Abstract
MYC is dysregulated in approximately 70% of human cancers, strongly suggesting its essential function in cancer. MYC regulates many biological processes, such as cell cycle, metabolism, cellular senescence, apoptosis, angiogenesis, and immune escape. MYC plays a central role in carcinogenesis and is a key regulator of tumor development and drug resistance. Therefore, MYC is one of the most alluring therapeutic targets for developing cancer drugs. Although the search for direct inhibitors of MYC is challenging, MYC cannot simply be assumed to be undruggable. Targeting the MYC-MAX complex has been an effective method for directly targeting MYC. Alternatively, indirect targeting of MYC represents a more pragmatic therapeutic approach, mainly including inhibition of the transcriptional or translational processes of MYC, destabilization of the MYC protein, and blocking genes that are synthetically lethal with MYC overexpression. In this review, we delineate the multifaceted roles of MYC in cancer progression, highlighting a spectrum of therapeutic strategies and inhibitors for cancer therapy that target MYC, either directly or indirectly.
Keywords: MYC, MYC inhibitors, MYC-Regulated biological processes, Oncogenic deregulation, Therapeutic strategies
Introduction
The MYC family consists of the oncogenes MYC (or c-Myc), MYCN, and MYCL.1 Although these three primary MYC family members have similar roles, their expression varies dependent on the kind of tissue and stage of development.2 The activity of MYC is often strictly regulated at the transcriptional, translational, and post-translational levels under physiological conditions, but up to 70% of human malignancies have abnormal expression of MYC, and many of these are extremely aggressive or have poor therapeutic response.3 As a pleiotropic transcription factor, the oncoprotein MYC controls global gene expression involved in many cellular processes, including metabolism, proliferation, senescence, cell metastasis, programmed cell death, angiogenesis, and ribosomal and protein biogenesis.4, 5, 6, 7, 8, 9, 10 In addition, MYC enables cancer cells to escape immune monitor through various methods.11 Moreover, the aberrant expression of MYC is associated with drug resistance in various tumors, emphasizing the need to target this oncogene to improve therapeutic outcomes.12 Due to the significant dysregulation of MYC and its direct involvement in the initiation and progression of cancer, it may be a feasible therapeutic approach to target MYC for the treatment of malignant tumors.13
Due to the inherent disordered structure of the MYC protein, the design of directly targeting MYC is challenging, but it is no longer deemed as an undruggable target.14 Many small molecules that directly target MYC-MAX have been tested in preclinical trials, and the OMO-103 cell-penetrating peptide has shown preliminary clinical efficacy in a phase I clinical trial.15 Alternatively, indirect targeting on MYC has been extensively explored to achieve desired anti-tumor effects.16,17 Studies of the key factors and mechanisms that regulate MYC (e.g., transcription, translation, stability, and activation) will provide new opportunities to treat cancer with targeted therapy. In the present review, we classify the current therapeutic strategies for targeting MYC into four categories: i) targeting the MYC/MAX complex; ii) inhibiting the transcription or translation processes of MYC; iii) decreasing MYC protein stability; iv) blocking genes synthetic lethal with MYC overexpression.
Structure of MYC protein
The MYC protein consists of 439 amino acids and is divided into three primary domains. The N-terminal region (1–143) contains the transactivation domain, the central region is responsible for nuclear localization and stability regulation, and the C-terminal region (357–439) is crucial for interacting with MAX (MYC-associated factor X) and binding to the promoters of target genes (Fig. 1A).18
Figure 1.
The structure and function of MYC protein. (A) MYC is comprised of three domains: the transactivation domain, the central region, and the DNA binding region. 0, I, II, IIIa, IIIb, and IV indicate MB0, MBI, MBII, MBIIIa, MBIIIb, and MBIV, respectively. The boxes illustrate proteins interacting with relevant MYC boxes. TFIIF, general transcription factor IIF subunit 1; AURKA, aurora kinase A; TRRAP, transformation/transcription domain associated protein; WDR5, WD repeat domain 5. (B) MYC and MAX combine to generate heterodimers that enhance the transcription of MYC target genes. GCN5, general control non-repressed 5 protein; TIP60 (KAT5), lysine (K) acetyltransferases 5.
The MYC box (MB) refers to the highly conserved sequences of MYC proteins.18,19 MYC possesses six MBs, MB0, MBI, MBII, MBIIIa, MBIIIb, and MBIV (Fig. 1A). MB0, MBI, and MBII are located within the transactivation domain, while the other MBs present in the central region.19 MYC implements distinct functions via binding to different proteins by MB domains. MB0 enhances transcription by interacting with the TFIIF transcription elongation complex, leading to the promotion of tumor growth.19,20 MB0 has a critical role in MYC-induced apoptosis, regardless of the presence of p53 20. MBI functions as a phosphodegron, participating in the ubiquitination and proteasomal destruction of MYC.21 The phosphorylation of threonine 58 (T58) and serine 62 (S62) within the MYC box I (MBI) region is crucial for controlling the activity and stability of MYC.22 MB0 and MBI interact with Aurora-A kinase, thereby inhibiting the binding of the ubiquitin ligase FBW7 (F-box and WD repeat domain-containing 7) to stabilize MYC protein.23 MBII directly interacts with TRRAP (transformation/transcription domain associated protein), a major component of the STAGA (SPT3-TAF9-GCN5 acetylase) and TIP60 (Tat-interactive protein 60 kDa) histone acetyltransferase complexes.24 The MBIIIa domain forms a connection with the histone deacetylase HDAC3 to suppress transcription.25 The MBIIIb domain interacts with WDR5 to choose its target genes based on genetic and epigenetic factors.26,27 Finally, MBIV is engaged in chromatin binding, facilitating the initiation of apoptosis and G2 cell arrest, hence increasing cell cycle progression.28,29 MBs interact with partners involved in multiple processes and pathways, such as cell proliferation and apoptosis.
