 36 weeks, the MQ scores even improved significantly more in the Tai Chi Chuan group compared to the fitness walking group [95].
The effect of low-intensity Qigong exercise on cognitive function in older T2DM patients was also investigated [96]. Qigong is an ancient Chinese exercise for mind–body integration and is often used in the prevention and treatment of chronic metabolic diseases [107]. The participants in the exercise group practiced Kinect-based Kaimai-style Qigong for 12 weeks. Based on MMSE scores at baseline and after the intervention, the Qigong group showed significant improvements in cognitive function compared to the control group [96].
These findings suggest that unconventional exercise, often involving the mind to a larger extent than conventional exercise, can contribute to improved cognition in T2DM.
Reviewing Targets and Mediators of the Effect of Exercise Training on the Brain in T2DM
To understand why certain exercise trainings cause cognitive improvement in T2DM, and others do not, there is a need for studies investigating the underlying biomedical mechanisms of the effect of exercise on the brain and cognition in T2DM. The following section discusses such studies, and aims to identify the different targets and mediators involved.
AD-Related Pathological Markers: Amyloid β Peptides and Hyperphosphorylated Tau
Increasing evidence and literature points to converging pathways and pathogenetic processes in T2DM and AD. Here we focus on studies showing effects on amyloid β and tau in T2DM due to exercise training. We refer to extensive reviews regarding T2DM and AD for further reading [24, 108, 109].
Leptin
Several studies have indicated that exercise is negatively associated with cerebral amyloid β and hyperphosphorylated tau in T2DM, and contributes to memory maintenance [110, 111]. The aspartyl protease β-site AβPP-cleaving enzyme 1 (BACE1) is responsible for catalysing the rate-limiting step of amyloid β production. It has been shown that the adipocytokine leptin reduces BACE1 activity and expression, thereby limiting the production of amyloid β [112, 113]. This is supported by Rezaei et al. (2023), who suggested leptin as a possible mediator of exercise-induced prevention of memory impairment in T2DM after finding elevated serum and hippocampal levels of this hormone together with decreased hippocampal levels of BACE1, amyloid β and hyperphosphorylated tau after 8 weeks of HIIT training in T2DM rats [114].
GSK3β
The maintenance of cognitive function in exercising T2DM rats treated with dexamethasone, demonstrated by De Sousa et al. (2020), was according to the authors presumably mediated by the observed lesser inhibition of the activation of hippocampal IRS-1 and higher concentration of GSK3β phosphorylated on serine-9 (Ser-9) [115]. GSK3β is namely inactivated by Ser-9 phosphorylation [116], which prevents GSK3β activation, and subsequently tau phosphorylation and associated neurotoxic effects [117, 118]. Accordingly, Rezaei et al. (2023) also found decreased hippocampal GSK3β dephosphorylation together with decreased hippocampal levels of amyloid β and phosphorylated tau in HIIT-trained T2DM rats [114]. GSK3β plays an important role in neurodegeneration in T2DM. Both glucolipotoxicity and insulin resistance contribute to GSK3β overactivation, leading to β-catenin phosphorylation and subsequent proteasomal degradation. This results in the inhibition of the expression of reactive oxygen species (ROS) scavenging enzymes, which leads to more oxidative stress and consequently the disruption of mitochondrial structure, function and axonal trafficking. Moreover, GSK3β overactivation also stimulates tau hyperphosphorylation, leading to microtubule destabilisation and thus disruption of axonal mitochondrial trafficking, as well as to a decrease in mitochondrial complex I and thus impaired energy production [118]. By inhibiting the activation of GSK3β through Ser-9 phosphorylation, exercise could thus contribute to the prevention of cognitive decline in T2DM.
Adiponectin
In the study of Rezaei et al. (2023), the T2DM rats showed decreased serum and hippocampal levels of insulin and adiponectin at baseline, as well as decreased levels of insulin receptors, adiponectin receptors and AMPK in the hippocampus, and increased hippocampal GSK3β and hyperphosphorylated tau. HIIT training counteracted these findings. Both serum insulin and adiponectin were significantly increased by HIIT, as well as the levels of hippocampal insulin and adiponectin receptors. HIIT training also increased hippocampal AMPK phosphorylation, and decreased hippocampal tau phosphorylation and GSK3β dephosphorylation. The authors suggest that the increased levels of adiponectin can contribute to the preservation of hippocampal volume and function through the stimulation of synaptic plasticity, as it has been shown by Pousti et al. (2018) and Weisz et al. (2017) respectively that adiponectin modulates synaptic plasticity in the hippocampal dentate gyrus and regulates hippocampal synaptic transmission [114, 119, 120]. Another study shows memory and learning impairments together with reduced AMPK phosphorylation and increased GSK3β activation in adiponectin-knockout mice [121], suggesting that the neuroprotective effects of adiponectin are mediated via AMPK phosphorylation and GSK3β inactivation, which is in line with the study of Rezaei et al. (2023). The exercise-induced decreased GSK3β Ser-9 dephosphorylation means that there is a higher ratio of inactive, phosphorylated GSK3β, preventing tau phosphorylation. In addition, the adiponectin-mediated AMPK phosphorylation could also be neuroprotective by inhibiting the inflammatory response of microglia to amyloid β [122].
Brain Volume
Several studies have demonstrated that exercise increases hippocampal volume in T2DM patients. One study showed that a higher step count, measured over 7 days, was associated with a larger hippocampal volume in T2DM patients [66]. In addition, one year of endurance training in T2DM patients with normal cognition significantly increased hippocampal volume, and prevented a decline in MMSE and MoCA scores. Hippocampal volume in the training group was significantly increased compared to baseline, as well as compared to the control group [84]. Another study showed a higher hippocampal CA1 and CA3 neuronal density in diabetic Sprague–Dawley (SD) rats after 6 weeks of exercise on a running wheel, probably contributing to the observed improved cognitive function [123]. Similarly, a pilot study in which T2DM patients performed 6 months of a progressive endurance- and resistance-training program showed improved hippocampal and total brain volumes, improved white matter integrity, and less decline in white matter volume in the intervention group compared to the control group [93].
Neuronal Survival
BDNF
BDNF is one of the most important neurotrophic factors. By binding the TrkB receptor, it regulates and promotes cell survival, neuroplasticity, and neurogenesis in the central nervous system [124, 125]. As already mentioned previously, BDNF plays an important role in the effect of exercise on cognitive function. A study investigating the effect of 4 weeks of swimming training on working, spatial, and recognition memory in diabetic C57BL/6 J mice, shows that higher PA is associated with increased hippocampal and prefrontal BDNF levels, together with an improvement in all memory aspects [99]. Similarly, Jesmin et al. (2022) showed significantly increased hippocampal BDNF levels together with improved memory function in OLETF rats after 4 months of endurance exercise [100]. This shows that also under diabetic conditions, BDNF is an important mediator of exercise effects on cognition.
Another study looked into the effect of 12 weeks of swimming training on BDNF/TrkB signalling and apoptosis in the cerebral cortex of male diabetic C57BL/6JNarl mice. The exercised diabetic mice showed less neural apoptosis compared to the control diabetic mice, accompanied by an increased activity of the BDNF/TrkB signaling pathway, and a decreased activity of the Fas/FasL-mediated and mitochondria-initiated apoptotic pathways. These findings indicate that exercise promotes neuronal survival in diabetic mice through the BDNF pathway [126].
Neuroplasticity and Neurogenesis
One study determined the effect of 7 weeks of treadmill exercise on neuroblast differentiation in the subgranular zone of the dentate gyrus (SZDG) in Zucker diabetic fatty (ZDF) rats and Zucker lean control (ZLC) rats. At 23 weeks old, the rats started to exercise for 7 weeks at 12-16 m/min. Compared to the non-exercising groups, the neuroblasts of both the exercising diabetic and exercising control rats showed a significant increase in tertiary dendrites. However, the number of neuroblasts only increased in the control rats. This shows that endurance exercise in diabetic rats can stimulate neuroplasticity, but not necessarily neuroproliferation [127]. A similar study tested the effect of 5 weeks of treadmill exercise on cell differentiation and proliferation in the SZDG of the same ZDF rat model. Proliferation was detected by means of the proliferation marker Ki67, and progenitor differentiation into neurons was detected by means of the differentiation marker doublecortin (DCX). In this study, the rats started exercising at 6 weeks old, and ran at 22 m/min. Compared to the sedentary ZDF rats, the exercised rats showed a significant increase in Ki67 positive cells and DCX-immunoreactive structures, indicating both increased proliferation and increased differentiation [128]. This confirms that exercise can stimulate neuronal differentiation in the diabetic SDZG, as shown by Hwang et al. (2010) [127]. The fact that, this time, exercise was able to stimulate neuronal proliferation as well, might indicate that exercise has to be performed at a certain intensity rather than a certain volume to achieve this effect.
BDNF
Increased hippocampal BDNF levels in combination with increased dendritic spine density on the secondary and tertiary dendrites of dentate granule neurons were found after voluntary wheel running in db/db mice [129]. This suggests that BDNF contributes to exercise-induced neuroplasticity in diabetic mouse models.
PI3K/Akt and AMPK/SIRT1
It was demonstrated that endurance exercise can upregulate synaptic plasticity-associated proteins in the hippocampus of T2DM rats, presumably via the activation of PI3K/Akt/mTOR and AMPK/SIRT1 signalling pathways and inhibition of the NFκB/NLRP3/IL-1β signalling pathway [130]. Under non-diabetic conditions, the PI3K/Akt/mTOR pathway is activated by insulin, resulting in the formation of dendritic spines and excitatory synapses in hippocampal neurons [131]. In T2DM, insulin resistance prevents this activation, contributing to reduced synaptic plasticity. Exercise thus could, to a certain extent, take over this role of insulin. The aforementioned BDNF also activates the PI3K/Akt pathway, constituting another mediator of the exercise-induced increase in synaptic plasticity-associated proteins [124]. Activation of the AMPK/SIRT1 pathway, on the other hand, contributes to synaptic plasticity by increasing insulin sensitivity and glucose uptake, regulating BDNF expression, promoting neuronal survival, etc. [132–136]. During exercise, AMPK activates SIRT1, after which SIRT1 deacetylates PGC-1α, resulting in its activation. PGC-1α is important for mitochondrial remodelling and biogenesis, contributing to synaptic plasticity [137]. Its activation leads to the upregulation of FNDC5, which in turn crosses the BBB and upregulates hippocampal BDNF expression [124].
Another study also found an increase in the synaptic plasticity proteins synaptophysin (SYN) and N-methyl-D-aspartate receptor (NMDAR) in the prefrontal cortex of diabetic SD rats after 4 weeks of treadmill training. They demonstrated that endurance exercise increased the level of phosphorylated PI3K, suggesting an increased activity of the PI3K/Akt pathway, and increased insulin sensitivity. In addition, they found a slight increase in the phosphorylation and a slight decrease in the acetylation of FOXO1, which is a transcription factor that promotes NF-kB activity and consequently the expression of proinflammatory factors. FOXO1 phosphorylation enables its cytoplasmic retention and thus prevents the transcription of its target inflammatory genes [138]. Since FOXO is a target of Akt, the increased PI3K/Akt activity might be the cause of the increased FOXO1 phosphorylation [139]. Nuclear NF-kB acetylation was found to be decreased as well in the rats that performed endurance exercise, reducing its DNA binding and transcriptional activity [138, 140]. These findings were confirmed by a study showing that 8 weeks of moderate intensity treadmill exercise in diabetic C57BL/ 6 mice improves cognitive dysfunction and increases the release of BDNF through the activation of the SIRT1/PGC-1α/FNDC5/BDNF pathway [101].
Cerebral Blood Flow
Zhao et al. (2023) investigated the effect of a 2-month moderate-intensity exercise program on brain oxygenation in sedentary older T2DM patients with cognitive impairment. They also looked into the effect of the exercise program on the hemodynamic responses to the Mini-Cog test, which assesses short-term memory and visuo-constructive abilities. The exercise program consisted of a combination of endurance and resistance exercise. They found that the exercise program improved the very low-frequency oscillations (< 0.05 Hz) during the Mini-Cog test, as assessed by near-infrared spectroscopy. Since very-low frequency oscillations are crucial for small-vessel function, this indicates that combined exercise has the power to improve cerebral vascular function in T2DM. In addition, the exercise program significantly reduced the oxyhemoglobin activation in the right superior frontal region during the Mini-Cog test, thereby reducing the overactivation usually observed in T2DM [141].
