Abstract
Elevation in CO2 can significantly impact the biology of various organisms, affecting life-history traits of both aquatic and terrestrial forms, including disease-vectoring mosquitoes. For mosquitoes, this effect is accentuated by egg quiescence duration, resulting in a change in foraging of adult females. Female mosquitoes rely on their olfactory system for locating resources, such as nectar and blood. This study employs a transcriptomic approach to investigate how a projected elevation in CO2 level, under a worst-case scenario, interacts with extended egg quiescence duration to modulate the molecular machinery of the peripheral olfactory system, the antennae and maxillary palps, of the yellow fever mosquito, Aedes aegypti. The transcriptome analysis demonstrates significant changes in the abundance of genes related to metabolism, xenobiotics degradation and chemosensory function, with the most pronounced effects observed in the CO2 sensing tissue, the maxillary palp. The study provides novel insights into how anthropogenic climate change can modulate the olfactory sensory system of disease vectors, which may have cascading effects on resource-seeking behaviour.
Keywords: Mosquitoes, Carbon dioxide, Climate change, Egg quiescence, Olfactory system, Transcriptome
Subject terms: Behavioural ecology, Climate-change ecology, Molecular ecology
Introduction
Global climate change, due to anthropogenic activities, is predicted to change the distribution and behaviour of insects, including mosquitoes that vector disease1–3. A key factor driving this change is the elevation in atmospheric carbon dioxide (CO2), which inadvertently affects life history traits across both aquatic and terrestrial stages of invertebrates4–6. For example, in the dengue vector, Aedes aegypti, an exponential increase in atmospheric CO2 level, reflecting those projected within recent time and those predicted under extreme conditions, if targets are not met, for the next century7, significantly affects key life-history traits, including larval survival and development, as well as adult survival and the feeding response of females6. These effects are further modulated by the extent of egg quiescence, i.e., the ability of eggs to withstand extended periods of desiccation or dormancy8, which is determined by environmental factors, such as temperature and humidity9–11. Prolonged egg quiescent duration increases the susceptibility of emerging larvae to abiotic stressors12,13, which may have significant effects on mosquito population dynamics and feeding behaviour, thus affecting vectorial capacity14. The feeding response of insects is the ultimate stage in a process regulating resource seeking, which for most insects is mediated predominantly by olfaction and influenced by the internal physiological state15,16. Resource-seeking behaviours, as well as the detection of ecologically relevant sensory cues, in insects are affected by both short- and long-term exposure to elevated levels of CO217–21.
Many insect species use CO2 as a reliable cue for nectar22,23, host24,25, and oviposition site seeking26, as well as threat avoidance27,28. An elevation in ambient CO2 negatively affects host-seeking in mosquitoes21 and oviposition site selection in moths, due to sensory constraints imposed on the CO2-sensory system21,26. Long-term developmental exposure to elevated CO2 also reduces the alarm-pheromone escape behaviour in aphids29, although no significant effects on olfactory perception have been described30. In addition, studies on aquatic invertebrates show an impairment in olfactory-guided behaviours as a consequence of elevated CO231, however, the underlying neuronal mechanism remains unclear32. Exposure to elevated CO2 in Helicoverpa moths affects the CO2-sensory neurons, which become promiscuous and respond to fluctuations in temperature, as well as to CO233, demonstrating that exposure to elevated CO2 likely has a broad effect on sensory systems and gene expression34. The aim of this study was to assess how predicted levels of elevated CO2 and extended egg quiescence affect chemosensory gene expression to identify molecular correlates underlying changes in resource-seeking behaviours in female Ae. aegypti.
The antennae and maxillary palps constitute the main peripheral olfactory system of mosquitoes, with hair-like structures, sensilla, on the surface acting as the smallest functional units35. Volatile odorants enter the sensilla, where they are recognized and transported by odorant binding proteins (OBPs) and chemosensory proteins (CSPs) to receptors in the dendritic membrane of olfactory sensory neurons (OSNs)36,37. Each OSN expresses one or a combination of olfactory receptor proteins from three different families: odorant receptors (ORs), ionotropic receptors (IRs) and gustatory receptors (GRs)38,39, as well as sensory neuron membrane proteins (SNMPs)36. The ORs and IRs form heterotetrameric complexes with conserved co-receptors, Orco, as well as Ir25a, Ir8a and Ir76b, respectively, and ligand-selective subunits, ORs and IRs40,41. The overall role of ORs and IRs in mosquitoes is to regulate host attraction and discrimination42–45. Although GRs are primarily involved in contact chemoreception, CO2 is detected by a heteromeric complex of Grs46,47, and involved in activation and attraction48,49. Apart from the canonical chemosensory gene families, pickpocket (PPK) and transient receptor potential (TRP) channels, involved in risk assessment50–52, are also expressed in the OSN dendritic membrane37. Several members of these chemosensory gene families are differentially regulated in response to a change in internal state of female mosquitoes16,53–55, however, there is currently limited information on how the external environment modulates the molecular machinery of the peripheral olfactory system.
To achieve the aim of this study, RNA sequencing was performed using antennal and maxillary palp tissues collected from females reared under current ambient and extreme CO2 conditions and originating from eggs following different egg quiescent periods. The transcriptome analysis demonstrated an overall effect on the differential expression within select gene ontologies, including metabolism, xenobiotics and chemosensory, predominantly in the maxillary palp, in response to elevated CO2 conditions, an affect exacerbated by egg quiescence duration. The findings of this study demonstrate that predicted changes in climate, driven by factors, such as elevation in CO2, affect the peripheral olfactory system of insects, which in turn may affect the resource-seeking behaviours.
Results
RNA sequencing
The RNA sequencing detected a total of 17,439 genes of the 19,804 annotated genes in the genome of Ae. aegypti, of which 10,226 were reliably expressed (Supplementary Table S1). Of these, 8,833 and 9,510 were reliably expressed in the antennae and maxillary palps, respectively. To assess the quality and depth of the sequencing, the core eukaryotic gene mapping approach was performed, demonstrating that 450 and 447 (of the total 450) genes were detected reliably above the 1 TPM expression level in the antennal and maxillary palp libraries, respectively (Supplementary Table S2).
Overall and differential expression
Overall gene expression was assessed using Principal Component Analysis (PCA) with the 29 libraries of tissue collected from females reared under ambient and elevated CO2 conditions, and shorter and extended egg quiescent periods (Fig. 1). The analysis revealed that 43.2% of the variance among libraries was based on the type of olfactory organ (PC 1), and 1.9% of the variance between maxillary palp libraries was based on CO2 condition (PC 9) (Fig. 1). There was no significant effect on overall antennal gene expression in response to CO2 level (F = 1.01, R2 = 0.064, p = 0.38) or egg quiescent duration (F = 1.68, R2 = 0.10, p = 0.17), individually or interactively (F = 2.07, R2 = 0.13, p = 0.09, Supplementary Figure S1). In contrast, the egg quiescence period (F = 3.43, R2 = 0.20, p = 0.02) significantly affected the overall gene expression in the maxillary palp (Supplementary Figure S1). However, neither CO2 level (F = 1.01, R2 = 0.06, p = 0.38) nor the interaction of the two stress factors (F = 2.07, R2 = 0.13, p = 0.09) had a significant effect on the overall maxillary palp gene expression (Supplementary Figure S1).