The C-terminal domain, which includes the basic region (BR), helix-loop-helix (HLH), and leucine zipper (LZ), is crucial to DNA binding (Fig. 1A). Subsequently, MYC forms a heterodimer with MAX through the conserved BR-HLH-LZ motif, which facilitates the interactions between DNA and proteins.2,30,31 The MYC-MAX heterodimers specifically attach to the conserved E-box DNA sequence (CACGTG) within the transcription regulatory region (promoter and enhancer) of target genes. Lastly, several coactivators, such as TRRAP, GCN5 (general control non-repressed 5 protein), and TIP60/KAT5, are recruited to the E-box elements to initiate transcription activation (Fig. 1B).2,32
The intrinsically disordered regions (IDRs) of MYC perform in a disordered state, characterized by continuous conformational fluctuations.33 In the absence of MAX, MYC performs as an intrinsically disordered protein (IDP).33 The disorder segment of MYC predicted by PONDR® VSL2 is amino acid 146–390. The intrinsic characteristics of IDPs/IDRs have a substantial impact on the onset and formation of liquid–liquid phase separation. Consequently, it is plausible to hypothesize that MYC is engaged in liquid–liquid phase separation and transcriptional regulation.34
Additionally, recent research reveals that in response to proteasome inhibition, heat shock, and transcription elongation perturbation, MYC forms multimers, often in sphere-like structures.35 MYC multimers stabilize the connection of replication fork-associated proteins and chromatin, thereby protecting the integrity of DNA double-stranded structures and promoting tumor cell proliferation under stress conditions.35 Thus, the multimerization of MYC contributes to its carcinogenic properties. The inhibition of MYC multimerization will be an alternative to treat MYC-addicted tumors.
Mechanisms on MYC activation in cancer
The regulation of MYC expression is precisely controlled in normal physiological settings. However, MYC is abnormally expressed in almost 70% of human malignancies.7, 8, 9 Numerous mechanisms, such as gene amplification, chromosomal translocation, retroviral insertion, activation of super enhancers, elevated cell signaling, and post-translational pathways, contribute to the abnormal activation of MYC in cancer cells.36, 37, 38, 39, 40 Genomic alterations, such as gene amplification and chromosomal translocations, can lead to increased MYC expression.40 Gene amplification occurs in 64% of ovarian cancer cases, 45.3% of esophageal cancer cases, 37.2% of squamous lung cancer cases, and 30% of breast cancer cases.36,41 The frequency of amplification of MYC, MYCL, or MYCN is 28% across 33 different forms of human cancer in The Cancer Genome Atlas (TCGA) project.36
In addition to the translocation or amplification, the up-regulation of MYC can be stimulated by multiple signaling pathways (including WNT, phosphoinositide 3-kinase/PI3K, extracellular signal-regulated kinase/ERK, Notch, etc.) at the mRNA or protein level (Fig. 2).42, 43, 44, 45 The activation of the WNT pathway initiates the nuclear translocation of β-catenin to form a complex with T-cell factor/lymphoid enhancer factor (TCF/LEF) to enhance the transcription of MYC.46 MYC is a target of the dysregulated Notch signaling pathways in T-cell leukemia.44 The PI3K/AKT (protein kinase B) signaling pathway enhances the nuclear translation of MYC by activating mechanistic target of rapamycin complex 1 (mTORC1) and prevents the degradation of MYC by inhibiting glycogen synthase kinase 3 beta (GSK3β).43 The RAS-MEK-ERK signaling pathway controls the function and durability of the MYC protein by modifying it through phosphorylation at S62.47 The expression of the MYC protein is minimal, and it has a transient half-life of only 30 min under normal physiological conditions.48 The post-translational modifications, such as phosphorylation and acetylation, increase the protein stability of MYC.49 On the contrary, the protein level of MYC is decreased via E3 ubiquitin ligase (e.g., FBW7 or SKP2/S-phase kinase-associated protein-2) and proteasomal degradation (Fig. 2).40,50 However, in cancer cells, the half-life of MYC is prolonged due to abnormal post-translational modifications, resulting in excessive accumulation.51
Figure 2.
The regulation of MYC activation via multiple signaling pathways. The receptor tyrosine kinases (RTKs) influence MYC translation and protein stability through the PI3K/Akt/mTOR and Ras/ERK signaling pathways, respectively. The WNT and Notch signaling pathways control the transcription of MYC. T58, threonine 58; S62, serine 62; P, phosphorylation; Ub, ubiquitination; PI3K, phosphoinositide 3-kinase; ERK, extracellular signal-regulated kinase; AKT, protein kinase B; mTOR, mechanistic target of rapamycin.
Roles of MYC in tumorigenesis
The oncoprotein MYC regulates numerous cellular processes, including proliferation, metabolism, cellular senescence, cell metastasis, ribosome and protein biosynthesis, programmed cell death, the immune response, and the tumor microenvironment.7,12,52, 53, 54 MYC is associated with drug resistance as well.55, 56, 57 In addition, MYC is involved in the biological activities and maintenance of cancer stem cells.58,59 The precise functions and regulatory mechanisms of MYC require further elucidation, which would be beneficial for precision medicine in cancer treatment.
MYC and cell cycle
MYC enables persistent cell proliferation by forcing cancer cells to re-enter the cell cycle.16,60 Additionally, MYC accelerates cell cycle progression by inhibiting the cell cycle checkpoints.61 MYC activates the transcription of telomerase (TERT), which sustains the proliferation of tumor cells.62 MYC induces critical positive cell-cycle regulators, such as cyclin-dependent kinases (CDKs), cyclins (cyclin A/B/D/E), and E2F transcription factors (E2F1/2/3) (Fig. 3).63,64 In addition to its direct impact on transcription, MYC activates cyclin-CDK complexes by stimulating the production of CDC25 phosphatases and CDK-activating kinase (CAK) (Fig. 3).65
Figure 3.
The regulation of cell cycle by MYC. The pointed arrows indicate that MYC promotes the transcription of cell cycle proteins (cyclin A, cyclin B, cyclin D, and cyclin E) and cell cycle-dependent kinases (CDK1, CDK2, CDK4, and CDK6). The red flat arrows show that MYC inhibits the activity of cell cycle inhibitors, such as p21 and p27. CAK, CDK activating kinase.