Glycometabolism
Lactate
Since dysregulated peripheral glycometabolism is a hallmark of T2DM, Shima et al. (2017) hypothesised that hippocampal glycometabolic dysfunction might contribute to memory impairment in T2DM [102]. As it has already been demonstrated in healthy animals that moderate exercise enhances memory function and hippocampal glycogen levels [142, 143], they wanted to determine if this was also the case in a T2DM animal model. At baseline, OLETF rats had higher hippocampal levels of glycogen and lower MCT2 expression compared to LETO control rats. They also showed impaired memory. After 4 weeks of treadmill running, the OLETF rats showed even higher hippocampal glycogen levels, in addition to normalised hippocampal MCT2 expression together with improved memory function. Since MCT2 is an important lactate transporter, this suggests that lactate plays a key role in the effect of exercise on glycometabolism and memory in T2DM [102]. In line with this, it was demonstrated that 4 months of treadmill running prevented the progression of cognitive decline in presymptomatic OLETF rats through improved hippocampal MCT2 expression, again confirming the role of lactate in the positive effect of exercise on cognition [100]. Another study by Shima et al. (2023) showed the same effect in an advanced stage T2DM mouse model. Ob/ob mice were subjected to 4 weeks of light-intensity exercise. The exercise program resulted in improved hippocampal MCT2 levels, accompanied by improved hippocampal memory retention [103]. As Soya et al. (2019) mention in their review discussing these findings, the upregulated MCT2 levels enable an increased uptake of L-lactate into neurons, where it can serve as an energy substrate, and act as a signalling molecule to induce the expression of neuroplasticity genes, eventually contributing to the increased memory function observed in these studies [144].
Inflammation
As inflammation is a very broad process, involving many different players, we would like to emphasize again that the pathways and mediators discussed in this review are only the ones that clearly have been shown to be involved in the effect of exercise on the brain in T2DM. For more information about the involvement of inflammation in T2DM, and the effect of exercise on it, we refer to more exhaustive reviews [78, 145].
AMPK/SIRT1
The AMPK/SIRT1 pathway is not only important for rescuing cognitive function by increasing BDNF levels, but also by counteracting inflammation. Treadmill exercise in diabetic C57BL/ 6 mice for 8 weeks reduced activation of hippocampal proinflammatory microglia M1, as well as the hippocampal levels of proinflammatory factors IL-1β, IL-6, TNF-α, and increased the expression levels of anti-inflammatory factors IL-10, TGF-β1. This co-occurred with an activation of the SIRT1/NF-κB pathway, which is known to be responsible for counteracting inflammation, suggesting the importance of this pathway in exercise-mediated anti-inflammatory actions in T2DM [101]. A different study also reported a reduction in hippocampal IL-1β and TNF-α, accompanied by cognitive improvement, after T2DM SD rats performed 6 weeks of endurance exercise on a running wheel. However, although we can assume that the AMPK/SIRT1 pathway is involved here as well, the authors did not elaborate on the pathways responsible for the observed reduction in pro-inflammatory factors [123].
Insulin Resistance
T2DM-associated insulin resistance is not only present in the periphery, but also in the brain, where it contributes to the cognitive deficits observed in T2DM patients via multiple mechanisms [132, 146–148]. For example, insulin resistance and the consequent hyperinsulinemia have been associated with increased tau hyperphosphorylation, increased deposition together with reduced breakdown of amyloid β, dysfunctional IGF-1 receptors (IGFRs), etc. This all contributes to neurodegeneration and cognitive impairment [146]. Studies have shown that the insulin-sensitising effect of exercise is also manifested in the brain, which gives exercise the power to prevent or decrease these cognitive deficits [58, 149]. One possible way in which this effect of exercise could be mediated, is by the aforementioned decrease in TNF-α. TNF-α activates JNK, which is a kinase that causes insulin resistance via serine phosphorylation of IRS-1 [78, 150]. IRS-1 is important for the signal transduction of insulin, ultimately leading to glycogen synthesis and glucose transporter 4 (GLUT-4) translocation. Serine phosphorylation of IRS-1 inhibits this insulin signal transduction and thus results in insulin resistance [151–153]. It could thus be hypothesised that by decreasing TNF-α, exercise could increase insulin sensitivity in the brain.
GLP-1
Park et al. (2019) investigated the effect of resistance exercise training on GLP-1R levels in the hypothalamus of OLETF rats. The exercise training comprised 12 weeks of ladder climbing. As a result of the resistance training, the rats showed increased levels of hypothalamic GLP-1R mRNA, protein kinase A (PKA), protein kinase B (PKB), glucose transporter 2 (GLUT-2), and decreased hypothalamic levels of protein kinase C-iota (PKC-ι) [154]. All these findings point in the direction of improved glycaemic control. PKB, also known as Akt, plays an important role in the PI3K/Akt pathway downstream of the IR, as mentioned before in this review [23, 27, 130]. PKA, on the other hand, is essential for the regulation of metabolism and triglyceride storage [155, 156], while PKC-ι is known to cause metabolic abnormalities in T2DM [157, 158]. GLP-1R, the main focus of this study, is a main receptor involved in T2DM. It is responsible for lowering blood glucose levels by, among other things, stimulating insulin secretion and suppressing glucagon secretion [159]. In addition, GLP-1R has been suggested to play a neuroprotective role in diabetes-related neurodegeneration by increasing insulin sensitivity [160]. Moreover, a different study has shown that recombinant human GLP-1 can reduce oxidative stress by activating PKA, which was found to be elevated in the study of Park et al. (2019), and by inhibiting PKC, which was found to be decreased, ultimately reversing diabetic nephropathy [161]. The increase in GLP-1 mRNA found by Park et al. (2019) thus indicates decreased oxidative stress and increased insulin sensitivity in the T2DM rats after resistance exercise training.
Mitochondrial Function
Mitochondrial dysfunction is an important contributor to T2DM pathology [162, 163]. Multiple studies have already demonstrated mitochondrial dysfunction in the T2DM brain, where it contributes to neurodegeneration and cognitive dysfunction [164, 165]. It is also known that exercise positively influences mitochondrial function [166–168]. Studies show that exercise can restore mitochondrial function in the muscle of T2DM patients [169–172]. However, little is known about the effect of exercise on mitochondrial function in the brain in T2DM.
Insulin Sensitivity
One study investigated the effect of endurance exercise training in combination with metformin on mitochondrial function in male C57BL/6 J mice with brain insulin resistance induced by a high-fat diet. They observed that the mitochondria in brain regions rich in insulin receptors produced less ATP and showed reduced activity of oxidative enzymes. The mice also showed elevated ROS production and reduced activity of antioxidant enzymes, accompanied by higher rates of mitochondrial fission, and accumulation of damaged mitochondrial proteins. Endurance exercise training together with metformin improved insulin sensitivity. This resulted in reduced ROS emission, less hippocampal mitochondrial fission, less mitochondrial protein oxidation, and increased ATP production in astrocytes and primary cortical neurons. The reduction in ROS emission and increased ATP production were counteracted by intranasal administration of the insulin receptor antagonist S961, proving that these mitochondrial ameliorations were mediated by increased insulin sensitivity [173]. Since metformin was also administered in this study, the observed effects are not fully attributable to exercise, however, it is clear that metformin and exercise have a synergistic effect, and that exercise can certainly be an added value in the treatment of T2DM.
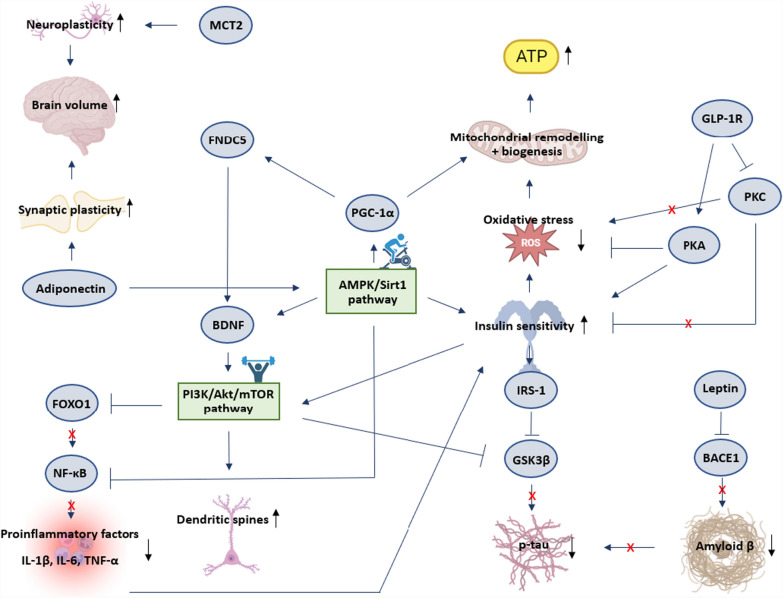
The figure below (Fig. 2) gives an overview of the interplay between all mediators and pathways previously mentioned.
Fig. 2.
Schematic representation of mediators and targets involved in the effect of exercise training on the brain in T2DM. Sharp blue arrows indicate stimulation. Blunt blue arrows indicate inhibition. Black arrows indicate increase or decrease. A red cross indicates a mediator’s inability to exert an effect due to previous inhibition of said mediator. Aβ = amyloid β; AMPK = AMP-activated Protein Kinase; ATP = Adenosine Triphosphate; BDNF = Brain-Derived Neurotrophic Factor; FNDC5 = Fibronectin type III Domain-Containing protein 5; FOXO1 = Forkhead box protein O1; GLP-1R = Glucagon-Like Peptide 1 Receptor; GSK3β = Glycogen Synthase Kinase-3 beta; IRS-1 = Insulin Receptor Substrate 1; IL-1β = Interleukin 1beta; IL-6 = Interleukin 6; MCT2 = Monocarboxylate Transporter 2; mTOR = mammalian Target Of Rapamycin; Nf-κB = Nuclear Factor kappa-light-chain-enhancer of activated B-cells; PGC-1α = Peroxisome proliferator-activated receptor Gamma Coactivator 1-alpha; PI3K = Phosphoinositide 3-Kinase; PKA = Protein Kinase A; PKC = Protein Kinase C; p-tau = phosphorylated tau; ROS = Reactive Oxygen Species; Sirt1 = Sirtuin 1; TNF-α = Tumor Necrosis Factor alpha
Discussion
In the systematic part of this review, 22 studies investigating the effect of exercise training on cognition in T2DM were included. Although mixed results were found, most studies (18 out of 22) demonstrated a significant positive effect of exercise training on cognition, which is consistent with findings of previous reviews on this topic [72, 73].
7 out of 10 studies investigating the effect of endurance exercise training were able to show cognitive improvement, indicating the effectiveness of this training modality. Leischik et al. showed that endurance exercise as simple as walking is already successful in improving cognition in T2DM [82]. However, this was not confirmed by Liu et al., who could not find any significant cognitive improvements after a 3-month walking intervention [97]. A small sample size and a short follow-up period were mentioned as possible reasons for the lack of a significant effect. However, sample size, duration of the exercise intervention, and frequency and length of the individual exercise sessions were comparable to the study of Leischik et al., where significant improvements were seen. This might suggest that the walking intensity in the study of Leischik et al. was more optimal, although this information was not disclosed.
Out of the two studies investigating the effect of resistance exercise on cognition in T2DM, one study failed to show cognitive improvement. This lack of effect could be due to the limited cognitive tests, or due to the absence of machine-based power training in this exercise intervention. Additional studies are required to clarify this.
The study of Teixeira et al. comparing endurance exercise training to resistance exercise training found similar results for both exercise forms. This suggests that exercise interventions in T2DM patients aimed at improving cognition do not have to be limited to one type of exercise, and can be adapted to the patient’s capacities. However, since only one study directly comparing these exercise modalities was identified, this conclusion should be interpreted with caution.
In addition, all included studies investigating the effect of combined (endurance + resistance (+ flexibility/balance)) exercise training were able to show significant cognitive improvements. Being in line with the exercise recommendations of the American College of Sports Medicine for older adults [174], this finding favours combined exercise to reach cognitive improvement in T2DM.
Finally, tai chi chuan and Kaimai style Qigong showed to improve cognition in T2DM. The beneficial effect of these exercise forms might be attributed to the fact that they are motorically more complex, and require a more extensive involvement of the mind.
Based on this review, one can thus conclude that both endurance and resistance exercise training, as well as exercise training involving the mind, are effective at improving cognition in T2DM. Combined exercise training is presumably most desirable, although studies comparing combined exercise training to endurance or resistance exercise training alone are lacking. It also seems that regarding resistance exercise training, machine-based power training is more effective in improving cognition than body weight exercises and exercises with elastic bands, but additional evidence is required.
The beneficial effect of exercise on cognitive function in T2DM was confirmed by previous reviews on this topic [73, 79]. A meta-analysis by Cai et al. found a significant positive effect of exercise on global cognitive function in older adults with T2DM, without a significant influence of intervention modality or duration [73]. In contrast, several reviews could not conclude a significant positive effect of exercise on cognition in T2DM [71, 74]. Four out of the six studies included in the systematic review of Zhao et al. found significant benefits of exercise for some aspects of cognition in adults with type 2 diabetes, insulin resistance or impaired glucose tolerance, but only 26% of the cognitive outcomes were significant across all studies [71]. Also Cooke et al. found no significant effect of exercise on executive function or memory after conducting a meta-analysis of 6 studies on the effect of exercise on cognition in T2DM. [74]. These contradictory results emphasize the need for additional qualitative studies on this topic.