Fig. 1.
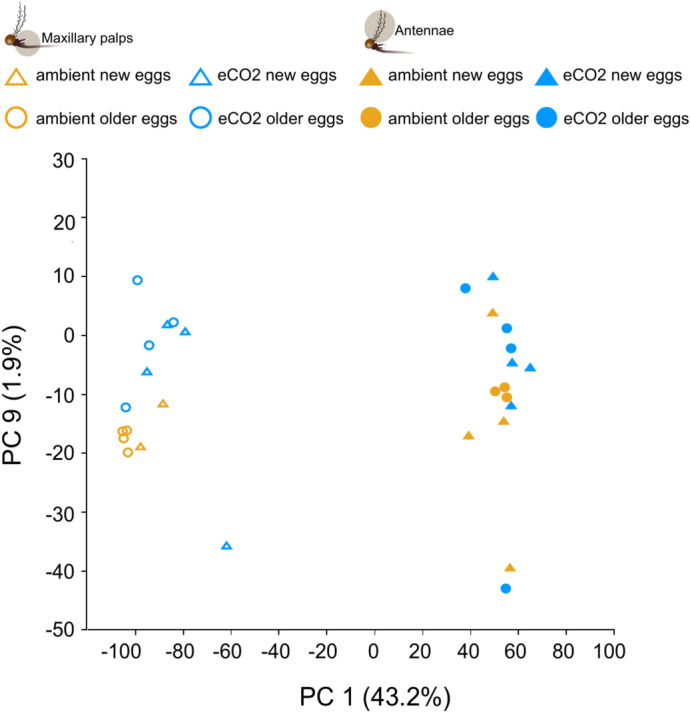
Elevated CO2, but not egg quiescence, differentially affects overall gene expression in the peripheral olfactory organs of Aedes aegypti. Principal component analysis of antennal and maxillary palp libraries of females emerging from new and older eggs, with short and extended egg quiescent duration, respectively, reared under ambient and elevated CO2 conditions. A total of 29 libraries were analysed to estimate the change in overall gene expression, in which Principal Component (PC) 1 (43.2%) and PC 9 (1.9%) accounted for the variance between the libraries.
The gene ontology (GO) analysis identified various molecular functional categories, based on differentially expressed genes (DEGs), which changed in both numbers and direction in the antennal and maxillary palp libraries in response to the interaction of an elevation in CO2 and extended egg quiescence (Fig. 2). Comparisons between ambient and elevated CO2, as well as between egg quiescence periods for antennal and maxillary palp libraries under ambient conditions, identified too few DEGs for drawing any overall findings (Fig. 2). In response to elevated CO2 and extended egg quiescence, > 85% of the DEGs in the antennal and maxillary palp libraries were categorised as molecular function (GO:0,003,674), followed by oxidoreductase activity (GO:0,016,491), peptidase activity (GO:0,008,233) and hydrolase activity, acting on carbon–nitrogen (but not peptide) bonds (GO:0,016,810) (Fig. 2, right). In addition, in the maxillary palp libraries, the 1% DEGs were categorised as hydrolase activity, acting on glycosyl bonds (GO:0,016,798) (Fig. 2). Within the molecular function category, several differentially expressed chemosensory genes, including Ors, Irs and Obps, were represented.
Fig. 2.
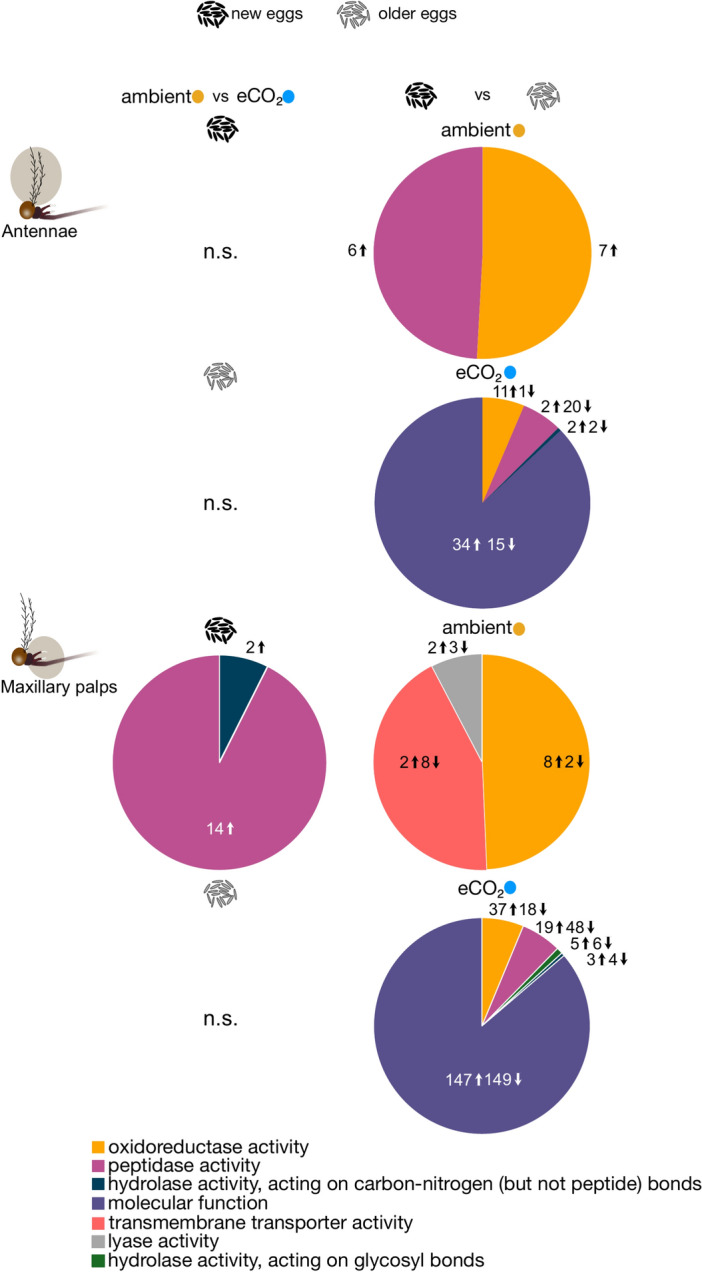
Gene ontology analysis of differentially expressed genes in the antennae and maxillary palps of Aedes aegypti. The olfactory tissues were collected from females reared under ambient and elevated CO2 conditions, as well as short and extended egg quiescence duration, referred to as new and older eggs, respectively. Pairwise comparisons are arranged in a matrix in response to CO2 conditions and egg quiescence period. The differentially expressed genes are classified into molecular function ontology, using gene ontology slim categorisation. n.s.: non-significant.