Moreover, MYC counteracts the function of CDK inhibitors, such as p21 and p27.66 The cell cycle inhibitors p21 (encoded by CDKN1A/cyclin-dependent kinase inhibitor 1A) and p27 (encoded by CDKN1B/cyclin-dependent kinase inhibitor 1B), play a major role in cell cycle control by blocking the kinase activity of the cyclin-CDK complex and inducing cell proliferation arrest in the G1 phase (Fig. 3).60 MYC represses CDKN1A transcription by binding to the zinc finger transcription factor Miz-1.67 MYC, Miz-1, and the DNA methyltransferase DMNT3A (DNA methyltransferase 3 alpha) form a ternary complex to repress CDKN1A expression by inducing CpG methylation within the promoter of CDKN1A.68 MYC induces miR-221/222 to silence p27 mRNA, resulting in the G1–S transition to promote the cell cycle.60
MYC in metabolic processes
The activation of MYC elevates nutrient uptake, promotes glycolysis and glutaminolysis, increases fatty acid and nucleotide synthesis, and enhances oxidative phosphorylation by facilitating mitochondrial biosynthesis.69 MYC coordinates the metabolic processes in cancer cells to accommodate the dynamic tumor microenvironment.
The regulation of glucose metabolism
The activation of MYC induces cancer cells to preferentially utilize glycolytic metabolism rather than oxidative phosphorylation, even under normoxic conditions, a phenomenon known as the Warburg effect.70 The aerobic glycolysis enables the tricarboxylic acid cycle intermediates to switch from ATP production to the synthesis of lipids, proteins, and nucleic acid precursors.71 In addition, ATP synthesis via the Warburg effect occurs at a significantly higher rate compared with oxidative phosphorylation in the presence of a sufficient glucose supply.71
MYC directly activates the transcription of almost all glycolytic genes, such as enolase 1 (ENO1), phosphofructokinase (PFK), phosphoglucose isomerase (GPI), hexokinase II (HK2), and lactate dehydrogenase A (LDHA), through binding to their E-box sequences.72 It up-regulates the glucose transporter SLC2A1 (solute carrier family 2 member 1) to enhance glucose uptake.73 In addition, MYC regulates lactate export by stimulating the expression of monocarboxylate transporter 1/2 (MCT1/2), hence controlling lactate levels within tumor cell.74 MYC stimulates the expression of glycolytic genes not only by controlling their transcription but also by influencing alternative splicing. For example, MYC maintains a high pyruvate kinase type M2 (PKM2)/pyruvate kinase type M1 (PKM1) ratio to ensure a high flux of glycolysis under aerobic conditions by stimulating the transcription of three splicing factors, namely, heterogeneous nuclear ribonucleoprotein A1/A2 (hnRNPA1/hnRNPA2) and polypyrimidine tract-binding protein (PTB).75 Since PKM2 facilitates the last stage of aerobic glycolysis, whereas PKM1 facilitates oxidative phosphorylation, MYC promotes glycolysis in cancer cells.76
The regulation of amino acid metabolism
MYC up-regulates the expression of solute carrier family 7 member 5 (SLC7A5) and solute carrier family 43 member 1 (SLC43A1), which elevates the uptake of the essential amino acids to promote tumor progression.77 In addition, MYC is essential in the metabolism of glutamine, which is another nutrient for cancer cells.78 MYC enhances glutamine absorption by activating the glutamine transporters SLC1A5 (solute carrier family 1 member 5) and SLC38A5 (solute carrier family 38 member 5).78 MYC suppresses the transcription of miR-23a/b to increase the production of glutaminase 1 (GLS1), resulting in the glutaminolysis increase.79
The regulation of lipid metabolism
MYC enhances the synthesis of citrate, a precursor of fatty acid in de novo synthesis, by increasing the activity of genes upstream of the tricarboxylic acid cycle. Additionally, MYC stimulates the transcription of fatty acid synthase (FASN), ATP citrate lyase (ACLY), acetyl-CoA carboxylase (ACC/ACACA), and stearoyl-CoA desaturase-1 (SCD1), which are involved in the process of fatty acid synthesis.69
The regulation of nucleotide metabolism
MYC increases the levels of glucose-6-phosphate dehydrogenase and transketolase in the pentose phosphate pathway and subsequently enhances the production of ribose 5-phosphate.80 Additionally, it stimulates the production of phosphoribosyl pyrophosphate synthetase 2 (PRPS2) and phosphoribosyl pyrophosphate sequentially. Phosphoribosyl pyrophosphate acts as a framework for de novo purine synthesis and the salvage pathway of pyrimidine synthesis.69 MYC directly activates the catalytic enzymes phosphoribosyl pyrophosphate amidotransferase (PPAT) and phosphoribosyl aminoimidazole succinocarboxamide synthetase to enable nitrogen introduction in purine synthesis.81 During pyrimidine synthesis, MYC stimulates the production of carbamoyl-phosphate synthetase (CAD), an enzyme responsible for catalyzing the initial three processes of pyrimidine biosynthesis. In addition, MYC promotes one-carbon metabolism and the folate cycle, both of which play a role in de novo nucleotide synthesis.82
The regulation of mitochondrial biogenesis
MYC up-regulates mitochondrial specific transcription factors and supports the structural and functional integrity of mitochondria.83 For instance, it up-regulates the expression of complement C1q binding protein (C1QBP), a constituent of the mitochondrial nucleoid, to promote the replication and transcription of mitochondrial DNA.84 In addition, AMPK-related protein kinase 5 (ARK5), a member of the AMP-activated protein kinase (AMPK) family and a target of MYC, plays a role in preserving the integrity of mitochondria and maintaining a balance in bioenergetics.85 SURF-1 (a respiratory complex assembly factor), TIM/TOM (inner/outer membrane transposase), and TFAM (a key regulator of mtDNA transcription and replication), as the targets of MYC, are involved in mitochondrial biogenesis as well.12,86
The regulation of ribosomal and protein biogenesis
MYC directly enhances protein synthesis by up-regulating the expression of various constituents of the protein synthesis apparatus, including ribosomal proteins, translation initiation factors, RNA polymerase III, and rDNA. In addition, MYC stimulates protein biogenesis by enhancing the transcription of genes encoding translation elongation factors, translation initiation factors, nucleolar assembly components, and the small and large ribosomal subunits.87 For example, MYC enhances the process of modifying and processing rRNA by directly controlling ribonucleases, enzymes that change rRNA, and nucleolar proteins involved in the creation of ribosomes, such as nucleophosmin (NPM), dyskerin pseudouridine synthase 1 (DKC1), Nop52, and Nop56.88 Furthermore, a segment of the MYC protein is found in the nucleolus and directly regulates the production of rRNA by attaching to E-box elements found in the rDNA promoter.87
The effects of MYC on the immune response and tumor microenvironment
MYC modifies the tumor microenvironment to enable cancer cells to escape from immune surveillance. Additionally, MYC stimulates the proliferation of stromal cells and induces angiogenesis.89 MYC is involved in the recruitment of various cytokines from the tumor microenvironment to promote the transition to a more aggressive and metastatic tumor phenotype.89