The mechanisms underlying cognitive improvement following exercise in T2DM have been explored in the second, narrative part of this review. Both the AMPK/Sirt1 pathway and PI3K/Akt/mTOR pathway have been identified as key players. The studies included in this review show that exercise-mediated activation of these pathways leads to beneficial brain changes in T2DM, such as increased neuroplasticity, brain volume, synaptic plasticity, dendritic spines, insulin sensitivity, mitochondrial remodelling and biogenesis, and ATP production, as well as decreased inflammation, oxidative stress, and amyloid β and p-tau production. BDNF, lactate, leptin, adiponectin, GSK3β and GLP-1 were identified as the most important factors mediating these changes. Although other factors such as IGF-1 have been suggested to play a role in the positive effect of exercise on cognition [175], no studies in T2DM have directly demonstrated this. Much more research is needed to identify the full spectrum of involved mediators and pathways, investigate interactions between different cell types, etc. In addition, it needs to be considered that the majority of the studies described in this review were conducted in rodents, and are yet to be confirmed in humans.
This review has various limitations that could interfere with the reliable interpretation of its findings. First of all, the fact that the studies included in this review use a variety of cognitive tests, makes it difficult to compare the different studies. Moreover, several studies merely use the MMSE or MoCA to test cognition, which are diagnostic tools designed to screen for MCI, and not to detect (subtle) changes in cognition [105, 106]. There is a need for a standardised cognitive test battery in T2DM to increase the comparability of future studies and draw more reliable conclusions. In addition, the age, medication use, and ethnicity of the patient population varied across studies. Since cognition is heavily influenced by age [176], and medication use [177, 178] and genetics [68] strongly affect the response to exercise, comparability of the included studies may have been limited by these inconsistencies. Regarding the narrative part of this review, an important limitation is that only studies directly demonstrating an involvement of certain mediators in the effect of exercise training on the brain in T2DM have been considered. This means the present review is a non-exhaustive presentation of the involved mediators and pathways. Finally, publication bias might have skewed the results of this review towards a positive effect of exercise on cognition in T2DM.
In order to define an exercise program that is specifically tailored to cognitive improvement in T2DM, future studies should directly compare exercise of different intensities and volumes, as well as different exercise modalities. In addition, as already mentioned, a standardised cognitive test battery should be used that is most sensitive to picking up exercise-induced cognitive changes in T2DM. Furthermore, medication use, disease stage, age, and genetics should be taken into account.
Conclusion
Overall, it can be concluded that exercise has a positive influence on cognition and brain structure in T2DM. There are few studies that fail to find a positive correlation between exercise training and cognition in T2DM, which can often be attributed to a small sample size or a limited cognitive test battery. From this review, we can assume that resistance and endurance exercise training have similar effects on cognition in T2DM, with resistance exercise training seemingly requiring machine-based exercises. We can also conclude that unconventional exercise training involving the mind, such as tai chi, is capable of inducing cognitive improvements as well. However, since the cognitive tests and exercise training programs used in studies investigating the effect of exercise on cognition in T2DM is so diverse, it is difficult to compare these studies and draw a definite conclusion on the best exercise program for cognitive improvement in T2DM. Future studies should focus on using a standardised cognitive test battery, and comparing similar exercise training interventions where only one parameter (intensity/duration/frequency/…) differs.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- Aβ
Amyloid β
- AD
Alzheimer’s disease
- AMPK
AMP-activated Protein Kinase
- APOE
Apolipoprotein E
- ATP
Adenosine Triphosphate
- BACE1
β-Site AβPP-cleaving enzyme 1
- BBB
Blood-brain barrier
- BDNF
Brain-Derived Neurotrophic Factor
- BNT
Boston Naming Test
- BVMT-R
Brief Visuospatial Memory Test-Revised
- CBS
Cambridge Brain Sciences
- CIND
Cognitive impairment without dementia
- CVLT-II
California Verbal Learning Test Second Edition
- D
Cohen’d
- DCX
Doublecortin
- DLPF
Dorsolateral prefrontal cortex
- DSC
Digit Symbol Coding
- DSST
Digit Symbol Substitution Test
- E group
Diabetes + exercise group
- FNDC5
Fibronectin type III Domain-Containing protein 5
- FOXO1
Forkhead box protein O1
- FPA
Frontal pursuit area
- GLP-1R
Glucagon-Like Peptide 1 Receptor
- GLUT
Glucose transporter
- GSK3β
Glycogen Synthase Kinase-3 beta
- HIFT
High-intensity low-volume functional training
- HRmax
Heart Rate maximum
- HRR
Heart Rate Reserve
- HVLT-D
Hopkins Verbal Learning Test revised
- I
Rest interval between sets and strength-type exercises
- ICE
Integrated concurrent exercise
- IGF-1
Insulin-like-growth-factor 1
- IGF-1R
Insulin-like-growth-factor 1 receptor
- IR
Insulin receptor
- IRS-1
Insulin Receptor Substrate 1
- IL-1β
Interleukin 1beta
- IL-6
Interleukin 6
- LETO
Long-Evans Tokushima
- LIFT
Low-intensity high-volume functional training
- MCE
Multicomponent exercise
- MCI
Mild cognitive impairment
- MCT2
Monocarboxylate Transporter 2
- MMSE
Mini mental state examination
- MoCA
Montreal cognitive assessment
- MQ
Weschler Memory Quotient
- MRI
Magnetic Resonance Imaging
- MRs
Maximal repetitions
- MTTS
Mental test and training system
- mTOR
Mammalian Target Of Rapamycin
- MWM
Morris water maze
- Nf-κB
Nuclear Factor kappa-light-chain-enhancer of activated B-cells
- NMDAR
N-methyl-D-aspartate receptor
- OFC
Orbitofrontal cortex
- OLETF
Otsuka-Long-Evans-Tokushima fatty
- PA
Physical activity
- PGC-1α
Peroxisome proliferator-activated receptor Gamma Coactivator 1-alpha
- PI3K
Phosphoinositide 3-Kinase
- PKA
Protein Kinase A
- PKC
Protein Kinase C
- p-tau
Phosphorylated tau
- ROCFT
Rey-Osterrieth Complex Figure Test
- ROS
Reactive Oxygen Species
- Ser-9
Serine-9
- SD
Sprague-Dawley
- SDMT
Symbol Digit Modalities Test
- Sirt1
Sirtuin 1
- SZDG
Subgranular zone of the dentate gyrus
- SYN
Synaptophysin
- T group
Diabetes group
- TMT
Trail Making Test
- TNF-α
Tumor Necrosis Factor alpha
- TUG
Timed up-and-go
- T2DM
Type 2 diabetes
- VEGF
Vascular-endothelial growth factor
- V1
Intervention midpoint
- V2
Postintervention
- v6MWT
Velocity in 6 min walking test
- ZDF
Zucker diabetic fatty
- ηp2
Partial eta-squared
- 1RM
1 Rep maximum
- 3MSE
Modified Mini Mental State Exam
Author contributions
JV conducted the literature search and quality control, and wrote the manuscript. DH, KC, and ID revised the manuscript and provided feedback. All authors read and approved the final version. Jitske Vandersmissen conducted the literature search and wrote the manuscript. Dominique Hansen, Koen Cuypers, and Ilse Dewachter revised the manuscript and provided feedback.
Funding
Internal funding of Hasselt University (23OWB01BOF) was received to assist with the preparation of this manuscript.
Availability of Data and Materials
The papers and associated datasets used for the current manuscript are available from PubMed, using the following search string: (exercise OR "physical activity" OR "aerobic exercise" OR "aerobic training" OR "resistance exercise" OR "resistance training" OR "combined exercise" OR "combined training" OR “endurance exercise” OR “endurance training”) AND (T2DM OR diabetes OR "type 2 diabetes") AND (memory OR "executive function*" OR "processing speed" OR attention OR brain OR cognition OR "cognitive function"). The following information was extracted from the studies: author(s), year of publication, age of the subjects, type of subjects (population), number of subjects in the intervention group, type of control group, number of subjects in the control group, type, duration, frequency, and intensity of the exercise intervention, duration of each exercise session, outcome measures (cognitive tests), and main cognitive results with their effect sizes. Exercise intervention characteristics, outcome measures and cognitive results can be found in Tables 3 and 4. Population characteristics are presented in supplementary Table 1 and 2 in the appendix.
Table 3.
Intervention characteristics and outcomes of included human studies
| Author | Type of exercise training | Duration of exercise intervention | Duration of each training session | Frequency of exercise training | Intensity of exercise training | Outcomes (cognitive tests) | Main cognitive results | Effect size | |
|---|---|---|---|---|---|---|---|---|---|
| Endurance exercise training | Leischik et al. [82] | Walking | 12 weeks | 40 min | 3x/week | ? | Cognitive attention/performance (FAIR-Test 2), verbal memory, non-verbal memory | Verbal memory, nonverbal memory, and cognitive performance significantly improved in the walking group and E-health group compared to the control group |
Cognitive performance: Walking vs. control: d = 0.73 E-health vs. control: d = 1.15 Quality value: Walking vs. control: d = 0.34 E-health vs. control: d = 0.41 Continuity value: Walking vs. control: d = 0.63 E-health vs. control: d = 1.05 Verbal memory: Walking vs. control: d = 0.73 E-health vs. control: d = 1.70 Non-verbal memory: Walking vs. control: d = 6.57 E-health vs. control: d = 6.32 |
| Ploydang et al. [83] | Aquatic Nordic walking | 12 weeks | 60 min | 3x/week |
First 6 weeks: 40–50% HRR Last 6 weeks: 50–60% HRR |
Visuospatial/executive function, naming, memory, attention, language, abstraction, delayed recall, and orientation (MoCA), orientation to time, orientation to place, registration, attention and calculation, word recall, language, and visual construction (MMSE), executive function (TMT part B), inhibition (Stroop colour and word test) | Significantly increased MoCA scores in Nordic walking group. No significant improvements in MMSE, Stroop test, or TMT |
MoCA: d = 0.46 MMSE: d = 0.05 Stroop: d = 0.12 TMT B: d = 0.03 |
|
| Wang et al. [84] | Outdoor aerobic dancing | 1 year | 60 min | 3x/week | ? | Cognitive screening (MMSE) + cognitive impairment (MoCA) | Aerobic training group had significantly increased MMSE and MoCA scores compared to the control group |
MMSE: d = 0.58 MoCA: d = 0.55 |
|
| Liu et al. [97] | Walking | 3 months | 50 min | 3x/week |
moderate intensity: 40–59% HRR |
Executive function (dimensional change card sort test) Episodic memory (picture sequence memory test) Working memory (List sorting working memory test) Processing speed (Pattern Comparison Processing Speed Test) |
Non-significant increases in executive function, episodic memory, working memory and processing speed scores |
Executive function: η2 = 0.027 Episodic memory: η2 = 0.013 Working memory: η2 = 0.008 Processing speed: η2 = 0.045 |
|
| Resistance exercise training | Zhao et al. [85] |
Whole-body, machine based power training (3 sets of 8 reps of lateral pulldown, chest press, upper back, leg press, knee extension, and knee flexion, hip extension and hip abduction) |
12 months | ? | 3 days/week |
High intensity (15–18 on Borg scale, 80% 1RM) |
Memory (word list memory, word list recall, and word list recognition subtests of the Consortium to Establish a Registry for Alzheimer’s Disease), attention/speed (TMT Part A (Trails A)), executive function: (TMT Part B (Trails B) + Trail Making Test B minus A (Trails B minus A)), global cognitive function (MMSE) | Cognition of both groups improved over time, with increased scores in the Trails A, Trails B, word list recall, and word list memory |
MMSE: d = − 0.52 TMT A: d = 0.10 TMT B: d = 0.16 TMT B – TMT A: d = 0.31 Word list memory: d = − 0.04 Word list recall: d = 0.32 Word list recognition: d = 0.00 |
| Yamamoto et al. [86] |
Body weight resistance training + exercises with elastic bands: tube fly, front raise, hammer curl, leg extension, calf raise, and squat Performed 20 times each |
48 weeks | ? | Daily | ? | Cognitive function (MMSE) | No significant changes in MMSE score | MMSE: d = 0.59 | |
| Endurance vs resistance exercise training | Teixeira et al. [87] |
Endurance exercise: elliptical, bike, treadmill, or upper-body cycle ergometer Resistance exercise |
12 weeks | 40 min | 3x/week |
Endurance: 60% HRmax resistance: 11–12 Borg scale |