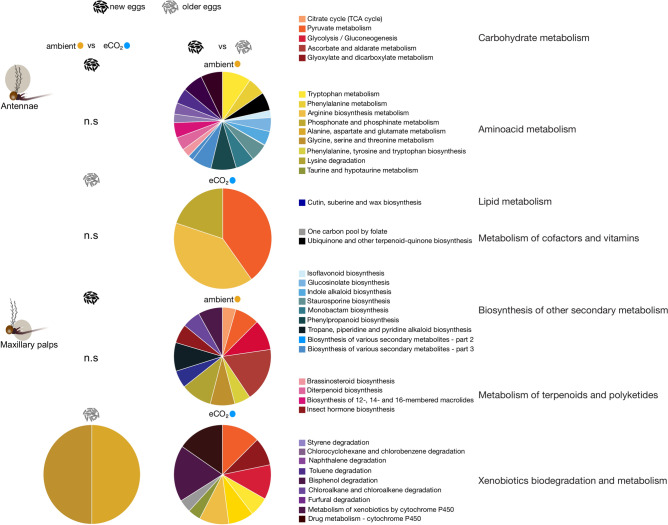
The KEGG pathway analysis identified 39 unique metabolic pathway terms, 17 of which were from the metabolism pathways, 9 from biosynthesis of secondary metabolites, four from metabolism of terpenoids and polyketides, and nine identified in the xenobiotic biodegradation pathway (Fig. 3). In the antennal libraries, four DEGs were categorised as xenobiotic response pathway in females reared under ambient CO2 conditions, in response to extended egg quiescence. Moreover, in the maxillary palp, six and 28 DEGs contributed to the xenobiotic biodegradation pathway, when reared under ambient and elevated CO2, in response to extended egg quiescence (Fig. 3, Supplementary Table S3). Within the xenobiotic response pathway, several stress response genes, including cytochrome P450 and UDP-glycosyl transferases, were represented across the comparisons in relation to CO2 conditions and egg quiescence period.
Fig. 3.
Kyoto Encyclopedia of Genes and Genomes pathway analysis of differentially expressed genes in the antennae and maxillary palps of Aedes aegypti. The olfactory tissues were collected from females reared under ambient and elevated CO2 conditions, as well as short and extended egg quiescence duration, referred to as new and older eggs, respectively. Pairwise comparisons are arranged in a matrix in relation to the response to CO2 conditions (eCO2) and egg quiescence period. The categories are annotated from Vectorbase and further classified into pathways designated by Kyoto Encyclopedia of Genes and Genomes database (https://www.genome.jp/kegg/).
Regulation of peripheral chemosensory genes
Elevation in CO2 and extended egg quiescence period differentially modulated the expression profile of chemosensory genes, with the highest differential regulation occurring in the maxillary palps.
Odorant receptors
Among the 97 annotated Ors, 88 and 3, including Orco, were reliably expressed in the antennae and maxillary palps of female Ae. aegypti, respectively (Supplementary Table S4). While Orco was not significantly regulated, the antennally-expressed Or50 and Or86 significantly increased in abundance in females emerging from older eggs, in response to elevated CO2 conditions (Fig. 4a). The three Ors expressed in the maxillary palps were not regulated in response to an elevation in CO2 or egg quiescence period (Fig. 4b).
Fig. 4.

Differential abundance of chemosensory genes in Aedes aegypti in response to elevated CO2 conditions and extended egg quiescence period. The olfactory tissues were collected from females reared under ambient and elevated CO2 conditions, as well as short and extended egg quiescence duration, referred to as new and older eggs, respectively. The abundance of reliably expressed (> 1 transcript per million) chemosensory genes compared between ambient and elevated CO2 (eCO2) levels, as well as egg quiescent periods, in the antennal (a) and maxillary palp (b) libraries, and demonstrated by fold-change (> 1.5-fold change; FDR > 0.05). Asterisks on fold change denote significant differences between pairwise comparisons.
Ionotropic receptors
Of the 52 annotated Irs, 33 and 4 were reliably expressed in the antennal and maxillary palp libraries, respectively (Supplementary Table S4). The three co-receptors were reliably expressed, with Ir25a having a significantly lower abundance in maxillary palps of females originating from eggs that underwent an extended egg quiescence period and then reared under elevated CO2 conditions (Supplementary Table S4, Fig. 4b). Of the 30 tuning Irs expressed in the antennal libraries, Ir75k increased in abundance in response to an extended egg quiescence period, when females were reared under ambient CO2 conditions (Fig. 4b).
Gustatory receptors
Among the 41 annotated Grs, 9 and 5 were reliably expressed in the antenna and maxillary palps libraries, respectively (Supplementary Table S4). No Grs were differentially regulated in the antennal or maxillary palp libraries (Supplementary Table S4).
Non-canonical chemoreceptor-related families
Of the 14 annotated Trps, 7 and 6 were reliably expressed in the antennal and maxillary palp libraries, respectively, none of which were differentially regulated (Supplementary Table S4). Similarly, of the 46 annotated pickpocket genes, 15 and 9 were reliably expressed in the antennal and maxillary palp libraries, respectively, none of which showed differential expression in response to elevated CO2 conditions or egg quiescence period (Supplementary Table S4).
The genes coding for sensory neuron membrane proteins (SNMPs), of which SNMP1 and SNMP2 were among the 10 and 11, out of the 13 annotated, reliably expressed SCRBs in the antennal and maxillary palp libraries, respectively (Supplementary Table S4). The expression of SCRB6 and SNMP1 was downregulated in the maxillary palps of females reared under elevated and ambient CO2 conditions, respectively, in response to extended egg quiescence period (Fig. 4b).
Soluble odorant-binding proteins
The genes encoding for OBPs and CSPs were highly abundant in the antennae and maxillary palps libraries. Out of the 52 annotated OBPs, 33 and 35 were reliably expressed in the antennal and maxillary palp libraries, respectively (Supplementary Table S4). Only one OBP, OBP25, increased in abundance in the antennae of females reared under elevated CO2 conditions in response to an extended egg quiescence period (Fig. 4a). In the maxillary palp libraries, OBPs were differentially regulated in response to elevated CO2: seven OBPs were significantly lower in abundance in females emerging from new eggs, while four OBPs were higher in abundance in females emerging from older eggs, in response to elevated CO2 conditions (Fig. 4b). In response to extended egg quiescence period, the abundance of OBPs were differentially regulated in relation to CO2 condition: seven out of the ten differentially expressed OBPs in the maxillary palp libraries of females reared under ambient CO2 conditions were lower in abundance, while nine OBPs were higher in abundance in females reared under elevated CO2 conditions (Fig. 4b).