MYC in angiogenesis
Vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α (HIF-1α) are crucial cytokines in angiogenesis, and their levels are increased when MYC is activated.90,91 HIF-1α governs the migration of macrophages to hypoxic areas inside the tumor and controls the expression of several genes implicated in tumor angiogenesis.90 MYC activation leads to the up-regulation of VEGF, which leads to loss of vascular permeability and integrity, therefore, VEGF is considered the main causative factor of MYC-induced angiogenesis.90 MYC up-regulates the G-protein-coupled adenosine A2B receptor (ADORA2B), which promotes angiogenesis by triggering the production of VEGF and the endothelial nitric oxide synthase (eNOS).91 MYC activates angiogenesis by increasing the expression of interleukin (IL)-1β, as well as by inhibiting thrombospondin-1 (TSP-1), which facilitates nutrient delivery to the cancer cells.90
Moreover, several microRNAs regulated by MYC are implicated in angiogenesis. The activation of miR-9 in human glioma is facilitated by MYC, leading to the promotion of angiogenesis through the HIF-1α/VEGF axis.92 The miR-17-92 cluster effectively promotes angiogenesis by directly inhibiting the expression of anti-angiogenic molecules, such as TSP-1 and connective tissue growth factor (CTGF).93
MYC and immune evasion
The immune cells infiltrated in tumor microenvironment include tumor-associated macrophages, dendritic cells, myeloid-derived suppressor cells, T cells, B cells, natural killer cells, neutrophils, etc.94 Upon the activation of MYC, not only more macrophages are infiltrated into the tumor, but also T, B, and natural killer cells are excluded from the tumor microenvironment.89
MYC promotes the expression of immune-checkpoint proteins, including cluster of differentiation 47 (CD47) and programmed cell death ligand 1 (PD-L1), to inhibit both innate and adaptive immune responses.11 Moreover, MYC causes cancer cells to secrete immune-inhibitory cytokines.95 Transforming growth factor-β (TGFβ) ligands, for instance, inhibit the function of cytolytic T cells.95 Furthermore, by transcriptionally suppressing signal transducer and activator of transcription 1/2 (STAT1 and STAT2) and the type I interferon pathway, MYC impairs natural killer cell-mediated immune surveillance.96 In addition, MYC increases the expression of many cytokines, including IL-23 and C–C motif chemokine ligand 2/9 (CCL2 and CCL9), which promote the recruitment of immunosuppressive macrophages and decrease the recruitment and activation of natural killer cells, B cells, and CD4+/CD8+ T cells.97
MYC and senescence
The suppression of MYC induces cellular senescence in different types of cancer cells, including osteosarcoma and hepatocellular carcinoma.98 MYC enables cancer cells to resist senescence by being dependent on cell cycle protein-dependent kinase 2 (CDK2).99 MYC inhibits senescence by activating long noncoding RNA (lncRNA) USP2-AS1 to stabilize E2F1 mRNA.100
MYC and cell metastasis
Metastasis requires the coordination of various molecules involved in invasion, chemotaxis, and contractile activity.101 MYC overexpression leads to the enhanced transcription of ezrin to induce the up-regulation of downstream genes, such as Akt and Ras homolog family member A (RhoA), which play an important role in cell invasion.102
MYC-nick is a truncated form of full-length MYC cleaved by the endogenous proteasome.103 MYC-nick enhances the production of fascin (an actin-bundling protein) and stimulates the activation of cell division cycle 42 (Cdc42) to rearrange the actin cytoskeleton to form filopodia, facilitating cell migration.103 In addition, MYC promotes cell migration by increasing the expression of genes associated with epithelial-to-mesenchymal transition, such as galectin 1 (LGALS1), osteopontin (OPN), and SNAIL.12,104 However, a study in Drosophila and lung adenocarcinoma cell lines discovered that MYC impedes migration and invasion mediated by Ras and lethal giant larvae (Lgl).105 The binding between urokinase (uPA) and urokinase receptor (uPAR) initiates the reorganization of cytoskeletal structure to impede cells' invasion into the extracellular matrix.106 MYC suppresses the expression of uPA and uPAR in cancer cells to stimulate metastasis.107
MYC and programmed cell death mechanisms
MYC is involved in various types of programmed cell death, including apoptosis, autophagy, pyroptosis, and ferroptosis. MYC regulates cell apoptosis through two main pathways: BCL-2 (B-cell lymphoma 2) pathway and p53 pathway. MYC inhibits BCL-2 and BCL-XL (BCL2 like 1) by promoting the transcription of BIM (BCL-2–interacting mediator of cell death), ultimately inducing apoptosis.108,109 The downstream activation of p53 is important for the apoptotic induction by MYC as well.108 The activation of MYC results in the overexpression of ADP-ribosylation factor (ARF), which enhances the levels of p53 to trigger apoptosis.12 MYC inhibition leads to defects in autophagosome formation and reduced delivery of autophagic substrates.110 Knockdown of MYC down-regulates the transcription of autophagy-related protein 7 (Atg7) and LC3-I (a cytosolic form of 1A/1B-light chain 3)/LC3-II (LC3-phosphatidylethanolamine conjugate), resulting in autolysosomal degradation.111 In multiple myeloma cells, the transcriptional inhibition on MYC results in caspase-1-dependent pyroptosis.112 By blocking nuclear receptor coactivator 4 (NCOA4)-mediated ferritin autophagy, MYC prevents ovarian cancer cells from ferroptosis.112 MYC also activates the expression of lymphoid-specific helicase, which inhibits ferroptosis.113
MYC and drug resistance
The expression of ATP-binding cassette (ABC) transporters, especially the P-glycoprotein (P-gp), can cause resistance to targeted chemotherapy and cytotoxic.114 MYC overexpression leads to the activation of several intermediate factors, such as small nucleolar RNA host gene 12 (SNHG12), HIF-1α, nuclear factor erythroid 2-related factor 2 (Nrf2), and miR-20a, which increase the levels of P-glycoprotein (P-gp) and promote the development of multidrug resistance.55, 56, 57 MYC also interacts with bromodomain PHD finger transcription factor (BPTF) to increase the expression of ABC transporter, resulting in multidrug resistance.115
The therapeutic exploration of MYC
Historically, most of the scientific community believed that MYC was essentially “undruggable”. Due to its intrinsically disordered regions, lacking binding pockets, and its sequestration within the nuclear compartment, it is challenging to explore MYC as a therapeutic target.116 Recently, the covalent ligand targeting of intrinsically disordered proteins and the antibody delivery system to the nucleus have been developed.117 In the absence of conventional binding pockets, drugs can bind to MYC proteins via cysteine-responsive covalent ligands.117,118 In 2023, scientists developed biodegradable silica nanocapsules to transfer antibodies directly to the nucleus for cancer therapy.119 This nuclear delivery system has the potential to be applied to the MYC targeting to cure cancer.