MTTS: attention and concentration(cognitrone), reaction time (determination test), selective attention (visual pursuit test) |
The group with both T2DM and hypertension showed a significantly increased number of reactions, but no significantly improved reaction time. No differences were observed between the endurance and resistance exercise group |
/ |
| Combined endurance and resistance exercise training | Silveira-Rodrigues et al. [88] |
6 resistance exercises (reverse grip lat pulldown, leg press, bench press machine, calf raises machine, dumbbell shoulder press, and abdominal crunches) + 15 min of treadmill walking, with increasing weight and repetition number over 8 weeks |
8 weeks | ? | 3x/week |
week 1–2: 20 min at 100% v6MWT (aerobic) 2 × 13–15 MRs, i = 60 s (strength) week 3–4 20 min at 105% v6MWT (aerobic) 3 × 13–15 MRs, i = 60 s (strength) week 5–6 25 min at 105% v6MWT (aerobic) 3 × 10–12 MRs, i = 60 s (strength) week 7–8 25 min at 105–110% v6MWT (aerobic) 3 × 8–10 MRs, i = 60 s (strength) |
Cognitive screening (MoCA), visuospatial memory (Taylor's complex figure test), processing speed (TMT A), cognitive flexibility (TMT B), verbal fluency (semantic and alternate word fluency), processing speed (digit symbol substitution), working memory (digit span test), attention/concentration/inhibitory control (Stroop colour test) | Significantly improved inhibitory control, working memory, cognitive flexibility, and attention in the exercise group compared to the control group |
Memory: d = − 0.07 Verbal fluency: d = − 0.01 Processing speed: d = − 0.10 Attention/concentration: d = 0.64 Cognitive flexibility: d = 0.67 Inhibitory control: d = 0.89 Working memory: d = 0.88 |
| Espeland et al. [89] | Walking, resistance training, and flexibility exercises | 2 years | 50 min | 3–4x/week | Moderate intensity | Global cognitive function (3MSE), psychomotor speed, attention, and working memory (the Wechsler Adult Intelligence Scale-III DSC test), delayed recall (HVLT-D), processing speed and executive function (computer tests: 1-back and 2-back tasks, the Eriksen Flanker task, and a task-switching paradigm) | Global cognitive function and delayed memory, significantly improved only in T2DM patients who received training |
3MSE: d = 3.24 DSC: d = 1.50 HVLT-D: d = 3.24 Executive function: d = 0.69 |
|
| Silveira-Rodrigues et al. [90] |
6 resistance exercises (reverse grip lat pulldown, leg press, bench press machine, calf raises machine, dumbbell shoulder press, and abdominal crunches) + treadmill walking, with increasing weight and repetition number over 8 weeks |
8 weeks | 40–60 min | 3x/week |
week 1–2: 20 min at 100% v6MWT (aerobic) 2 × 13–15 MRs, i = 60 s (strength) week 3–4 20 min at 105% v6MWT (aerobic) 3 × 13–15 MRs, i = 60 s (strength) week 5–6 25 min at 105% v6MWT (aerobic) 3 × 10–12 MRs, i = 60 s (strength) week 7–8 25 min at 105–110% v6MWT (aerobic) 3 × 8–10 MRs, i = 60 s (strength) |
Executive function: cognitive flexibility (TMT), inhibitory control (Stroop Colour Task), working memory (Digit Span) Long-term memory: simplified Taylor Complex Figure Test |
Executive function was significantly improved in exercise group |
Executive function: d = 1.31 Inhibitory control: d = 0.87 Working memory: d = 0.40 Cognitive flexibility: d = 0.96 Long-term memory: d = 0.22 |
|
| Ghodrati et al. [91] | Combined training: endurance + resistance + balance exercises | 12 weeks | 65 min | 3x/week |
Endurance: 55–75% HRmax resistance: 65–85% 1RM |
Cognitive function (MoCA), information processing speed + overall cognitive functioning (DSST), short-term memory (forward digit span test) | Intervention group scored significantly higher on MoCA than control group, and increased score with 3.1 compared to baseline. No improvement in DSST or forward digit span test |
MoCA: d = 0.18 DSST: d = 0.05 Forward digit span: d = 0.16 |
|
| Martinez-Velilla et al. [92] |
Combination of resistance, balance, and walking exercises Squats rising from a chair, leg press, and bilateral knee extension, seated bench press semitandem foot standing, line walking, stepping practice, walking with small obstacles, proprioceptive exercises on unstable surfaces, altering the base of support, and weight transfer from one leg to the other, knee extension and flexion, hip abduction, walking along corridor |
8 days | 20 min |
2x/day 5–7 days/week |
? | MMSE | After the intervention, the exercise group scored on average 1.6 points higher than the control group (23.7 vs. 22.1), significant | MMSE: d = 0.69 | |
| Callisaya et al. [93] | Combination of endurance and resistance exercise | 6 months | 1 h | 3x/week |
Started at low to moderate, progressed to moderate to vigorous |
Cognitive function: Victoria Stroop test, TMT (shifting score B-A), the DSC Test, digit span subtest of the Wechsler Adult Intelligence Scale (third edition), Controlled Oral Word Association Test, three part HVLT-D (revised), Rey Complex Figure copy and delay | Better global cognitive score, better performance on the DSC Test, Rey Complex Copy test, Stroop C-D, Trail Making Test A and B, Hopkins intermediate and recognition, and Controlled Oral Word Association Test |
Cognitive global score: d = 2.43 RCF copy: d = 1.67 RCF delay: d = 0.5 Stroop C-D: d = − 2.84 Trails B-A: d = − 0.48 DSC: d = 1.77 Digit span: d = − 3.43 Hopkins I: d =− 0.53 Hopkins D: d = 0.6 Hopkins R: d = 2 COWAT word: d = 1.57 COWAT category: d = 0.84 |
|
| Ghahfarrokhi et al. [94] | Endurance exercises, upper and lower body strength, balance exercises and maintaining posture, and hip control exercises and mid-body stability | 6 weeks |
30–35 min (HIFT) 40–45 min (LIFT) |
3x/week (HIFT) 5x/week (LIFT) |
HIFT: 100–120% lactate threshold LIFT: 70–75% lactate threshold |
Cognitive screening (MMSE), processing speed (SDMT), learning (CVLT-II), memory (BVMT-R), attention (Stroop tests) | MMSE, Stroop, SDMT, CVLT-II and BVMT-R scores improved significantly in the HIFT group. MMSE and Stroop scores improved significantly in the LIFT group. Only change in Stroop scores in the HIFT group was significantly different from the control group |
MMSE: HIFT: d = 0.73 LIFT: d = 0.71 Control: d = − 0.09 Stroop: HIFT: d = 0.82 LIFT: d = 0.72 Control: d = − 0.24 SDMT: HIFT: d = 0.55 LIFT: d = 0.22 Control: d = 0.08 CVLT-II: HIFT: d = 0.61 LIFT: d = 0.42 Control: d = 0.06 BVMT-R: HIFT: d = 0.66 LIFT: d = 0.45 Control: d = − 0.15 |
|
| Other exercise types | Chen et al. [95] | 24-form simplified Tai Chi Chuan | 36 weeks | 60 min | 3x/week | Moderate intensity | Global cognition (MoCA), MQ, DSST, TMT-B, BNT, ROCFT |
Both interventions significantly increased MoCA scores, with a significantly larger effect of Tai Chi Chuan compared to fitness walking. 24 weeks: DSST score was more effectively improved by the Tai Chi Chuan group compared to the fitness walking group. No significant differences in MQ, TMT-B, BNT or ROCFT between Tai Chi Chuan and fitness walking group. MoCA, MQ, DSST, and TMT-B scores improved significantly more in Tai Chi Chuan group compared to control group. MoCA scores in fitness walking group improved significantly more than in control group 36 weeks: MQ scores improved significantly more in Tai Chi Chuan group compared to fitness walking group. MQ, DSST, and TMT-B scores improved significantly more in Tai Chi Chuan group compared to control group. MoCA scores in fitness walking group improved significantly more than in control group |
MoCA: Tai Chi Chuan vs. control: d = 0.63 Fitness walking vs. control: d = 0.33 Wechsler Memory Quotient (MQ): Tai Chi Chuan vs. control: d = 0.47 Fitness walking vs. control: d = 0.15 DSST: Tai Chi Chuan vs. control: d = 0.31 Fitness walking vs. control: d = 0.14 TMT B: Tai Chi Chuan vs. control: d = − 0.37 Fitness walking vs. control: d = − 0.06 BNT: Tai Chi Chuan vs. control: d = 0.21 Fitness walking vs. control: d = 0.16 ROCFT: Tai Chi Chuan vs. control: d = 0.07 Fitness walking vs. control: d = − 0.12 ROCFT delayed recall: Tai Chi Chuan vs. control: d = 0.01 Fitness walking vs. control: d = 0.00 |
| Cai et al. [96] | Kinect-based Kaimai-style Qigong | 12 weeks | 30 min | 3x/week | Low intensity | Cognitive function (MMSE) | Qigong group showed significant improvements in cognitive function compared to the control group | MMSE: d = 1.75 |
BNT Boston Naming Test, BVMT-R Brief Visuospatial Memory Test-Revised, CBS Cambridge Brain Sciences, CVLT-II California Verbal Learning Test II, d cohen’s d, DSC digit symbol coding, DSST Digit Symbol Substitution Test, HIFT High-Intensity low-volume Functional Training, HRmax Heart Rate maximum, HRR heart rate reserve, HVLT-D Hopkins Verbal Learning Test revised, I rest interval between sets and strength-type exercises, LIFT Low-Intensity High-volume Functional Training, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment, MQ Weschler Memory Quotient, MRI Magnetic Resonance Imaging, MRs maximal repetitions, MTTS Mental Test and Training System, ROCFT Rey-Osterrieth Complex Figure Test, SDMT Symbol Digit Modalities test, TMT Trail Making Test, TUG timed up-and-go, V1 intervention midpoint, V2 postintervention, v6MWT velocity in 6 min walking test, ηp2 = partial eta-squared, 1RM = 1 Rep Maximum, 3MSE = Modified Mini Mental State Exam
Table 4.
Intervention characteristics and outcomes of included animal studies
| Author | Type of exercise training | Duration of exercise intervention | Duration of each training session | Frequency of exercise training | Intensity of exercise training | Outcomes (cognitive tests) | Main cognitive results | Effect size |
|---|---|---|---|---|---|---|---|---|
| Parsa et al. [98] | Swimming | 12 weeks | 40 min | 5 days/week | ? |
Cognitive memory (novel object recognition test and elevated plus maze) Aversive memory and learning (passive avoidance learning test) |
Swimming training alone insignificantly improved exploratory behaviour, locomotor activity, and passive avoidance memory, and non-spatial cognitive memory compared to sedentary diabetic rats | / |
| Shekarchian et al. [99] | Swimming | 4 weeks |
Week 1–2: 3 × 10 min Week 3–4: 6 × 10 min |
5 days/week | ? |
Working memory (Y maze) Recognition memory (novel object recognition) Spatial memory (MWM) |
Exercise improved all memory aspects in exercising T2DM mice compared to non-exercising T2DM mice |
Elevated plus maze test: Percentage of spontaneous alternation: η2 = 0.02 Total arm entries: η2 = 0.01 Novel object recognition test: Familiar recognition ratio: η2 = 0.04 Novel recognition ratio: η2 = 0.08 MWM: Escape latency: η2 = 0.10 Platform quadrant time: η2 = 0.01 Platform crossings: η2 = 0.01 |
| Jesmin et al. [100] | Treadmill running | 4 months | 30 min/day | 5 days/week | Light intensity: OLETF 7.0 m/min, LETO 10.0 m/min; Moderate intensity: OLETF 12.5 m/min, LETO 20 m/min | Spatial learning memory and retention (MWM) | Shortened swim length and escape latency in both light and moderate exercise groups improved memory |
MWM: Escape latency: η2 = 0.11 Swim length: η2 = 0.08 Memory retention: η2 = 0.16 |
| Lang et al. [101] | Treadmill running | 8 weeks | 37 min/day | 5 days/week | Moderate intensity | Spatial learning and memory (MWM) | Compared with T group, the escape latency of E group was significantly reduced on day 4–5, treadmill exercise significantly increased the number of platform crossings in E group |
MWM: Escape latency: d = − 1.39 Platform quadrant time: d = 1.01 Platform crossings: d = 1.32 |
| Shima et al. [102] | Treadmill running | 4 weeks | 30 min/day | 5 days/week | Moderate intensity (OLETF rats, 12.5 m/min; LETO rats, 20 m/min) | Memory (MWM) |
Escape latency and swim path length of exercising OLETF rats were shortened, without alteration of the speed of swimming, at trials on days 2, 3 and 4 The time spent by OLETF rats in the platform area significantly improved after 4 weeks of exercise |
MWM: Escape latency: η2 = 0.11 Swim length: η2 = 0.15 Platform quadrant time: η2 = 0.16 |
| Shima et al. [103] | Running on a forced exercise wheel bed | 4 weeks | 30 min/day | 5 days/week | Light intensity | Memory (MWM) | No effect of exercise on the escape latency, swim distance or speed in ob/ob mice. The times of crossing the target platform during the probe test in C57BL/6 mice were significantly greater than that in sedentary ob/ob mice, but not than that in exercised ob/ob mice |
MWM: Swim length: η2 = 0.02 Swim duration: η2 = 0.05 |
E group diabetes + exercise group, LETO Long Evans Tokushima Otsuka, MWM Morris Water Maze, ob/ob obese-hyperglycemic, OLETF Otsuka Long-Evans Tokushima Fatty, T group diabetes group, T2DM type 2 diabetes
Declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors confirm that there were no conflicts of interest. The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Koen Cuypers and Dominique Hansen are shared authorship.