Out of the 17 annotated CSPs, seven and ten were reliably expressed in the antennal and maxillary palp libraries, respectively (Supplementary Table S4). The CSPs did not display any differential expression in response to CO2 conditions and egg quiescence period in the antennal libraries. However, in the maxillary palp libraries, one and three CSPs decreased in abundance in females when reared under ambient and elevated CO2 conditions, respectively, in response to an extended egg quiescence period (Fig. 4b).
Discussion
Based on this transcriptome analysis, the effect of an elevation in CO2 level, to that predicted under extreme conditions7, appears to be gene-family specific, while egg quiescent duration has a distinct and overall impact on gene expression, particularly in the maxillary palp. Differential expression of genes in both antennae and maxillary palps involved in metabolism and xenobiotics emphasise a stress response as a consequence of elevated CO2 and extended egg quiescence duration, similar to the systemic response shown in other insects to environmental stressors56. Contrasting regulation of select members of chemosensory gene families, ORs, IRs, SNMPs, OBPs and CSPs, in the antennae and maxillary palp, may regulate the observed differences in resource-seeking behaviour in response to the two external stressors in female Ae. aegypti6. Overall, this study provides insights into how environmental stress impacts the peripheral olfactory system of insects and ensuing behaviour.
The differential feeding behaviour of Ae. aegypti as a result of different egg quiescence durations, and when reared under elevated CO26, while appearing to have no significant generalised effect on gene expression, is likely a result of more targeted regulation of genes as indicated in the GO slim and KEGG analyses. The high number of significant DEGs, characterised by GO slim analysis, emphasises an interactive effect of elevated CO2 conditions and extended egg quiescence period on gene regulation in the peripheral olfactory system. The differentially regulated genes, predominantly in the maxillary palp, divides into categories including energy metabolism and xenobiotic response pathways, which is highlighted through KEGG analysis, and emphasises a significant transcriptional regulation of stress-induced genes in an organ that is involved in the detection of CO2 and other host-related chemosensory signals57,58. A similar transcriptional regulation of metabolic genes, in response to elevated CO2, has been demonstrated in aquatic invertebrates and insects59,60. Tissue-specific effect on gene expression regulation in the olfactory system, in response to elevated CO2, has also been demonstrated in salmon61. While elevated CO2 levels do not appear to directly trigger the xenobiotic response pathways, elevated CO2 upregulates the transcription of genes encoding for detoxifying enzymes, including cytochrome P450s62,63, [this study]. Xenobiotic response genes, including members of the cytochrome P450 family, are regulated in response to a variety of environmental stressors, including volatile compounds64,65, prolonged exposure to insecticides66,67, and abiotic stressors68–70. Cytochrome P450s act as odorant degrading enzymes in the insect peripheral olfactory system71. Hence, the oxidative stress and potential acidification of the sensillum lymph, as a result of the conversion of CO2 into carbonic acid72, may explain the observed response in this degradation pathway. Acidification of the sensillum lymph influences the folding of OBPs73–75, which can lead to alterations in protein function. Although mosquitoes acid–base regulate under varying pH conditions76,77, it remains unclear how the buffering capacity is impacted by prolonged exposure to elevated CO2. Furthermore, how this affects the membrane-bound receptors78, and the cascading effects on neuronal signalling79,80, remains to be studied.
Elevated CO2 levels, accentuated by egg quiescence duration, differentially affected the expression of soluble and membrane-bound chemosensory genes, which may directly affect the behaviour of disease-transmitting mosquitoes6, [this study]. Of the soluble odorant-binding proteins, insect OBPs facilitate odorant transport, odorant-receptor interactions and gain control81, as well as xenobiotic adaptations82. The significant differential regulation of OBPs, predominantly in the maxillary palp, emphasises the important role of these genes in response to elevated CO2 levels and extended egg quiescence duration. Of the 12 differentially regulated OBPs, only OBP22 and OBP39 have been functionally characterised, and demonstrated to detect long-chain fatty acids involved in host- and oviposition-site seeking, respectively83,84. The abundance of a subset of OBPs, including OBP56, OBP39, OBP34 and OBP38, shifted in response to elevated CO2, from low in new eggs to high in older eggs, suggesting a conserved regulatory pathway for these OBPs in response to stress. Considering the role of OBPs, the demonstrated regulation of genes will likely affect the interaction between odorant ligands and the membrane-bound receptors.
Among the membrane-bound receptors, the differential regulation of Ors in the antenna provides an insight into the regulatory mechanism regulating Or expression in response to environmental stress85, despite the unknown functional relevance of these changes for Ae. aegypti44,86–89. The absence of regulation in other Ors in both antennae53,90 and maxillary palps91 suggests that core Or-mediated sensory detection remains largely unaffected, as is the case for other membrane-bound receptors. Among the differentially regulated Irs that have been functionally characterised, the Ir co-receptor Ir25a is involved in the detection of amines92,93, whereas the tuning Ir, Ir75k, is sensitive to short-chain carboxylic acids94. These chemical classes play important roles in host- and oviposition-site selection95,96, and the differential regulation of the receptors detecting these odorants may affect the efficient resource seeking by mosquitoes. In Drosophila, Ir25a is required for context-dependent attraction to CO297, and in female Ae. aegypti, Ir25a is co-expressed in the maxillary palp CO2 sensitive OSN38. The functional significance of the lower abundance of Ir25a in response to stress requires further investigation. While short-term exposure to elevated CO2 significantly impact host seeking, as a consequence of sensory constraint21, the genes encoding for the subunits forming the CO2 receptor98 were not regulated in response to developmental exposure to high CO2. Whether long-term exposure to high CO2 levels has a similar affect, and how this is regulated is yet unknown. Taken together, the interaction of elevated CO2 and extended egg quiescence differentially affect the expression of chemosensory genes that likely play key roles in regulating mosquito behaviours.
This study provides evidence that anthropogenic climate change factors, such as elevated CO2, interact with other stress factors, such as egg quiescence duration, elicit a stress response in the peripheral olfactory system of mosquitoes and that the capacity of females to detect ecologically-relevant volatile organic compounds may be hampered. While previous studies have demonstrated negative effects of elevated CO2 and egg quiescence duration on life-history parameters of both aquatic and terrestrial stages of Ae. aegypti, and subsequent carry-over effect on the feeding response of adult females, future experiments are required to assess how these stress factors affect odour-mediated behaviour and physiology.