Although targeting MYC is challenging, it cannot simply be assumed that MYC is “undruggable”.14 Currently, effective approaches to MYC targeting include interfering with the MYC-MAX complex, blocking MYC transcription and translation, promoting MYC proteasomal degradation, and utilizing synthetic lethality. Next, we outline various approaches to targeting MYC, along with recent advances in MYC inhibitors (Table 1).
Table 1.
Summary on MYC targeting agents and the mechanisms of action (MOA).
| Strategy | MOA | Agents | Malignancy | Preclinical/Clinical stage | References |
|---|---|---|---|---|---|
| Targeting the MYC-MAX complex directly | Blocking MYC-MAX interaction | Omomyc (OMO-103) | Advanced solid tumors | Phase I/II: NCT04808362 |
15 |
| 3jc48-3 | Patient-derived prostate cancer xenografts (PDX) mouse models | Preclinical | 121 | ||
| KJ-Pyr-9 | MDA-MB-231 breast cancer CDX model | Preclinical | 123 | ||
| MYCMI-6 | Neuroblastoma xenograft tumor model | Preclinical | 124 | ||
| MYCi361 and MYCi975 | MyC-CaP allograft/xenograft mouse prostate model | Preclinical | 122 | ||
| EN4 | MDA-MB-231 breast cancer CDX model | Preclinical | 118 | ||
| Stabilizing MAX-MAX homodimers | KI-MS2-008 | T-cell acute lymphoblastic leukemia and hepatocellular carcinoma mouse models. |
Preclinical | 125 | |
| Blocking MYC-MAX from binding to DNA | KSI-3716 | Orthotopic bladder xenografts | Preclinical | 127 | |
| ME47 | Breast cancer CDX model | Preclinical | 126 | ||
| Silencing the transcription of MYC | Stabilizing G-quadruplex DNA | CX-5461 | Solid tumors | Phase I: NCT02719977 | 130 |
| IZCZ-3 | SiHa, HeLa, Huh7, A375 cell lines and human cervical squamous | Preclinical | 131 | ||
| QN-1 | Triple-negative breast cancer (TNBC) mouse model | Preclinical | 132 | ||
| IZTZ-1 | Breast cancer xenograft mouse model | Preclinical | 133 | ||
| BRD4 inhibitors | ZEN-3694 | Metastatic castration-resistant prostate cancer (mCRPC) | Phase I/II: NCT02711956 | 137 | |
| OTX015/MK-8628 | Advanced solid tumors | Phase I: NCT02698176 Phase II: NCT02296476 |
140 | ||
| AZD5153 | Malignant solid tumors, lymphoma and breast cancer | Phase I: NCT03205176 | 138,139 | ||
| GSK525762/I-BET762 | Neoplasms | Phase I/II: NCT01943851 | 141 | ||
| CDK7 inhibitors | CT7001 (samuraciclib) | Advanced solid malignancies | Phase I: NCT03363893 | 146 | |
| LY3405105 | Solid tumors | Phase I/II: NCT03770494 | 148 | ||
| SY-1365 | Advanced solid tumors | Phase I: NCT03134638 | 149 | ||
| SY-5609 | Advanced solid tumors | Phase I: NCT04247126 | 150 | ||
| CDK9 inhibitors | KB-0742 | Relapsed or refractory solid tumors | Phase I/II:NCT04718675 | 151 | |
| CYC065 | Advanced cancers | Phase I: NCT02552953 | 153 | ||
| SCH 727965 (Dinaciclib) | Advanced breast and lung Cancers | Phase II: NCT00732810 | 154 | ||
| Inhibiting the MYC protein biosynthesis | mTOR inhibitors | MLN0128 | Metastatic castration-resistant prostate cancer | Phase II: NCT02091531 | 156 |
| Rapamycin | Lymphangioleiomyomatosis | clinical use | 155 | ||
| Temsirolimus and everolimus | Advanced renal cell carcinoma (RCC) | clinical use | 155 | ||
| eIF4A inhibitor | eFT226 | Solid tumors | Phase I-II: NCT04092673 |
158 | |
| Decreasing the MYC stability | USP7 inhibitors | XL177A | Ewing sarcoma and malignant rhabdoid tumor (MRT) cell lines | Preclinical | 162 |
| GNE-6640, GNE-6776 | EOL-1 xenograft models | Preclinical | 163 | ||
| FT671 | Colorectal carcinoma (HCT116) or bone osteosarcoma (U2OS) cell lines | Preclinical | 164 | ||
| USP36 inhibitor | Cinobufotalin | Colon cancer cell lines and xenograft models | Preclinical | 165 | |