References
- 1.Harman MF, Martin MG. Epigenetic mechanisms related to cognitive decline during aging. J Neurosci Res. 2020;98(2):234–46. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y, et al. Cognitive decline associated with aging. Adv Exp Med Biol. 2023;1419:25–46. [DOI] [PubMed] [Google Scholar]
- 3.Grimm A, Eckert A. Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem. 2017;143(4):418–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Todorova V, Blokland A. Mitochondria and synaptic plasticity in the mature and aging nervous system. Curr Neuropharmacol. 2017;15(1):166–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergado JA, Almaguer W. Aging and synaptic plasticity: a review. Neural Plast. 2002;9(4):217–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harada CN, Natelson Love MC, Triebel KL. Normal cognitive aging. Clin Geriatr Med. 2013;29(4):737–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oschwald J, et al. Brain structure and cognitive ability in healthy aging: a review on longitudinal correlated change. Rev Neurosci. 2019;31(1):1–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blinkouskaya Y, et al. Brain aging mechanisms with mechanical manifestations. Mech Ageing Dev. 2021;200: 111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geula C, et al. Aging renders the brain vulnerable to amyloid beta-protein neurotoxicity. Nat Med. 1998;4(7):827–31. [DOI] [PubMed] [Google Scholar]
- 10.Dickstein DL, et al. Dendritic spine changes associated with normal aging. Neuroscience. 2013;251:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antal B, et al. Type 2 diabetes mellitus accelerates brain aging and cognitive decline: complementary findings from UK Biobank and meta-analyses. Elife. 2022;11:e73138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik A, et al. Cognitive impairment in type 2 diabetes mellitus. Cureus. 2022;14(2): e22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roy S, et al. Cognitive function and control of type 2 diabetes mellitus in young adults. N Am J Med Sci. 2015;7(5):220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zilliox LA, et al. Diabetes and cognitive impairment. Curr Diab Rep. 2016;16(9):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biessels GJ, et al. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2(3):246–55. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborty A, et al. Age related prevalence of mild cognitive impairment in type 2 diabetes mellitus patients in the indian population and association of serum lipids with cognitive dysfunction. Front Endocrinol (Lausanne). 2021;12: 798652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng G, et al. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J. 2012;42(5):484–91. [DOI] [PubMed] [Google Scholar]
- 18.Sato N, Morishita R. Brain alterations and clinical symptoms of dementia in diabetes: abeta/tau-dependent and independent mechanisms. Front Endocrinol (Lausanne). 2014;5:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karvani M, et al. Neurocognitive impairment in type 2 diabetes mellitus. Hormones (Athens). 2019;18(4):523–34. [DOI] [PubMed] [Google Scholar]
- 20.Li ZY, et al. Changes of brain function in patients with type 2 diabetes mellitus measured by different analysis methods: a new coordinate-based meta-analysis of neuroimaging. Front Neurol. 2022;13: 923310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, et al. Reduced gray matter volume in patients with type 2 diabetes mellitus. Front Aging Neurosci. 2017;9:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui Y, et al. Aberrant functional connectivity of default-mode network in type 2 diabetes patients. Eur Radiol. 2015;25(11):3238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tumminia A, et al. Type 2 diabetes mellitus and Alzheimer’s disease: role of insulin signalling and therapeutic implications. Int J Mol Sci. 2018;19(11):3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michailidis M, et al. Alzheimer’s disease as type 3 diabetes: common pathophysiological mechanisms between Alzheimer’s disease and type 2 diabetes. Int J Mol Sci. 2022;23(5):2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steen E, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J Alzheimers Dis. 2005;7(1):63–80. [DOI] [PubMed] [Google Scholar]
- 26.Femminella GD, et al. Antidiabetic drugs in Alzheimer’s disease: mechanisms of action and future perspectives. J Diabetes Res. 2017;2017:7420796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blazquez E, et al. Insulin in the brain: its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front Endocrinol (Lausanne). 2014;5:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104(6):1433–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talbot K, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farris W, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100(7):4162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sousa L, et al. Insulin-degrading enzyme: an ally against metabolic and neurodegenerative diseases. J Pathol. 2021;255(4):346–61. [DOI] [PubMed] [Google Scholar]
- 32.Song D, Yu DSF. Effects of a moderate-intensity aerobic exercise programme on the cognitive function and quality of life of community-dwelling elderly people with mild cognitive impairment: a randomised controlled trial. Int J Nurs Stud. 2019;93:97–105. [DOI] [PubMed] [Google Scholar]
- 33.Baker LD, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67(1):71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gomez-Pinilla F, Hillman C. The influence of exercise on cognitive abilities. Compr Physiol. 2013;3(1):403–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y, et al. Treadmill exercise exerts neuroprotection and regulates microglial polarization and oxidative stress in a streptozotocin-induced rat model of sporadic Alzheimer’s disease. J Alzheimers Dis. 2017;56(4):1469–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang R, Holsinger RMD. Exercise-induced brain-derived neurotrophic factor expression: therapeutic implications for Alzheimer’s dementia. Ageing Res Rev. 2018;48:109–21. [DOI] [PubMed] [Google Scholar]
- 37.Izquierdo M, et al. Cytokine and hormone responses to resistance training. Eur J Appl Physiol. 2009;107(4):397–409. [DOI] [PubMed] [Google Scholar]
- 38.de Sousa CV, et al. The antioxidant effect of exercise: a systematic review and meta-analysis. Sports Med. 2017;47(2):277–93. [DOI] [PubMed] [Google Scholar]
- 39.Demnitz N, et al. Hippocampal maintenance after a 12-month physical activity intervention in older adults: the REACT MRI study. Neuroimage Clin. 2022;35: 102762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ten Brinke LF, et al. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. Br J Sports Med. 2015;49(4):248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris JK, et al. Aerobic exercise for Alzheimer’s disease: a randomized controlled pilot trial. PLoS ONE. 2017;12(2): e0170547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi SH, et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science. 2018;361(6406):eaan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burns JM, et al. Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology. 2008;71(3):210–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lautenschlager NT, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–37. [DOI] [PubMed] [Google Scholar]
- 46.Buchman AS, et al. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78(17):1323–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu L, et al. Brain metabolism in Alzheimer’s disease: biological mechanisms of exercise. Transl Neurodegener. 2023;12(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang J, et al. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc Natl Acad Sci U S A. 2014;111(33):12228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.El Hayek L, et al. Lactate mediates the effects of exercise on learning and memory through SIRT1-dependent activation of hippocampal brain-derived neurotrophic factor (BDNF). J Neurosci. 2019;39(13):2369–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brinkmann C, et al. Effects of cycling and exergaming on neurotrophic factors in elderly type 2 diabetic men: a preliminary investigation. Exp Clin Endocrinol Diabetes. 2017;125(7):436–40. [DOI] [PubMed] [Google Scholar]
- 51.Morland C, et al. Exercise induces cerebral VEGF and angiogenesis via the lactate receptor HCAR1. Nat Commun. 2017;8:15557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morland C, et al. The lactate receptor, G-protein-coupled receptor 81/hydroxycarboxylic acid receptor 1: expression and action in brain. J Neurosci Res. 2015;93(7):1045–55. [DOI] [PubMed] [Google Scholar]
- 53.Villamil-Parra W, Moscoso-Loaiza L. Effects of physical exercise on Irisin and BDNF concentrations, and their relationship with cardiometabolic and mental health of individuals with Metabolic Syndrome: a Systematic Review. Exp Gerontol. 2024;198: 112640. [DOI] [PubMed] [Google Scholar]
- 54.Lourenco MV, et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med. 2019;25(1):165–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gokce E, Gun N. The relationship between exercise, cathepsin b, and cognitive functions: systematic review. Percept Mot Skills. 2023;130(4):1366–85. [DOI] [PubMed] [Google Scholar]
- 56.Huang B, Chen K, Li Y. Aerobic exercise, an effective prevention and treatment for mild cognitive impairment. Front Aging Neurosci. 2023;15:1194559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moon HY, et al. Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab. 2016;24(2):332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malin SK, et al. Brain insulin resistance and cognitive function: influence of exercise. J Appl Physiol. 2022;133(6):1368–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shen J, et al. Potential molecular mechanism of exercise reversing insulin resistance and improving neurodegenerative diseases. Front Physiol. 2024;15:1337442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kjeldsen EW, Frikke-Schmidt T. Causal cardiovascular risk factors for dementia: insights from observational and genetic studies. Cardiovasc Res 2024. [DOI] [PMC free article] [PubMed]
- 61.Mok VCT, Cai Y, Markus HS. Vascular cognitive impairment and dementia: mechanisms, treatment, and future directions. Int J Stroke. 2024;19(8):838–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sousa RAL, Improta-Caria AC, Souza BSF. Exercise-linked Irisin: consequences on mental and cardiovascular health in type 2 diabetes. Int J Mol Sci. 2021;22(4):2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chacon D, Fiani B. A review of mechanisms on the beneficial effect of exercise on atherosclerosis. Cureus. 2020;12(11): e11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Madarshahian F, Hassanabadi M, Koshniat Nikoo M. Cognitive status and foot self care practice in overweight diabetics, engaged in different levels of physical activity. J Diabetes Metab Disord. 2014;13(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rabinowitz Y, et al. Physical activity is associated with slower cognitive decline in older adults with type 2 diabetes. J Prev Alzheimers Dis. 2023;10(3):497–502. [DOI] [PubMed] [Google Scholar]
- 66.Zabetian-Targhi F, et al. The association between physical activity intensity, cognition, and brain structure in people with type 2 diabetes. J Gerontol A Biol Sci Med Sci. 2021;76(11):2047–53. [DOI] [PubMed] [Google Scholar]
- 67.Shih IF, et al. Physical activity modifies the influence of apolipoprotein E epsilon4 allele and type 2 diabetes on dementia and cognitive impairment among older Mexican Americans. Alzheimers Dement. 2018;14(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu T, et al. The influence of the BDNF Val66Met polymorphism on the association of regular physical activity with cognition among individuals with diabetes. Biol Res Nurs. 2021;23(3):318–30. [DOI] [PubMed] [Google Scholar]
- 69.Silveira-Rodrigues JG, et al. Acute bouts of aerobic and resistance exercise similarly alter inhibitory control and response time while inversely modifying plasma BDNF concentrations in middle-aged and older adults with type 2 diabetes. Exp Brain Res. 2023;241(4):1173–83. [DOI] [PubMed] [Google Scholar]