Methods
Mosquito rearing and tissue collection
For general colony maintenance, Ae. aegypti (Rockefeller) were maintained under 27 ± 2 °C, 65 ± 5% relative humidity and a 12 h: 12 h light: dark cycle. The adults had ad libitum access to 10% sucrose (w/v). Females were blood fed with defibrinated sheep blood (Håtunalab AB, Bro, Sweden), using a membrane feeding system (Hemotek Ltd, Blackburn, UK) for egg production. The eggs, deposited on moist filter paper, were collected, dried, labelled and stored for subsequent experiments to account for different egg quiescent periods. The CO2 acclimatization experiments were conducted in two high-precision climate chambers, in which temperature, humidity and light conditions were maintained as above. The CO2 concentration in the chambers was 400 ppm (current ambient), and 1000 ppm (elevated CO2), respectively, in which pure CO2 (Strandmöllen, Ljungby, Sweden) was delivered and mixed into the ventilation system. Age-controlled eggs (2-week or 3–6-month quiescent periods) were introduced to each experimental chamber, in which eggs from the same cohort were divided equally between two chambers, resulting in a larval density of 100 larvae per 600 ml of water, in each rearing tray. The larvae were fed with fish food (TetraMin® Flakes, Melle, Germany) daily (1 mg larvae-1), normalised for larval mortality. Upon pupation, individual pupae were collected into small (30 ml) plastic cups with distilled water and placed into Bugdorm cages (17.5 cm × 17.5 cm × 17.5 cm; Megaview Science Co., Ltd, Taichung, Taiwan). The emerging adults had ad libitum access to water until tissue was collected.
Collection of teneral (30 ± 6 h) female antennae and maxillary palp were done at Zeitgeber time 10–12, i.e., the peak diel activity period of Ae. aegypti99. For the dissection, females were anesthetised on ice, and the tissues removed using a pair of fine sterilised forceps, with separate pairs of forceps used for each olfactory tissue type, and then placed into 200 µl of RNAlater (Thermo Fisher Scientific, Gothenburg, Sweden). Forceps were sterilised in between each biological replicate using 70% ethanol. The tissue was stored at room temperature overnight, then at -20 °C overnight, and thereafter at -80 °C until RNA extraction. A total of 16 antennal libraries were generated, with each library comprising pooled tissues from 50 individuals per replicate from different cohorts, across two CO₂ conditions, two egg quiescence periods, and four biological replicates (50 tissues × 2 CO₂ levels × 2 quiescent periods × 4 replicates = 800 pairs of tissues). Similarly, 16 maxillary palp libraries were constructed using the same pooling strategy, yielding an additional 800 pairs of tissues. In total, 1,600 pairs of tissues were collected for all (32) libraries.
RNA extraction and sequencing
Total RNA extraction was performed using the RNeasy microRNA kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol with an additional step of quick freezing with liquid nitrogen to facilitate the homogenisation of the tissues. The RNA extracted was immediately stored at -80 °C and later quantified using the TapeStation system 4150 (Agilent Technologies, Inc, Santa Clara, US). The samples were shipped on dry ice to Eurofins Genomics (Constance, Germany), where INVIEW ultra-low transcriptome libraries were constructed using NovaSeq Illumina genome sequencing technology (Illumina NovaSeq 6000 S4 PE150 XP). The cDNA library construction was realised using Eurofins proprietary protocol, generating 2 × 150 bp coverage paired-end reads with a depth of 20 million paired-end reads (Supplementary Table S5).
Read mapping and annotation
Prior to the quantitative assessment of the library sequences, the samples underwent quality control steps involving the removal of adapter sequences, and discarding sequences with a Phred score of below 40, using CLC Genomics Workbench (23.0.5, Qiagen, Aarhus, Denmark). Three libraries were removed from further analysis due to cross-contamination between tissues (Supplementary Table 1)100. The sequences were mapped to the Ae. aegypti reference genome (VectorBase: Aedes aegypti LVP_AGWG, AaegL5.3).
PCA analysis
Principal component analysis (PCA) was performed to estimate the effect of elevated CO2 and egg quiescence period on the overall expression profile. The high-dimensional dataset containing the antennal and maxillary palp libraries was projected onto two-dimensional components to determine the variance between libraries using the toolbox for RNA-seq data in CLC Genomics Workbench. The individual and interactive effect of CO2 level and egg quiescent period on each olfactory tissue was assessed through permutational multivariate analysis of variance (PERMOVA) using “adonis2” function under the vegan package in RStudio.
RNA seq and differential expression analysis
For the transcriptome analysis, transcripts per million (TPM) was used, with a reliable expression of genes determined to be above a threshold of 1 TPM. Differential transcript abundance was analysed using a negative binomial distribution with a gamma-Poisson mixed distribution in CLC Genomics Workbench (https://digitalinsights.qiagen.com/). To account for false positives during the statistical tests, the false discovery rate (FDR) with p-value correction was performed using the Benjamin-Hochberg method101. The analysis generated a mean abundance value, fold change (FC) and FDR p-values that were accessed for differential expression. Genes were considered significantly differentially expressed when fold change > 1.5 and FDR p-value < 0.05.
Functional enrichment analyses
To assess the effects of elevated CO2 and extended egg quiescence period on molecular function level and metabolic pathways, a gene ontology (GO) analysis and KEGG (Kyoto Encyclopedia of Genes and Genomes) analysis were performed. The GO and KEGG terms used for the identified differentially expressed genes (DEGs) in the antennae and maxillary palps, were identified from VectorBase (AaegL5.3, Release 68). The VectorBase GO enrichment tool was used for assessing the molecular function, with both computed and curated evidence limited to GO slim terms. The VectorBase metabolic pathway enrichment was used for KEGG analysis. The significance cut-off was set to alpha (α) = 0.05.
Supplementary Information
Acknowledgements
We acknowledge the help provided by the Biotron maintenance staff at SLU.
Author contributions
SN and RI conceived the idea, SN, SRH and RI designed the methodology and SN collected and analysed the data. SN drafted the manuscript, and RI critically revised the manuscript. All authors gave final approval for publication.
Funding
Open access funding provided by Swedish University of Agricultural Sciences.
Max Planck Center next Generation Insect Chemical Ecology.
Data availability
All data generated are presented in the publication. The transcriptome data generated and analysed during this study is available in the NCBI project database, with BioProject ID: PRJNA1195965. https://www.ncbi.nlm.nih.gov/sra/PRJNA1195965.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-98159-w.