| PP2A activators | SMAP | KRAS-driven non-small cell lung cancer and triple-negative breast cancer xenograft models | Preclinical | 168,169 | |
| PLK1 inhibitors | BI6727 | NSCLC | Phase II: NCT00824408 | 171 | |
| BI2356 | Neoplasms | Phase I: NCT02211872 | 172 | ||
| NMS-1286937 | Advanced solid tumors | Phase I: NCT01014429 | 173 | ||
| GSK461364 | Advanced solid tumors or lymphoma | Phase I: NCT00536835 | 174 | ||
| PIN1 inhibitor | Sulfopin | Murine models of neuroblastoma and pancreatic cancer | Preclinical | 177 | |
| PROTAC | PROTAC targeting MYC | TNBC cells | Preclinical | 179 | |
| ProMyc | Xenograft tumor models | Preclinical | 180 | ||
| Targeting synthetic-lethal genes with MYC | CDK1 inhibitor | RO-3306 | Ovarian cancer cells and a transgenic mouse model of ovarian cancer | Preclinical | 184 |
| CHK1 inhibitors | CBP501 | Advanced solid tumors | Phase I: NCT03113188 | 187 | |
| LY2603618 | Non-small cell lung cancer | Phase II: NCT00988858 | 188 | ||
| AR323 and AR678 | Melanoma cell lines | Preclinical | 189 | ||
| PRKDC inhibitor | Peposertib (M3814) | Advanced solid tumors or chronic lymphocytic leukemia | Phase I: NCT02316197 | 190 | |
| ATR inhibitor | Ceralasertib (AZD6738) | Advanced solid tumors | Phase I: NCT04497116 | 191 | |
| IMPDH2 inhibitor | AVN-944 | Refractory solid tumors | Phase I: NCT00923728 | 192 |
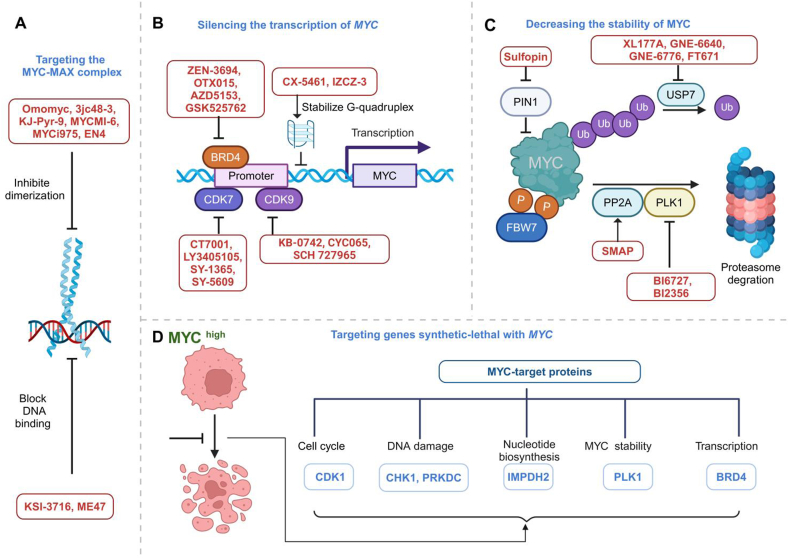
Targeting the MYC-MAX complex
The MYC-MAX complex binds to DNA to activate the target gene's transcription.2 To inhibit MYC signaling, small molecules have been developed to target the interface between MYC and MAX. These molecules can stabilize the MAX-MAX homodimer or disrupt the binding of MYC-MAX to DNA (Fig. 4A). The only direct inhibitor of MYC in clinical phase I/II trials is Omomyc (OMO-103), which heterodimers with MAX to prevent transcription of MYC targets.120 Recently, results from a dose-escalation phase I study in solid tumors showed initial indications of the safety and the drug activity of OMO-103.15 3jc48-3 was well tolerated and effective in reducing tumor growth rates in mouse models.121 The MYC inhibitors 361 and 975 (MYCi361 and MYCi975) exhibit substantial anti-tumor effectiveness by disrupting the MYC-MAX and promoting MYC phosphorylation on threonine-58.122 Compared with MYCi361, MYCi975 showed increased tolerance at significantly higher doses.122 KJ-Pyr-9 and MYCMI-6, small molecules inhibiting MYC-MAX interactions, induce massive apoptosis and reduce proliferation in xenograft mouse models with MYC-driven tumors.123,124 EN4, which was found through a cysteine-responsive covalent ligand screen, targets cysteine 171 (C171) within the intrinsically disordered region of MYC.118 In the MDA-MB-231 breast cancer cell line-derived xenograft model, EN4 decreased MYC-MAX DNA binding, resulting in compromised cell proliferation and tumor growth in vivo.118 The compound KI-MS2-008 stabilizes the MAX–MAX homodimers to decrease the expression of MYC target genes and decreases tumor growth in vivo.125
Figure 4.
The therapeutic strategies for targeting MYC. (A) Small compounds directly suppress MYC by preventing MYC-MAX from dimerizing or by preventing MYC-MAX from binding to specific DNA regions. (B) Indirect targeting of MYC by silencing the transcription of MYC. (C) Indirect targeting of MYC by decreasing the stability of MYC. (D) Inhibiting synthetic lethal genes with MYC. BRD4, bromodomain-containing protein 4; PP2A, protein phosphatase 2A; PLK1, polo-like kinase-1; CDK, cyclin-dependent kinase; CHK1, checkpoint kinase 1; IMPDH, inosine-5-monophosphate dehydrogenase; GLS, glutaminase; Me, methylation; P, phosphorylation; Ub, ubiquitylation.