- 70.Wang H, Tang W, Zhao Y. Acute effects of different exercise forms on executive function and the mechanism of cerebral hemodynamics in hospitalized T2DM patients: a within-subject study. Front Public Health. 2023;11:1165892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao RR, O’Bullivan AJ, Fiatarone Singh MA. Exercise or physical activity and cognitive function in adults with type 2 diabetes, insulin resistance or impaired glucose tolerance: a systematic review. Eur Rev Aging Phys Act. 2018;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang R, et al. The effect of physical activity interventions on cognition function in patients with diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2021;37(7): e3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai YH, et al. Effect of exercise on the cognitive function of older patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Front Hum Neurosci. 2022;16: 876935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cooke S, et al. Effects of exercise, cognitive, and dual-task interventions on cognition in type 2 diabetes mellitus: a systematic review and meta-analysis. PLoS ONE. 2020;15(5): e0232958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dyer AH, et al. Non-pharmacological interventions for cognition in patients with Type 2 diabetes mellitus: a systematic review. QJM. 2020;113(3):155–61. [DOI] [PubMed] [Google Scholar]
- 76.Callisaya M, Nosaka K. Effects of exercise on type 2 diabetes mellitus-related cognitive impairment and dementia. J Alzheimers Dis. 2017;59(2):503–13. [DOI] [PubMed] [Google Scholar]
- 77.Yi SS. Effects of exercise on brain functions in diabetic animal models. World J Diabetes. 2015;6(4):583–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bertram S, Brixius K, Brinkmann C. Exercise for the diabetic brain: how physical training may help prevent dementia and Alzheimer’s disease in T2DM patients. Endocrine. 2016;53(2):350–63. [DOI] [PubMed] [Google Scholar]
- 79.Podolski N, et al. Effects of regular physical activity on the cognitive performance of type 2 diabetic patients: a systematic review. Metab Syndr Relat Disord. 2017;15(10):481–93. [DOI] [PubMed] [Google Scholar]
- 80.Smart NA, et al. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc. 2015;13(1):9–18. [DOI] [PubMed] [Google Scholar]
- 81.Macleod MR, et al. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35(5):1203–8. [DOI] [PubMed] [Google Scholar]
- 82.Leischik R, et al. Exercise improves cognitive function-a randomized trial on the effects of physical activity on cognition in type 2 diabetes patients. J Pers Med. 2021;11(6):530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ploydang T et al. Nordic walking in water on cerebrovascular reactivity and cognitive function in elderly patients with type 2 diabetes. Med Sci Sports Exerc 2023. [DOI] [PubMed]
- 84.Wang Y, et al. Aerobic training increases hippocampal volume and protects cognitive function for type 2 diabetes patients with normal cognition. Exp Clin Endocrinol Diabetes 2023. [DOI] [PubMed]
- 85.Zhao RR, et al. Effect of high-intensity power training on cognitive function in older adults with type 2 diabetes: secondary outcomes of the GREAT2DO study. J Gerontol A Biol Sci Med Sci. 2022;77(10):1975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamamoto Y, et al. Effects of resistance training using elastic bands on muscle strength with or without a leucine supplement for 48 weeks in elderly patients with type 2 diabetes. Endocr J. 2021;68(3):291–8. [DOI] [PubMed] [Google Scholar]
- 87.Teixeira RB, et al. Evaluating the effects of exercise on cognitive function in hypertensive and diabetic patients using the mental test and training system. World J Biol Psychiatry. 2019;20(3):209–18. [DOI] [PubMed] [Google Scholar]
- 88.Silveira-Rodrigues JG, et al. Combined exercise training improves specific domains of cognitive functions and metabolic markers in middle-aged and older adults with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2021;173: 108700. [DOI] [PubMed] [Google Scholar]
- 89.Espeland MA, et al. Effects of physical activity intervention on physical and cognitive function in sedentary adults with and without diabetes. J Gerontol A Biol Sci Med Sci. 2017;72(6):861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Silveira-Rodrigues JG, et al. Combined training improves executive functions without changing brain-derived neurotrophic factor levels of middle-aged and older adults with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2023;131(6):345–53. [DOI] [PubMed] [Google Scholar]
- 91.Ghodrati N, et al. Effect of combined exercise training on physical and cognitive function in women with type 2 diabetes. Can J Diabetes. 2023;47(2):162–70. [DOI] [PubMed] [Google Scholar]
- 92.Martinez-Velilla N, et al. Effects of a tailored exercise intervention in acutely hospitalized oldest old diabetic adults: an ancillary analysis. J Clin Endocrinol Metab. 2021;106(2):e899–906. [DOI] [PubMed] [Google Scholar]
- 93.Callisaya ML, et al. Feasibility of a multi-modal exercise program on cognition in older adults with Type 2 diabetes: a pilot randomised controlled trial. BMC Geriatr. 2017;17(1):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ghahfarrokhi MM, et al. Feasibility and preliminary efficacy of different intensities of functional training in elderly type 2 diabetes patients with cognitive impairment: a pilot randomised controlled trial. BMC Geriatr. 2024;24(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Y, et al. Effects of Tai Chi Chuan on cognitive function in adults 60 years or older with type 2 diabetes and mild cognitive impairment in China: a randomized clinical trial. JAMA Netw Open. 2023;6(4): e237004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cai H, et al. Effect of low-intensity, Kinect-based Kaimai-style qigong exercise in older adults with type 2 diabetes. J Gerontol Nurs. 2019;45(2):42–52. [DOI] [PubMed] [Google Scholar]
- 97.Liu T, et al. The effects of aerobic exercise on cognitive function in middle-aged and older individuals with type 2 diabetes: a pilot randomized controlled trial. Geriatr Nurs. 2024;60:677–85. [DOI] [PubMed] [Google Scholar]
- 98.Parsa H, et al. Swimming training and Plantago psyllium ameliorate cognitive impairment and glucose tolerance in streptozotocin-nicotinamide-induced type 2 diabetic rats. J Physiol Sci. 2021;71(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shekarchian M, Peeri M, Azarbayjani MA. Physical activity in a swimming pool attenuates memory impairment by reducing glutamate and inflammatory cytokines and increasing BDNF in the brain of mice with type 2 diabetes. Brain Res Bull. 2023;201: 110725. [DOI] [PubMed] [Google Scholar]
- 100.Jesmin S, et al. Long-term light and moderate exercise intervention similarly prevent both hippocampal and glycemic dysfunction in presymptomatic type 2 diabetic rats. Am J Physiol Endocrinol Metab. 2022;322(3):E219–30. [DOI] [PubMed] [Google Scholar]
- 101.Lang X, et al. Treadmill exercise mitigates neuroinflammation and increases BDNF via activation of SIRT1 signaling in a mouse model of T2DM. Brain Res Bull. 2020;165:30–9. [DOI] [PubMed] [Google Scholar]
- 102.Shima T, et al. Moderate exercise ameliorates dysregulated hippocampal glycometabolism and memory function in a rat model of type 2 diabetes. Diabetologia. 2017;60(3):597–606. [DOI] [PubMed] [Google Scholar]
- 103.Shima T, et al. Light-intensity exercise improves memory dysfunction with the restoration of hippocampal MCT2 and miRNAs in type 2 diabetic mice. Metab Brain Dis. 2023;38(1):245–54. [DOI] [PubMed] [Google Scholar]
- 104.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nasreddine ZS, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. [DOI] [PubMed] [Google Scholar]
- 106.Arevalo-Rodriguez I, et al. Mini-Mental State Examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev. 2021;7(7):CD010783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li X, et al. Effects of fitness qigong and tai chi on middle-aged and elderly patients with type 2 diabetes mellitus. PLoS ONE. 2020;15(12): e0243989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stanciu GD, et al. Link between diabetes and Alzheimer’s disease due to the shared amyloid aggregation and deposition involving both neurodegenerative changes and neurovascular damages. J Clin Med. 2020;9(6):1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li L, Holscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev. 2007;56(2):384–402. [DOI] [PubMed] [Google Scholar]
- 110.He XF, et al. Voluntary exercise promotes glymphatic clearance of amyloid beta and reduces the activation of astrocytes and microglia in aged mice. Front Mol Neurosci. 2017;10:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu L, et al. Treadmill exercise promotes E3 ubiquitin ligase to remove amyloid beta and P-tau and improve cognitive ability in APP/PS1 transgenic mice. J Neuroinflam. 2022;19(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marwarha G, Ghribi O. Leptin signaling and Alzheimer’s disease. Am J Neurodegener Dis. 2012;1(3):245–65. [PMC free article] [PubMed] [Google Scholar]
- 113.Marwarha G, et al. Leptin attenuates BACE1 expression and amyloid-beta genesis via the activation of SIRT1 signaling pathway. Biochim Biophys Acta. 2014;1842(9):1587–95. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 114.Rezaei MH et al. Leptin signaling could mediate hippocampal decumulation of beta-amyloid and tau induced by high-intensity interval training in rats with type 2 diabetes. Cell Mol Neurobiol, 2023. [DOI] [PMC free article] [PubMed]
- 115.De Sousa RAL, et al. High-intensity resistance training induces changes in cognitive function, but not in locomotor activity or anxious behavior in rats induced to type 2 diabetes. Physiol Behav. 2020;223: 112998. [DOI] [PubMed] [Google Scholar]
- 116.Fang X, et al. Phosphorylation and inactivation of glycogen synthase kinase 3 by protein kinase A. Proc Natl Acad Sci U S A. 2000;97(22):11960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hernandez F, Lucas JJ, Avila J. GSK3 and tau: two convergence points in Alzheimer’s disease. J Alzheimers Dis. 2013;33(Suppl 1):S141–4. [DOI] [PubMed] [Google Scholar]
- 118.Rolev KD, Shu XS, Ying Y. Targeted pharmacotherapy against neurodegeneration and neuroinflammation in early diabetic retinopathy. Neuropharmacology. 2021;187: 108498. [DOI] [PubMed] [Google Scholar]
- 119.Pousti F, et al. Adiponectin modulates synaptic plasticity in hippocampal dentate gyrus. Neurosci Lett. 2018;662:227–32. [DOI] [PubMed] [Google Scholar]
- 120.Weisz F, et al. The role of adiponectin receptors in the regulation of synaptic transmission in the hippocampus. Synapse. 2017;71(5):e21964. [DOI] [PubMed] [Google Scholar]
- 121.Ng RC, et al. Chronic adiponectin deficiency leads to Alzheimer’s disease-like cognitive impairments and pathologies through AMPK inactivation and cerebral insulin resistance in aged mice. Mol Neurodegener. 2016;11(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jian M, et al. Adiponectin suppresses amyloid-beta oligomer (AbetaO)-induced inflammatory response of microglia via AdipoR1-AMPK-NF-kappaB signaling pathway. J Neuroinflam. 2019;16(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mehta BK, Singh KK, Banerjee S. Effect of exercise on type 2 diabetes-associated cognitive impairment in rats. Int J Neurosci. 2019;129(3):252–63. [DOI] [PubMed] [Google Scholar]
- 124.Colucci-D’Amato L, Speranza L, Volpicelli F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int J Mol Sci. 2020;21(20):7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jamali A, Shahrbanian S, Morteza Tayebi S. The Effects of exercise training on the brain-derived neurotrophic factor (BDNF) in the patients with type 2 diabetes: a systematic review of the randomized controlled trials. J Diabetes Metab Disord. 2020;19(1):633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cheng SM, Lee SD. Exercise training enhances BDNF/TrkB signaling pathway and inhibits apoptosis in diabetic cerebral cortex. Int J Mol Sci. 2022;23(12):6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hwang IK, et al. Effects of age and treadmill exercise in chronic diabetic stages on neuroblast differentiation in a rat model of type 2 diabetes. Brain Res. 2010;1341:63–71. [DOI] [PubMed] [Google Scholar]
- 128.Yi SS, et al. Effects of treadmill exercise on cell proliferation and differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res. 2009;34(6):1039–46. [DOI] [PubMed] [Google Scholar]
- 129.Stranahan AM, et al. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19(10):951–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li J, et al. Mechanisms of aerobic exercise upregulating the expression of hippocampal synaptic plasticity-associated proteins in diabetic rats. Neural Plast. 2019;2019:7920540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee CC, Huang CC, Hsu KS. Insulin promotes dendritic spine and synapse formation by the PI3K/Akt/mTOR and Rac1 signaling pathways. Neuropharmacology. 2011;61(4):867–79. [DOI] [PubMed] [Google Scholar]
- 132.Barone E, et al. The interplay among oxidative stress, brain insulin resistance and AMPK dysfunction contribute to neurodegeneration in type 2 diabetes and Alzheimer disease. Free Radic Biol Med. 2021;176:16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu J, et al. Brain SIRT1 mediates metabolic homeostasis and neuroprotection. Front Endocrinol (Lausanne). 2018;9:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Banerjee J, Bruckbauer A, Zemel MB. Activation of the AMPK/Sirt1 pathway by a leucine-metformin combination increases insulin sensitivity in skeletal muscle, and stimulates glucose and lipid metabolism and increases life span in Caenorhabditis elegans. Metabolism. 2016;65(11):1679–91. [DOI] [PubMed] [Google Scholar]