References
- 1.Pureswaran, D. S., Roques, A. & Battisti, A. Forest insects and climate change. Curr. Forestry Rep.4, 35–50 (2018). [Google Scholar]
- 2.Halsch, C. A. et al. Insects and recent climate change. Proc. Natl. Acad. Sci. U S A. 118, 2002543117, (2021). [DOI] [PMC free article] [PubMed]
- 3.Tjaden, N. B., Caminade, C., Beierkuhnlein, C. & Thomas, S. M. Mosquito-borne diseases: advances in modelling climate-change impacts. Trends Parasitol.34, 227–245 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Weiss, L. C. et al. Rising pCO2 in freshwater ecosystems has the potential to negatively affect predator-induced defenses in Daphnia. Curr. Biol.28, 327–332 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Sun, Y. F. & Ge, F. How do aphids respond to elevated CO2?. J. Asia-Pac. Entomol.14, 217–220 (2011). [Google Scholar]
- 6.Nalikkaramal, S., Hill, S. R. & Ignell, R. Effect of extended egg quiescence and elevation in carbon dioxide on life history traits of Aedes aegypti. Sci Rep15, 9310. 10.1038/s41598-025-92193-4 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen, M. R. et al. Framing and context. In Global warming of 1.5°c. an IPCC special report on the impacts of global warming of 1.5°c above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty (ed. Masson-Delmotte, V. et al.). 49–92 (Cambridge University Press). 10.1017/9781009157940.003. (2018).
- 8.Rezende, G. L. et al. Embryonic desiccation resistance in Aedes aegypti: presumptive role of the chitinized serosal cuticle. BMC Dev. Biol.8, 82 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clements, A. N. The biology of mosquitoes, volume 1(Chapman & Hall, 1992).
- 10.Farnesi, L. C., Martins, A. J., Valle, D. & Rezende, G. L. Embryonic development of Aedes aegypti (Diptera: Culicidae): influence of different constant temperatures. Mem. Inst. Oswaldo Cruz.104, 124–126 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Christophers, S. R. Aedes aegypti (L.) the yellow fever mosquito: its life history, bionomics and structure (Cambridge Univ. Press, 1960). [Google Scholar]
- 12.Perez, M. H. & Noriega, F. G. Aedes aegypti pharate 1st instar quiescence affects larval fitness and metal tolerance. J. Insect Physiol.58, 824–829 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez, M. H. & Noriega, F. G. Aedes aegypti pharate 1st instar quiescence: a case for anticipatory reproductive plasticity. J. Insect Physiol.59, 318–324 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis, J. et al. Intrinsic factors driving mosquito vector competence and viral evolution: a review. Front. Cell. Infect. Microbiol.13, 1330600 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chadee, D. D., Sutherland, J. M. & Gilles, J. R. L. Diel sugar-feeding and host-seeking rhythms in mosquitoes (Diptera: Culicidae) under laboratory conditions. Acta. Trop.132, S86–S90 (1992). [DOI] [PubMed] [Google Scholar]
- 16.Hill, S. R. & Ignell, R. Modulation of odour-guided behaviour in mosquitoes. Cell Tissue Res.383, 195–206 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whittaker, J. B. Impacts and responses at population level of herbivorous insects to elevated CO2. Eur. J. Entomol.96, 149–156 (1999). [Google Scholar]
- 18.Nicolas, G. & Sillans, D. Immediate and latent effects of carbon dioxide on insects. Ann. Rev. Entomol.34, 97–116 (1989). [Google Scholar]
- 19.Abrell, L., Guerenstein, P. G., Mechaber, W. L., Stange, G. & Christensen, T. A. Effect of elevated atmospheric CO2 on oviposition behaviour in Manduca sexta moths. Global. Change Biol.11, 1272–1282 (2005). [Google Scholar]
- 20.Stange, G. Effects of changes in atmospheric carbon dioxide on the location of hosts by the moth Cactoblastis cactorum. Oecologia110, 539–545 (1997). [DOI] [PubMed] [Google Scholar]
- 21.Majeed, S., Hill, S. R. & Ignell, R. Impact of elevated CO2 background levels on the host-seeking behaviour of Aedes aegypti. J. Exp. Biol.217, 598–604 (2014). [DOI] [PubMed] [Google Scholar]
- 22.Peach, D. A. H., Gries, R., Zhai, H., Young, N. & Gries, G. Multimodal floral cues guide mosquitoes to tansy inflorescences. Sci. Rep.9, 3908 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guerenstein, P. G. & Hildebrand, J. G. Roles and effects of environmental carbon dioxide in insect life. Annu. Rev. Entomol.53, 161–178 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Gillies, M. T. The role of carbon dioxide in host-finding by mosquitoes (Diptera: Culicidae): a review. Bull. Entomol. Res.70, 525–532 (1980). [Google Scholar]
- 25.Barrozo, R. B. & Lazzari, C. R. The response of the blood-sucking bug Triatoma infestans to carbon dioxide and other host odours. Chem. Senses.29, 319–329 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Stange, G., Monro, J., Stowe, S. & Osmond, C. B. The CO2 sense of the moth Cactoblastis cactorum and its probable role in the biological control of the CAM plant Opuntia stricta. Oecologia102, 341–352 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Suh, G. S. B. et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature431, 854–859 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Faucher, C., Forstreuter, M., Hilker, M. & de Bruyne, M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J. Exp. Biol.209, 2739–2748 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Mondor, E. B., Tremblay, M., Awmack, C. & Lindroth, R. L. Divergent pheromone-mediated insect behaviour under global atmospheric change. Glob. Change Biol.10, 1820–1824 (2004). [Google Scholar]
- 30.Boullis, A. et al. Elevated carbon dioxide concentration reduces alarm signaling in aphids. J. Chem. Ecol.43, 164–171 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Thomas, J. T., Munday, P. L. & Watson, S. A. Toward a mechanistic understanding of marine invertebrate behaviour at elevated CO2. Front. Mar. Sci.7, 345 (2020). [Google Scholar]
- 32.Heuer, R. M., Hamilton, T. J. & Nilsson, G. E. The physiology of behavioural impacts of high CO2. In Fish Physiology. (eds. Grosell, M., Munday, P. L., Farrell, A. P. & Brauner, C. J.) 37, 161–194 (2019).