Unlike small compounds that impede heterodimerization, compound KSI-3716 and ME47 specifically prevent the binding of MYC-MAX to DNA.126,127 In a cell line-derived xenograft model of breast cancer, ME47 decreases cell proliferation, slows tumor growth, and increases survival.126 In murine orthotopic bladder xenografts, KSI-3716 suppresses tumor growth without significant systemic toxicity.127
Silencing the transcription of MYC
The transcription of MYC can be inhibited in two ways: the first is to stabilize the MYC G-quadruplex structure, and the second is to target the transcriptional complex (Fig. 4B). The G-quadruplex structure is a noncanonical DNA structure near the promoter region of oncogenes, such as MYC. The three-dimensional structure of G-quadruplex provides a natural binding pocket for small molecules to inhibit transcription.128 G-quadruplexes regulate gene expression, including 90% of MYC expression, thereby making them potential drug targets for MYC addictive cancers.128 Small molecules and peptides have been identified to bind to and stabilize the MYC G4 structure, such as CX-5461, IZCZ-3, QN-1, and IZTZ-1.129 For the treatment of solid cancers, CX-5461 was well tolerated in a phase I clinical trial.130 The three remaining inhibitors showed significant anti-tumor effects in both cancer cell lines and mouse models.131, 132, 133
Super-enhancers (SEs) are clusters of enhancers densely occupied by transcription factors and chromatin regulators.134 In addition to SEs, the transcription of MYC is regulated by a variety of transcriptional complexes, such as CDKs, bromodomain and extra-terminal (BETs) domains, and RNA polymerase II.135 Bromodomain-containing protein 4 (BRD4), a member of BETs, plays a major role in MYC transcription.136 BRD4 stimulates the transcriptional activation and elongation of MYC via the recruitment of positive transcription elongation factor b (P-TEFb).136 Several BET inhibitors are currently being evaluated in phase I or phase II clinical trials for cancer treatment, including ZEN-3694, OTX015/MK-8628, AZD5153, and GSK525762/I-BET762.137, 138, 139, 140, 141 Due to the limited therapeutic activity of BET inhibitors, it is alternative to combine with other epigenetic reagents.142
Unlike the traditional cell cycle CDKs mainly involved in cell cycle transition, CDK7 and CDK9 play crucial roles in the initiation and elongation of transcription.143,144 CDK7 functions as the catalytic component of the transcription factor IIH complex (TFIIH), while CDK9 serves as the kinase component of P-TEFb.143,144 CDK7 and CDK9 phosphorylate the serine residues on the carboxy-terminal of RNA polymerase II, which helps to initiate transcription, release pauses, and facilitate elongation efficiently.145 The CDK7 inhibitor, CT7001, has undergone clinical phase I trials in advanced breast cancer with an acceptable safety profile and preliminary evidence of efficacy.146 In addition, the CDK7 inhibitors LY3405105, SY-1365, and SY-5609 have entered phase I/II clinical trials in solid tumors.147, 148, 149, 150 CDK9 inhibitors, such as KB-0742, CYC065, and SCH 727965 (dinaciclib) have demonstrated anti-tumor activity in vivo and are currently being investigated in phase I/II clinical trials.151, 152, 153, 154
Inhibition of the MYC protein biosynthesis
Under typical physiological circumstances, mTORC1 phosphorylates eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4EBP1) to release the eukaryotic initiation factor 4G (eIF4G)-binding sites in eIF4E and start the translation of MYC. Rapamycin, a pharmaceutical inhibitor of mTORC1, decreases the expression of MYC and has been in clinical use.155 Rapamycin derivatives, temsirolimus and everolimus, retain the skeletal structure of rapamycin but have improved solubility and pharmacokinetic properties.155 MLN0128 (sapanisertib), a novel mTOR inhibitor as the next generation of rapamycin and its analogues, has been evaluated in phase II clinical trials, but its efficacy is limited.156
The helicase activity of eukaryotic initiation factor 4A (eIF4A) is crucial for overcoming structural barriers caused by G-quadruplexes, therefore ensuring the continuous translation of MYC mRNA.157 eFT226, an inhibitor of eIF4A helicase, effectively reduces MYC protein levels without affecting MYC mRNA levels and is in a phase I-II clinical trial.158 This study has shown that eFT226 is effective in advanced solid tumors.
Decrease in MYC stability
The stability of MYC is controlled by phosphorylation and ubiquitination. Phosphorylation of Ser62 enhances the stability of MYC, while phosphorylation of Thr58 leads to its destruction.49 The MYC protein is tightly regulated by the ubiquitin-proteasome system, leading to a relatively short half-life of approximately 30 min under normal physiological conditions. MYC proteins are degraded by the ubiquitin-proteasome system in two stages. First, poly-ubiquitin is covalently tagged onto the target protein. Second, the 26S proteasome recognizes and accepts the tagged protein with the right ubiquitin linkages. Then, the tagged protein is deubiquitinated, unfolded, and degraded into tiny peptide fragments by the proteasome.49
One strategy for MYC degradation is to target deubiquinating enzymes (DUBs).159 DUBs, including USP7, USP13, USP22, USP28, USP36, USP37, and USP43, deubiquitinate and stabilize MYC family proteins.160,161 The inhibition of these DUBs increases the proteasomal degradation of MYC and reduces the transcription of MYC-driven genes.160 The inhibitors targeting the above DUBs are currently in preclinical studies. USP7 inhibitors, including GNE6776, XL177A, FT671, and GNE-6640, effectively hinder the proliferation of many cancer cell lines and impede tumor growth in xenograft models.162, 163, 164 The newly discovered USP36 inhibitor, cinobufotalin, suppresses the malignant phenotypes of colon cancer cells both in vitro and in vivo.165 For the remaining five DUBs, no specific inhibitors have yet been reported.
T58 undergoes phosphorylation by GSK3β as a result of a complicated signaling cascade.49 The cascade is triggered by mitogen-activated protein kinases (MAPKs) and CDKs, which cause the addition of a phosphate group to serine 62 (S62) on MYC. GSK3β phosphorylates T58 after the phosphorylation of S62. However, the dephosphorylation of S62 by the protein phosphatase PP2A (protein phosphatase 2A) is necessary to recognize MYC by F-box and WD repeat domain-containing 7 (FBW7).49,166 Several methods have been explored to improve the degradation of MYC, including the inhibition of PI3K to promote the action of GSK3β and using small-molecule activators of PP2A (Fig. 4C).167 SMAP (small-molecule activators of PP2A) treatment inhibited tumor growth in xenograft models of KRAS-driven non-small cell lung cancer and triple-negative breast cancer.168,169
By directly binding and phosphorylation, Polo-like kinase-1 (PLK1) promotes FBW7's polyubiquitination and destruction, resulting in the stabilization of MYC (Fig. 4C).170 There have been more than a dozen available PLK1-specific inhibitors, of which BI6727, BI2356, NMS-1286937, and GSK461364, have entered clinical trials in solid tumors. Although they have acceptable safety profiles, their efficacy is limited and they may induce drug resistance.171, 172, 173, 174, 175 Additionally, PIN1 is a member of the peptidylprolyl cis/trans isomerase (PPIase) family and increases the stability and transcriptional activity of MYC (Fig. 4C).176 Sulfopin, a covalent inhibitor of PIN1, reduces tumor progression in mouse models of neuroblastoma and pancreatic cancer.177
As a novel drug design strategy, proteolysis targeting chimera (PROTAC) is being explored to cure cancer.178 A PROTAC based on TNA (threose nucleic acid) and DNA has been developed to effectively target and degrade MYC in triple-negative breast cancer cells, which provides a promising therapeutic intervention for triple-negative breast cancer.179 In addition, a recent study identified a specific anti-MYC aptamer MA9C1 and further developed a multifunctional aptamer-based PROTAC for the proteolysis of MYC (ProMyc).180 ProMyc not only significantly degrades MYC, but also reduces MAX proteins subsequently.180 The circular PA1-ProMyc chimeras achieved tumor regression in xenograft tumor models, laying the foundation for the clinical development of effective MYC degraders.180
Targeting genes synthetic lethal with MYC overexpression
Synthetic lethal interactions occur between two genes when the individual perturbation of either gene makes the cell/organism viable, but the simultaneous perturbation of both genes results in lethality.181 Synthetic lethality has the potential to selectively target oncogenic MYC to minimize harm to normal cells.