- 135.Wang R, et al. Deciphering therapeutic options for neurodegenerative diseases: insights from SIRT1. J Mol Med (Berl). 2022;100(4):537–53. [DOI] [PubMed] [Google Scholar]
- 136.Lin L, et al. Aerobic exercise improves Type 2 diabetes mellitus-related cognitive impairment by inhibiting JAK2/STAT3 and enhancing AMPK/SIRT1 pathways in mice. Dis Markers. 2022;2022:6010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang X, et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1alpha signalling pathway. Adipocyte. 2020;9(1):484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang Q, et al. Aerobic exercise improves synaptic-related proteins of diabetic rats by inhibiting FOXO1/NF-kappaB/NLRP3 inflammatory signaling pathway and ameliorating pi3k/akt insulin signaling pathway. J Mol Neurosci. 2019;69(1):28–38. [DOI] [PubMed] [Google Scholar]
- 139.Hemmings BA, Restuccia DF. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4(9): a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Greene WC, Chen LF. Regulation of NF-kappaB action by reversible acetylation. Novartis Found Symp. 2004;259:208–17 (discussion 218–25). [PubMed] [Google Scholar]
- 141.Zhao F, Tomita M, Dutta A. Operational modal analysis of near-infrared spectroscopy measure of 2-month exercise intervention effects in sedentary older adults with diabetes and cognitive impairment. Brain Sci. 2023;13(7):1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.van Praag H, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Matsui T, et al. Brain glycogen supercompensation following exhaustive exercise. J Physiol. 2012;590(3):607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Soya M, et al. Dysregulation of glycogen metabolism with concomitant spatial memory dysfunction in type 2 diabetes: potential beneficial effects of chronic exercise. Adv Neurobiol. 2019;23:363–83. [DOI] [PubMed] [Google Scholar]
- 145.Karstoft K, Pedersen BK. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol. 2016;94(2):146–50. [DOI] [PubMed] [Google Scholar]
- 146.Dutta BJ, et al. Inside the diabetic brain: Insulin resistance and molecular mechanism associated with cognitive impairment and its possible therapeutic strategies. Pharmacol Res. 2022;182: 106358. [DOI] [PubMed] [Google Scholar]
- 147.Arnold SE, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol. 2018;14(3):168–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao H, et al. Insulin resistance is a risk factor for mild cognitive impairment in elderly adults with T2DM. Open Life Sci. 2019;14:255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kullmann S, et al. Exercise restores brain insulin sensitivity in sedentary adults who are overweight and obese. JCI Insight. 2022;7(18):e161498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li H, Yu X. Emerging role of JNK in insulin resistance. Curr Diabetes Rev. 2013;9(5):422–8. [DOI] [PubMed] [Google Scholar]
- 151.Sykiotis GP, Papavassiliou AG. Serine phosphorylation of insulin receptor substrate-1: a novel target for the reversal of insulin resistance. Mol Endocrinol. 2001;15(11):1864–9. [DOI] [PubMed] [Google Scholar]
- 152.Tanti JF, et al. Alteration in insulin action: role of IRS-1 serine phosphorylation in the retroregulation of insulin signalling. Ann Endocrinol (Paris). 2004;65(1):43–8. [DOI] [PubMed] [Google Scholar]
- 153.Bevan P. Insulin signalling. J Cell Sci. 2001;114(Pt 8):1429–30. [DOI] [PubMed] [Google Scholar]
- 154.Park SH, et al. Resistance exercise training attenuates the loss of endogenous GLP-1 receptor in the hypothalamus of Type 2 diabetic rats. Int J Environ Res Public Health. 2019;16(5):830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Enns LC, Ladiges W. Protein kinase A signaling as an anti-aging target. Ageing Res Rev. 2010;9(3):269–72. [DOI] [PubMed] [Google Scholar]
- 156.Chen CJ, et al. Protein kinase a and lipid metabolism. Sheng Li Ke Xue Jin Zhan. 2016;47(5):321–9. [PubMed] [Google Scholar]
- 157.Sajan MP, Farese RV. Insulin signalling in hepatocytes of humans with type 2 diabetes: excessive production and activity of protein kinase C-iota (PKC-iota) and dependent processes and reversal by PKC-iota inhibitors. Diabetologia. 2012;55(5):1446–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sajan MP, et al. Correction of metabolic abnormalities in a rodent model of obesity, metabolic syndrome, and type 2 diabetes mellitus by inhibitors of hepatic protein kinase C-iota. Metabolism. 2012;61(4):459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–42. [DOI] [PubMed] [Google Scholar]
- 160.Cheng D, et al. The role of glucagon-like peptide-1 receptor agonists (GLP-1 RA) in diabetes-related neurodegenerative diseases. Drug Des Devel Ther. 2022;16:665–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yin W, et al. Protein kinase C and protein kinase A are involved in the protection of recombinant human glucagon-like peptide-1 on glomeruli and tubules in diabetic rats. J Diabetes Investig. 2019;10(3):613–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rovira-Llopis S, et al. Mitochondrial dynamics in type 2 diabetes: Pathophysiological implications. Redox Biol. 2017;11:637–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wada J, Nakatsuka A. Mitochondrial dynamics and mitochondrial dysfunction in diabetes. Acta Med Okayama. 2016;70(3):151–8. [DOI] [PubMed] [Google Scholar]
- 164.Luo JS, et al. The role of mitochondrial quality control in cognitive dysfunction in diabetes. Neurochem Res. 2022;47(8):2158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cheng H, et al. Mitochondrial dysfunction plays a key role in the development of neurodegenerative diseases in diabetes. Am J Physiol Endocrinol Metab. 2020;318(5):E750–64. [DOI] [PubMed] [Google Scholar]
- 166.Gusdon AM, et al. Exercise increases mitochondrial complex I activity and DRP1 expression in the brains of aged mice. Exp Gerontol. 2017;90:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Amar D. et al. The mitochondrial multi-omic response to exercise training across tissues. bioRxiv, 2023. [DOI] [PMC free article] [PubMed]
- 168.Mendham AE, et al. Exercise training improves mitochondrial respiration and is associated with an altered intramuscular phospholipid signature in women with obesity. Diabetologia. 2021;64(7):1642–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Meex RC, et al. Restoration of muscle mitochondrial function and metabolic flexibility in type 2 diabetes by exercise training is paralleled by increased myocellular fat storage and improved insulin sensitivity. Diabetes. 2010;59(3):572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hey-Mogensen M, et al. Effect of physical training on mitochondrial respiration and reactive oxygen species release in skeletal muscle in patients with obesity and type 2 diabetes. Diabetologia. 2010;53(9):1976–85. [DOI] [PubMed] [Google Scholar]
- 171.Toledo FG, et al. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56(8):2142–7. [DOI] [PubMed] [Google Scholar]
- 172.Baekkerud FH, et al. High intensity interval training ameliorates mitochondrial dysfunction in the left ventricle of mice with type 2 diabetes. Cardiovasc Toxicol. 2019;19(5):422–31. [DOI] [PubMed] [Google Scholar]
- 173.Ruegsegger GN, et al. Exercise and metformin counteract altered mitochondrial function in the insulin-resistant brain. JCI Insight. 2019;4(18):e130681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.American College of Sports M. et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc 2009;41(7):1510–30. [DOI] [PubMed]
- 175.Cassilhas RC, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. 2007;39(8):1401–7. [DOI] [PubMed] [Google Scholar]
- 176.Murman DL. The impact of age on cognition. Semin Hear. 2015;36(3):111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Peel C, Mossberg KA. Effects of cardiovascular medications on exercise responses. Phys Ther. 1995;75(5):387–96. [DOI] [PubMed] [Google Scholar]
- 178.Powles AC. The effect of drugs on the cardiovascular response to exercise. Med Sci Sports Exerc. 1981;13(4):252–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The papers and associated datasets used for the current manuscript are available from PubMed, using the following search string: (exercise OR "physical activity" OR "aerobic exercise" OR "aerobic training" OR "resistance exercise" OR "resistance training" OR "combined exercise" OR "combined training" OR “endurance exercise” OR “endurance training”) AND (T2DM OR diabetes OR "type 2 diabetes") AND (memory OR "executive function*" OR "processing speed" OR attention OR brain OR cognition OR "cognitive function"). The following information was extracted from the studies: author(s), year of publication, age of the subjects, type of subjects (population), number of subjects in the intervention group, type of control group, number of subjects in the control group, type, duration, frequency, and intensity of the exercise intervention, duration of each exercise session, outcome measures (cognitive tests), and main cognitive results with their effect sizes. Exercise intervention characteristics, outcome measures and cognitive results can be found in Tables 3 and 4. Population characteristics are presented in supplementary Table 1 and 2 in the appendix.
Table 3.
Intervention characteristics and outcomes of included human studies
| Author | Type of exercise training | Duration of exercise intervention | Duration of each training session | Frequency of exercise training | Intensity of exercise training | Outcomes (cognitive tests) | Main cognitive results | Effect size | |
|---|---|---|---|---|---|---|---|---|---|
| Endurance exercise training | Leischik et al. [82] | Walking | 12 weeks | 40 min | 3x/week | ? | Cognitive attention/performance (FAIR-Test 2), verbal memory, non-verbal memory | Verbal memory, nonverbal memory, and cognitive performance significantly improved in the walking group and E-health group compared to the control group |
Cognitive performance: Walking vs. control: d = 0.73 E-health vs. control: d = 1.15 Quality value: Walking vs. control: d = 0.34 E-health vs. control: d = 0.41 Continuity value: Walking vs. control: d = 0.63 E-health vs. control: d = 1.05 Verbal memory: Walking vs. control: d = 0.73 E-health vs. control: d = 1.70 Non-verbal memory: Walking vs. control: d = 6.57 E-health vs. control: d = 6.32 |
| Ploydang et al. [83] | Aquatic Nordic walking | 12 weeks | 60 min | 3x/week |
First 6 weeks: 40–50% HRR Last 6 weeks: 50–60% HRR |
Visuospatial/executive function, naming, memory, attention, language, abstraction, delayed recall, and orientation (MoCA), orientation to time, orientation to place, registration, attention and calculation, word recall, language, and visual construction (MMSE), executive function (TMT part B), inhibition (Stroop colour and word test) | Significantly increased MoCA scores in Nordic walking group. No significant improvements in MMSE, Stroop test, or TMT |
MoCA: d = 0.46 MMSE: d = 0.05 Stroop: d = 0.12 TMT B: d = 0.03 |
|
| Wang et al. [84] | Outdoor aerobic dancing | 1 year | 60 min | 3x/week | ? | Cognitive screening (MMSE) + cognitive impairment (MoCA) | Aerobic training group had significantly increased MMSE and MoCA scores compared to the control group |
MMSE: d = 0.58 MoCA: d = 0.55 |
|
| Liu et al. [97] | Walking | 3 months | 50 min | 3x/week |
moderate intensity: 40–59% HRR |
Executive function (dimensional change card sort test) Episodic memory (picture sequence memory test) Working memory (List sorting working memory test) Processing speed (Pattern Comparison Processing Speed Test) |
Non-significant increases in executive function, episodic memory, working memory and processing speed scores |
Executive function: η2 = 0.027 Episodic memory: η2 = 0.013 Working memory: η2 = 0.008 Processing speed: η2 = 0.045 |
|
| Resistance exercise training | Zhao et al. [85] |
Whole-body, machine based power training (3 sets of 8 reps of lateral pulldown, chest press, upper back, leg press, knee extension, and knee flexion, hip extension and hip abduction) |
12 months | ? | 3 days/week |
High intensity (15–18 on Borg scale, 80% 1RM) |
Memory (word list memory, word list recall, and word list recognition subtests of the Consortium to Establish a Registry for Alzheimer’s Disease), attention/speed (TMT Part A (Trails A)), executive function: (TMT Part B (Trails B) + Trail Making Test B minus A (Trails B minus A)), global cognitive function (MMSE) | Cognition of both groups improved over time, with increased scores in the Trails A, Trails B, word list recall, and word list memory |
MMSE: d = − 0.52 TMT A: d = 0.10 TMT B: d = 0.16 TMT B – TMT A: d = 0.31 Word list memory: d = − 0.04 Word list recall: d = 0.32 Word list recognition: d = 0.00 |
| Yamamoto et al. [86] |
Body weight resistance training + exercises with elastic bands: tube fly, front raise, hammer curl, leg extension, calf raise, and squat Performed 20 times each |