- 33.Stange, G. & Wong, C. Moth response to climate. Nature365, 699 (1993). [Google Scholar]
- 34.Heleniusa, I. T. et al. Elevated CO2 suppresses specific Drosophila innate immune responses and resistance to bacterial infection. Proc. Natl. Acad. Sci. U.S.A.106, 18710–18715 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McIver, S. Sensilla of mosquitoes (Diptera: Culicidae). J. Med. Entomol.19, 489–535 (1982). [DOI] [PubMed] [Google Scholar]
- 36.Leal, W. S. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol.58, 373–391 (2013). [DOI] [PubMed] [Google Scholar]
- 37.Wheelwright, M., Whittle, C. R. & Riabinina, O. Olfactory systems across mosquito species. Cell Tissue Res.383, 75–90 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herre, M. et al. Non-canonical odour coding in the mosquito. Cell185, 3104–3123 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adavi, E. D. et al. Olfactory receptor coexpression and co-option in the dengue mosquito. Biorxiv10.1101/2024.08.21.608847 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benton, R., Sachse, S., Michnick, S. W. & Vosshall, L. B. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol.4, e20 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larsson, M. C. et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron43, 703–714 (2004). [DOI] [PubMed] [Google Scholar]
- 42.DeGennaro, M. et al. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature498, 487–491 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Obaldia, M. E. et al. Differential mosquito attraction to humans is associated with skin-derived carboxylic acid levels. Cell185, 4099–4116 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McBride, C. S. et al. Evolution of mosquito preference for humans linked to an odorant receptor. Nature515, 222–227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raji, J. I. et al. Aedes aegypti mosquitoes detect acidic volatiles found in human odor using the IR8a pathway. Curr Biol.29, 1253–1262 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones, W. D., Cayirlioglu, P., Kadow, I. G. & Vosshall, L. B. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature445, 86–90 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Kumar, A. et al. Contributions of the conserved insect carbon dioxide receptor subunits to odour detection. Cell Rep.31, 107510 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMeniman, C. J., Corfas, R. A., Matthews, B. J., Ritchie, S. A. & Vosshall, L. B. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell156, 1060–1071 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dekker, T., Geier, M. & Carde, R. T. Carbon dioxide instantly sensitises female yellow fever mosquitoes to human skin odours. J. Exp. Biol.208, 2963–2972 (2005). [DOI] [PubMed] [Google Scholar]
- 50.Greppi, C. et al. Mosquito heat seeking is driven by an ancestral cooling receptor. Science365, 681–684 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matthews, B. J., Younger, M. A. & Vosshall, L. B. The ion channel ppk301 controls freshwater egg-laying in the mosquito Aedes aegypti. eLife. 8, e43963 (2019). [DOI] [PMC free article] [PubMed]
- 52.Corfas, R. A. & Vosshall, L. B. The cation channel TRPA1 tunes mosquito thermotaxis to host temperatures. eLife. 4, e11750 (2015). [DOI] [PMC free article] [PubMed]
- 53.Hill, S. R., Taparia, T. & Ignell, R. Regulation of the antennal transcriptome of the dengue vector, Aedes aegypti, during the first gonotrophic cycle. BMC Genomics22, 71 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tallon, A. K., Hill, S. R. & Ignell, R. Sex and age modulate antennal chemosensory-related genes linked to the onset of host seeking in the yellow-fever mosquito Aedes aegypti. Sci. Rep.9, 43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Omondi, A. B., Ghaninia, M., Dawit, M., Svensson, T. & Ignell, R. Age-dependent regulation of host seeking in Anopheles coluzzii. Sci. Rep.9, 9699 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rix, R. R. & Cutler, G. C. Review of molecular and biochemical responses during stress induced stimulation and hormesis in insects. Sci. Total Environ.827, 154085 (2022). [DOI] [PubMed] [Google Scholar]
- 57.Majeed, S., Hill, S. R., Birgersson, G. & Ignell, R. Detection and perception of generic host volatiles by mosquitoes modulate host preference: context dependence of (R)-1-octen-3-ol. R. Soc. Open Sci.3, 160467 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vainer, Y. et al. A conserved odorant receptor underpins borneol-mediated repellency in culicine mosquitoes. Biorxiv10.1101/2023.08.01.548337 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strader, M. E., Wong, J. M. & Hofmann, G. E. Ocean acidification promotes broad transcriptomic responses in marine metazoans: a literature survey. Front. Zool.17, 7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu, W., Li, Z., Zhang, S., Ke, Y. & Hou, Y. Transcriptome response to elevated atmospheric CO2 concentration in the Formosan subterranean termite, Coptotermes formosanus Shiraki (Isoptera: Rhinotermitidae). PeerJ4, e2527 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams, C. R. et al. Elevated CO2 impairs olfactory-mediated neural and behavioral responses and gene expression in ocean-phase coho salmon (Oncorhynchus kisutch). Glob. Chang. Biol.25, 963–977 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fan, Z. F. et al. Effects of elevated CO2 on activities of protective and detoxifying enzymes in Frankliniella occidentalis and F. intonsa under spinetoram stress. Pest Manag. Sci.78, 274–286 (2022). [DOI] [PubMed] [Google Scholar]
- 63.Lu, Z. et al. Effects of Elevated CO2 concentration on host adaptability and chlorantraniliprole susceptibility in Spodoptera frugiperda. Insects.13, 1029 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.López, M. F., Cano-Ramírez, C., Cesar-Ayala, A. K., Ruiz, E. A. & Zúñiga, G. Diversity and expression of P450 genes from Dendroctonus valens LeConte (Curculionidae: Scolytinae) in response to different kairomones. Insect Biochem. Mol. Biol.43, 417–432 (2013). [DOI] [PubMed] [Google Scholar]
- 65.Mappin, F., Bellantuono, A. J., Ebrahimi, B. & DeGennaro, M. Odor-evoked transcriptomics of Aedes aegypti mosquitoes. Biorxiv10.1371/journal.pone.0293018 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang, Y. et al. Response of xenobiotic biodegradation and metabolic genes in Tribolium castaneum following eugenol exposure. Mol. Genet. Genomics.297, 801–815 (2022). [DOI] [PubMed] [Google Scholar]
- 67.Low, W. Y. et al. Recognition and detoxification of the insecticide DDT by Drosophila melanogaster glutathione S-transferase D1. J. Mol. Biol.399, 358–366 (2010). [DOI] [PubMed] [Google Scholar]
- 68.Li, H. et al. Comparative transcriptome analysis of the heat stress response in Monochamus alternatus Hope (Coleoptera: Cerambycidae). Front. Physiol.10, 1568 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang, Q. et al. Short-term particulate matter contamination severely compromises insect antennal olfactory perception. Nat. Commun.14, 4112 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li, N., Li, Y., Zhang, S., Fan, Y. & Liu, T. Effect of elevated CO2 concentration and temperature on antioxidant capabilities of multiple generations of Bemisia tabaci MEAM1 (Hemiptera: Aleyrodidae). J. Insect. Physiol.103, 91–97 (2017). [DOI] [PubMed] [Google Scholar]
- 71.Feyereisen, R. Insect CYP genes and P450 enzymes. In Insect Molecular Biology and Biochemistry (ed. Gilbert, L. I.) 236–316 (Academic Press, 2012)
- 72.Badre, N. H., Martin, M. E. & Cooper, R. L. The physiological and behavioral effects of carbon dioxide on Drosophila melanogaster larvae. Comp. Biochem. Physiol. A Mol. Integr. Physiol.140, 363–376 (2005). [DOI] [PubMed] [Google Scholar]
- 73.mechanisms of ligand release. Zubkov, S., Gronenborn, A. M., Byeon, In-Ja. L. & Mohanty, S. Structural consequences of the pH-induced conformational switch in A. polyphemus pheromone-binding protein. J. Mol. Biol.354, 1081–1090 (2005). [DOI] [PubMed] [Google Scholar]
- 74.Mam, B. et al. Influence of pH on indole-dependent heterodimeric interactions between Anopheles gambiae odorant-binding proteins OBP1 and OBP4. Int. J. Biol. Macromol.245, 125422 (2023). [DOI] [PubMed] [Google Scholar]
- 75.Manoharan, M., Fuchs, P. F. J., Sowdhamini, R. & Offmann, B. Insights on pH-dependent conformational changes of mosquito odorant binding proteins by molecular dynamics simulations. J. Biomol. Struct. Dyn.32, 1742–1751 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Clark, T. M., Vieira, M. A. L., Huegel, K. L., Flury, D. & Carper, M. Strategies for regulation of hemolymph pH in acidic and alkaline water by the larval mosquito Aedes aegypti (L.) (Diptera; Culicidae). J. Exp. Biol. 210, 4359–4367 (2007). [DOI] [PubMed]
- 77.Clark, T. M., Flis, B. J. & Remold, S. K. pH tolerances and regulatory abilities of freshwater and euryhaline Aedine mosquito larvae. J. Exp. Biol.207, 2297–2304 (2004). [DOI] [PubMed] [Google Scholar]
- 78.Bobkov, Y. V., Walker, W. B. III. & Cattaneo, A. M. Altered functional properties of the codling moth Orco mutagenized in the intracellular loop-3. Sci. Rep.11, 3893 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Porteus, C. S. et al. Near-future CO2 levels impair the olfactory system of a marine fish. Nat. Clim. Change.8, 737–743 (2018). [Google Scholar]
- 80.Porteus, C. S., Roggatz, C. C., Velez, Z., Hardege, J. D. & Hubbard, P. C. Acidification can directly affect olfaction in marine organisms. J. Exp. Biol.10.1242/jeb.237941 (2021). [DOI] [PubMed] [Google Scholar]
- 81.Rihani, K., Ferveur, J. F. & Briand, L. The 40-Year mystery of insect odorant-binding proteins. Biomolecules11, 509 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abendroth, J. A., Moural, T. W., Wei, H. & Zhu, F. Roles of insect odorant-binding proteins in communication and xenobiotic adaptation. Front. Insect. Sci.3, 1274197 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang, J., Murphy, E. J., Nix, J. C. & Jones, D. N. M. Aedes aegypti odorant-binding protein 22 selectively binds fatty acids through a conformational change in its C-terminal tail. Sci. Rep.10, 3300 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leal, G. M. & Leal, W. S. Binding of a fluorescence reporter and a ligand to an odorant-binding protein of the yellow fever mosquito, Aedes aegypti. F1000Res. 3, 305 (2014). [DOI] [PMC free article] [PubMed]
- 85.Jafari, S. & Alenius, M. Cis-regulatory mechanisms for robust olfactory sensory neuron class-restricted odorant receptor gene expression in Drosophila. PLoS Genet.11, e1005051 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zeng, F., Xu, P. & Leal, W. S. Odorant receptors from Culex quinquefasciatus and Aedes aegypti sensitive to floral compounds. Insect Biochem. Mol. Biol.113, 103213 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bernier, U. R., Kline, D. L., Barnard, D. R., Schreck, C. E. & Yost, R. A. Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Anal. Chem.72, 747–756 (1999). [DOI] [PubMed]
- 88.Bohbot, J. D. et al. Conservation of indole responsive odorant receptors in mosquitoes reveals an ancient olfactory trait. Chem. Senses.36, 149–160 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruel, D. M., Yakir, E. & Bohbot, J. D. Supersensitive odorant receptor underscores pleiotropic roles of indoles in mosquito ecology. Front. Cell. Neurosci.12, 533 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matthews, B. J., McBride, C. S., DeGennaro, M., Despo, O. & Vosshall, L. B. The neurotranscriptome of the Aedes aegypti mosquito. BMC Genomics17, 32 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bohbot, J. D., Sparks, J. T. & Dickens, J. C. The maxillary palp of Aedes aegypti, a model of multisensory integration. Insect Biochem. Mol. Biol.48, 29–39 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Raji, J. I., Konopka, J. K. & Potter, C. J. A spatial map of antennal-expressed ionotropic receptors in the malaria mosquito. Cell Rep.42, 112101 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vulpe, A. & Menuz, K. Ir76b is a co-receptor for amine responses in Drosophila olfactory neurons. Front. Cell. Neurosci.15, 759238 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pitts, R., Derryberry, S. L., Zhang, Z. & Zwiebel, L. J. Variant ionotropic receptors in the malaria vector mosquito Anopheles gambiae tuned to amines and carboxylic acids. Sci. Rep.7, 40297 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ponnusamy, L. et al. Identification of bacteria and bacteria-associated chemical cues that mediate oviposition site preferences by Aedes aegypti. Proc. Natl. Acad. Sci. U.S.A.105, 9262–9267 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Navarro-Silva, M. A., Marques, F. A. & Duque L, J. E. Review of semiochemicals that mediate the oviposition of mosquitoes: a possible sustainable tool for the control and monitoring of Culicidae. Rev. Bras. Entomol.53, 1–6 (2009).
- 97.van Breugel, F., Huda, A. & Dickinson, M. H. Distinct activity-gated pathways mediate attraction and aversion to CO2 in Drosophila. Nature564, 420–424 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.von der Weid, B. et al. Large-scale transcriptional profiling of chemosensory neurons identifies receptor-ligand pairs in vivo. Nat. Neurosci.18, 1455–1463 (2015). [DOI] [PubMed] [Google Scholar]
- 99.Taylor, B. & Jones, M. D. R. The circadian rhythm of flight activity in the mosquito Aedes aegypti (L.): the phase setting effects of light-on and light-off. J. Exp. Biol. 51, 59-70 (1968). [DOI] [PubMed]
- 100.Rinker, D. C. et al. RNAseq in the mosquito maxillary palp: a little antennal RNA goes a long way. Biorxiv10.1101/016998 (2015). [Google Scholar]
- 101.Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B (Methodol.)57, 289–300 (1995). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated are presented in the publication. The transcriptome data generated and analysed during this study is available in the NCBI project database, with BioProject ID: PRJNA1195965. https://www.ncbi.nlm.nih.gov/sra/PRJNA1195965.