48 weeks | ? | Daily | ? | Cognitive function (MMSE) | No significant changes in MMSE score | MMSE: d = 0.59 | |
| Endurance vs resistance exercise training | Teixeira et al. [87] |
Endurance exercise: elliptical, bike, treadmill, or upper-body cycle ergometer Resistance exercise |
12 weeks | 40 min | 3x/week |
Endurance: 60% HRmax resistance: 11–12 Borg scale |
MTTS: attention and concentration(cognitrone), reaction time (determination test), selective attention (visual pursuit test) |
The group with both T2DM and hypertension showed a significantly increased number of reactions, but no significantly improved reaction time. No differences were observed between the endurance and resistance exercise group |
/ |
| Combined endurance and resistance exercise training | Silveira-Rodrigues et al. [88] |
6 resistance exercises (reverse grip lat pulldown, leg press, bench press machine, calf raises machine, dumbbell shoulder press, and abdominal crunches) + 15 min of treadmill walking, with increasing weight and repetition number over 8 weeks |
8 weeks | ? | 3x/week |
week 1–2: 20 min at 100% v6MWT (aerobic) 2 × 13–15 MRs, i = 60 s (strength) week 3–4 20 min at 105% v6MWT (aerobic) 3 × 13–15 MRs, i = 60 s (strength) week 5–6 25 min at 105% v6MWT (aerobic) 3 × 10–12 MRs, i = 60 s (strength) week 7–8 25 min at 105–110% v6MWT (aerobic) 3 × 8–10 MRs, i = 60 s (strength) |
Cognitive screening (MoCA), visuospatial memory (Taylor's complex figure test), processing speed (TMT A), cognitive flexibility (TMT B), verbal fluency (semantic and alternate word fluency), processing speed (digit symbol substitution), working memory (digit span test), attention/concentration/inhibitory control (Stroop colour test) | Significantly improved inhibitory control, working memory, cognitive flexibility, and attention in the exercise group compared to the control group |
Memory: d = − 0.07 Verbal fluency: d = − 0.01 Processing speed: d = − 0.10 Attention/concentration: d = 0.64 Cognitive flexibility: d = 0.67 Inhibitory control: d = 0.89 Working memory: d = 0.88 |
| Espeland et al. [89] | Walking, resistance training, and flexibility exercises | 2 years | 50 min | 3–4x/week | Moderate intensity | Global cognitive function (3MSE), psychomotor speed, attention, and working memory (the Wechsler Adult Intelligence Scale-III DSC test), delayed recall (HVLT-D), processing speed and executive function (computer tests: 1-back and 2-back tasks, the Eriksen Flanker task, and a task-switching paradigm) | Global cognitive function and delayed memory, significantly improved only in T2DM patients who received training |
3MSE: d = 3.24 DSC: d = 1.50 HVLT-D: d = 3.24 Executive function: d = 0.69 |
|
| Silveira-Rodrigues et al. [90] |
6 resistance exercises (reverse grip lat pulldown, leg press, bench press machine, calf raises machine, dumbbell shoulder press, and abdominal crunches) + treadmill walking, with increasing weight and repetition number over 8 weeks |
8 weeks | 40–60 min | 3x/week |
week 1–2: 20 min at 100% v6MWT (aerobic) 2 × 13–15 MRs, i = 60 s (strength) week 3–4 20 min at 105% v6MWT (aerobic) 3 × 13–15 MRs, i = 60 s (strength) week 5–6 25 min at 105% v6MWT (aerobic) 3 × 10–12 MRs, i = 60 s (strength) week 7–8 25 min at 105–110% v6MWT (aerobic) 3 × 8–10 MRs, i = 60 s (strength) |
Executive function: cognitive flexibility (TMT), inhibitory control (Stroop Colour Task), working memory (Digit Span) Long-term memory: simplified Taylor Complex Figure Test |
Executive function was significantly improved in exercise group |
Executive function: d = 1.31 Inhibitory control: d = 0.87 Working memory: d = 0.40 Cognitive flexibility: d = 0.96 Long-term memory: d = 0.22 |
|
| Ghodrati et al. [91] | Combined training: endurance + resistance + balance exercises | 12 weeks | 65 min | 3x/week |
Endurance: 55–75% HRmax resistance: 65–85% 1RM |
Cognitive function (MoCA), information processing speed + overall cognitive functioning (DSST), short-term memory (forward digit span test) | Intervention group scored significantly higher on MoCA than control group, and increased score with 3.1 compared to baseline. No improvement in DSST or forward digit span test |
MoCA: d = 0.18 DSST: d = 0.05 Forward digit span: d = 0.16 |
|
| Martinez-Velilla et al. [92] |
Combination of resistance, balance, and walking exercises Squats rising from a chair, leg press, and bilateral knee extension, seated bench press semitandem foot standing, line walking, stepping practice, walking with small obstacles, proprioceptive exercises on unstable surfaces, altering the base of support, and weight transfer from one leg to the other, knee extension and flexion, hip abduction, walking along corridor |
8 days | 20 min |
2x/day 5–7 days/week |
? | MMSE | After the intervention, the exercise group scored on average 1.6 points higher than the control group (23.7 vs. 22.1), significant | MMSE: d = 0.69 | |
| Callisaya et al. [93] | Combination of endurance and resistance exercise | 6 months | 1 h | 3x/week |
Started at low to moderate, progressed to moderate to vigorous |
Cognitive function: Victoria Stroop test, TMT (shifting score B-A), the DSC Test, digit span subtest of the Wechsler Adult Intelligence Scale (third edition), Controlled Oral Word Association Test, three part HVLT-D (revised), Rey Complex Figure copy and delay | Better global cognitive score, better performance on the DSC Test, Rey Complex Copy test, Stroop C-D, Trail Making Test A and B, Hopkins intermediate and recognition, and Controlled Oral Word Association Test |
Cognitive global score: d = 2.43 RCF copy: d = 1.67 RCF delay: d = 0.5 Stroop C-D: d = − 2.84 Trails B-A: d = − 0.48 DSC: d = 1.77 Digit span: d = − 3.43 Hopkins I: d =− 0.53 Hopkins D: d = 0.6 Hopkins R: d = 2 COWAT word: d = 1.57 COWAT category: d = 0.84 |
|
| Ghahfarrokhi et al. [94] | Endurance exercises, upper and lower body strength, balance exercises and maintaining posture, and hip control exercises and mid-body stability | 6 weeks |
30–35 min (HIFT) 40–45 min (LIFT) |
3x/week (HIFT) 5x/week (LIFT) |
HIFT: 100–120% lactate threshold LIFT: 70–75% lactate threshold |
Cognitive screening (MMSE), processing speed (SDMT), learning (CVLT-II), memory (BVMT-R), attention (Stroop tests) | MMSE, Stroop, SDMT, CVLT-II and BVMT-R scores improved significantly in the HIFT group. MMSE and Stroop scores improved significantly in the LIFT group. Only change in Stroop scores in the HIFT group was significantly different from the control group |
MMSE: HIFT: d = 0.73 LIFT: d = 0.71 Control: d = − 0.09 Stroop: HIFT: d = 0.82 LIFT: d = 0.72 Control: d = − 0.24 SDMT: HIFT: d = 0.55 LIFT: d = 0.22 Control: d = 0.08 CVLT-II: HIFT: d = 0.61 LIFT: d = 0.42 Control: d = 0.06 BVMT-R: HIFT: d = 0.66 LIFT: d = 0.45 Control: d = − 0.15 |
|
| Other exercise types | Chen et al. [95] | 24-form simplified Tai Chi Chuan | 36 weeks | 60 min | 3x/week | Moderate intensity | Global cognition (MoCA), MQ, DSST, TMT-B, BNT, ROCFT |
Both interventions significantly increased MoCA scores, with a significantly larger effect of Tai Chi Chuan compared to fitness walking. 24 weeks: DSST score was more effectively improved by the Tai Chi Chuan group compared to the fitness walking group. No significant differences in MQ, TMT-B, BNT or ROCFT between Tai Chi Chuan and fitness walking group. MoCA, MQ, DSST, and TMT-B scores improved significantly more in Tai Chi Chuan group compared to control group. MoCA scores in fitness walking group improved significantly more than in control group 36 weeks: MQ scores improved significantly more in Tai Chi Chuan group compared to fitness walking group. MQ, DSST, and TMT-B scores improved significantly more in Tai Chi Chuan group compared to control group. MoCA scores in fitness walking group improved significantly more than in control group |
MoCA: Tai Chi Chuan vs. control: d = 0.63 Fitness walking vs. control: d = 0.33 Wechsler Memory Quotient (MQ): Tai Chi Chuan vs. control: d = 0.47 Fitness walking vs. control: d = 0.15 DSST: Tai Chi Chuan vs. control: d = 0.31 Fitness walking vs. control: d = 0.14 TMT B: Tai Chi Chuan vs. control: d = − 0.37 Fitness walking vs. control: d = − 0.06 BNT: Tai Chi Chuan vs. control: d = 0.21 Fitness walking vs. control: d = 0.16 ROCFT: Tai Chi Chuan vs. control: d = 0.07 Fitness walking vs. control: d = − 0.12 ROCFT delayed recall: Tai Chi Chuan vs. control: d = 0.01 Fitness walking vs. control: d = 0.00 |
| Cai et al. [96] | Kinect-based Kaimai-style Qigong | 12 weeks | 30 min | 3x/week | Low intensity | Cognitive function (MMSE) | Qigong group showed significant improvements in cognitive function compared to the control group | MMSE: d = 1.75 |
BNT Boston Naming Test, BVMT-R Brief Visuospatial Memory Test-Revised, CBS Cambridge Brain Sciences, CVLT-II California Verbal Learning Test II, d cohen’s d, DSC digit symbol coding, DSST Digit Symbol Substitution Test, HIFT High-Intensity low-volume Functional Training, HRmax Heart Rate maximum, HRR heart rate reserve, HVLT-D Hopkins Verbal Learning Test revised, I rest interval between sets and strength-type exercises, LIFT Low-Intensity High-volume Functional Training, MMSE Mini-Mental State Examination, MoCA Montreal Cognitive Assessment, MQ Weschler Memory Quotient, MRI Magnetic Resonance Imaging, MRs maximal repetitions, MTTS Mental Test and Training System, ROCFT Rey-Osterrieth Complex Figure Test, SDMT Symbol Digit Modalities test, TMT Trail Making Test, TUG timed up-and-go, V1 intervention midpoint, V2 postintervention, v6MWT velocity in 6 min walking test, ηp2 = partial eta-squared, 1RM = 1 Rep Maximum, 3MSE = Modified Mini Mental State Exam
Table 4.
Intervention characteristics and outcomes of included animal studies
| Author | Type of exercise training | Duration of exercise intervention | Duration of each training session | Frequency of exercise training | Intensity of exercise training | Outcomes (cognitive tests) | Main cognitive results | Effect size |
|---|---|---|---|---|---|---|---|---|
| Parsa et al. [98] | Swimming | 12 weeks | 40 min | 5 days/week | ? |
Cognitive memory (novel object recognition test and elevated plus maze) Aversive memory and learning (passive avoidance learning test) |
Swimming training alone insignificantly improved exploratory behaviour, locomotor activity, and passive avoidance memory, and non-spatial cognitive memory compared to sedentary diabetic rats | / |
| Shekarchian et al. [99] | Swimming | 4 weeks |
Week 1–2: 3 × 10 min Week 3–4: 6 × 10 min |
5 days/week | ? |
Working memory (Y maze) Recognition memory (novel object recognition) Spatial memory (MWM) |
Exercise improved all memory aspects in exercising T2DM mice compared to non-exercising T2DM mice |
Elevated plus maze test: Percentage of spontaneous alternation: η2 = 0.02 Total arm entries: η2 = 0.01 Novel object recognition test: Familiar recognition ratio: η2 = 0.04 Novel recognition ratio: η2 = 0.08 MWM: Escape latency: η2 = 0.10 Platform quadrant time: η2 = 0.01 Platform crossings: η2 = 0.01 |
| Jesmin et al. [100] | Treadmill running | 4 months | 30 min/day | 5 days/week | Light intensity: OLETF 7.0 m/min, LETO 10.0 m/min; Moderate intensity: OLETF 12.5 m/min, LETO 20 m/min | Spatial learning memory and retention (MWM) | Shortened swim length and escape latency in both light and moderate exercise groups improved memory |
MWM: Escape latency: η2 = 0.11 Swim length: η2 = 0.08 Memory retention: η2 = 0.16 |
| Lang et al. [101] | Treadmill running | 8 weeks | 37 min/day | 5 days/week | Moderate intensity | Spatial learning and memory (MWM) | Compared with T group, the escape latency of E group was significantly reduced on day 4–5, treadmill exercise significantly increased the number of platform crossings in E group |
MWM: Escape latency: d = − 1.39 Platform quadrant time: d = 1.01 Platform crossings: d = 1.32 |
| Shima et al. [102] | Treadmill running | 4 weeks | 30 min/day | 5 days/week | Moderate intensity (OLETF rats, 12.5 m/min; LETO rats, 20 m/min) | Memory (MWM) |
Escape latency and swim path length of exercising OLETF rats were shortened, without alteration of the speed of swimming, at trials on days 2, 3 and 4 The time spent by OLETF rats in the platform area significantly improved after 4 weeks of exercise |
MWM: Escape latency: η2 = 0.11 Swim length: η2 = 0.15 Platform quadrant time: η2 = 0.16 |
| Shima et al. [103] | Running on a forced exercise wheel bed | 4 weeks | 30 min/day | 5 days/week | Light intensity | Memory (MWM) | No effect of exercise on the escape latency, swim distance or speed in ob/ob mice. The times of crossing the target platform during the probe test in C57BL/6 mice were significantly greater than that in sedentary ob/ob mice, but not than that in exercised ob/ob mice |
MWM: Swim length: η2 = 0.02 Swim duration: η2 = 0.05 |
E group diabetes + exercise group, LETO Long Evans Tokushima Otsuka, MWM Morris Water Maze, ob/ob obese-hyperglycemic, OLETF Otsuka Long-Evans Tokushima Fatty, T group diabetes group, T2DM type 2 diabetes