The crucial factor in harnessing synthetic lethal interactions for cancer therapy lies in the discovery of synthetic lethal genes (Fig. 4D). Large-scale genetic screens have explored noncanonical sensitivities in MYC-driven cancers, and the range of potential synthetic lethal targets is expanded. With the ongoing advancement of high-throughput screening technologies, over a hundred candidate genes could be synthetically lethal with the dysregulated oncoprotein MYC.182
Potential synthetic lethal targets are genes that function together with MYC to promote cell cycle progression or metabolism. CDK1 is a synthetic lethal target of MYC in cancer cells.183 CDK1 inhibitor RO-3306 has anti-tumor effects in ovarian cancer cells and mouse xenograft models implanted with ovarian cancer cells.184 MYC has been found to target DNA damage checkpoint regulators, such as checkpoint kinase 1 (CHK1), TPX2 (microtubule nucleation factor), catalytic subunit (PRKDC), and ataxia telangiectasia and Rad3-related protein (ATR), as synthetic lethal targets to counteract the effects of increased proliferation-related elevated mitotic stress and DNA damage.185,186 CBP501 and LY2603618, the CHK1 inhibitors, had acceptable safety and pharmacokinetic profiles in clinical phase I trials, but they did not present promising anti-cancer effects in clinical phase II trials.187,188 More CHK1 inhibitors, such as AR323 and AR678, are still in preclinical development.189 The PRKDC inhibitor peposertib and ATR inhibitor ceralastetib were well tolerated in phase I clinical trials.190,191
Since MYC-driven tumors heavily depend on glucose and glutamine, the simultaneous suppression of both glycolysis and glutaminolysis could potentially provide a promising synergistic treatment for MYC-driven malignancies. Enhanced production of nucleic acid and protein is required to sustain MYC-induced cellular proliferation. Inosine-5-monophosphate dehydrogenase 2 (IMPDH2) is a crucial enzyme involved in nucleotide biosynthesis and is identified as a synthetic lethal target of MYC.182 The inhibitor of IMPDH2, AVN-944, is currently in a phase I clinical trial, under the evaluation of safety and efficacy.192 Numerous genes described above that regulate MYC expression and stability have also been identified as synthetic lethal targets for MYC-driven tumors, including PLK1 and BRD4.182 Studies on inhibitors of PLK1 and BRD4 have been described previously.
The development of medications that target MYC has made significant strides. However, due to the extensive influence of MYC on cells, it is crucial to assess the side effects of MYC inhibition in cancer treatment. The mice with MYC knockout exhibit early aging and their age-sensitive abilities decline, but they also live noticeably longer than wild-type mice and have a 3-to-4-fold lower cancer incidence.193 MYC knockout resulted in electron transport chain dysfunction and increased production of reactive oxygen species.193 The mitochondrial abnormalities may potentially accelerate aging in MYC knockout mice.193 Therefore, we need to be mindful of the possible adverse effects of premature aging and mitochondrial dysfunction, particularly when MYC inhibitors are applied in children's cancers.
Conclusions
MYC is a potential therapeutic target for cancer since it controls both the immune system's responses and the intrinsic development of tumor cells. Cancer growth is slowed in a number of preclinical cancer models when MYC expression or activity is removed. We outline many approaches to cancer treatment that target MYC both directly and indirectly. Nevertheless, several challenges still exist in the therapeutic exploration of MYC. First, the development and optimization of direct MYC inhibitors are still hampered by the intricate structure of the MYC protein. Recent developments in protein structure prediction could be a significant step forward in the improvement of imperfect crystallographic data. However, most research institutions may find it difficult to meet the increasing demand for computer resources, and no model can now fully understand the intricacy of intrinsically disordered domains. Secondly, it is both chemically and pharmacologically feasible to suppress MYC expression by targeting the regulatory factors. However, a lot of other genes or proteins are targeted simultaneously as well, and this pleiotropic effect makes it difficult to find drugs with minimal side effects. Targeting the stability of the MYC protein with selectivity is also difficult. Therefore, it is debatable if these techniques' ability to selectively target MYC is what makes them therapeutically effective. Third, due to MYC’s interactions with so many distinct proteins, which are often context-dependent, the effects of indirect MYC inhibition are probably specific to certain tumor types. Ultimately, given MYC's pervasive role in cancer, it is crucial to contemplate the potential adverse effects of MYC inhibition, such as premature aging.
Combination therapies are commonly used to enhance the efficacy of cancer treatment, as resistance to single therapies has become a significant issue. Emerging treatments that target MYC, its downstream genes, or synthetic lethal partners could potentially be integrated into future clinical strategies for patient stratification and the selection of combination therapy regimens.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 32270610, 82072499, 31801094 to Chunyan Li) and the Fundamental Research Funds for the Central Universities (China) (No. YWF-21-BJ-J-T105 to Chunyan Li).
CRediT authorship contribution statement
Yingying Duan: Investigation, Visualization, Writing – original draft, Writing – review & editing. Zhaoshuo Liu: Visualization. Qilin Wang: Investigation, Writing – review & editing. Junyou Zhang: Investigation, Writing – review & editing. Jiaxin Liu: Writing – review & editing. Ziyi Zhang: Writing – review & editing. Chunyan Li: Conceptualization, Funding acquisition, Writing – review & editing.
Declaration of Generative AI and AI-assisted technologies in the writing process
During the preparation of this work, the authors used Kimi to improve the writing and readability of some sentences in the manuscript. After using these tools, the authors reviewed and edited the content as needed. The authors take full responsibility for the content of the publication.
Conflict of interests
The authors declared no conflict of interests.
Acknowledgements
We apologize for not including all relevant publications due to space limitations. We deeply thank Caixia Guo and Ceshi Chen for their helpful suggestions during the manuscript preparation. All figures are created with BioRender.com.
Footnotes
Peer review under the responsibility of the Genes & Diseases Editorial Office, in alliance with the Association of Chinese Americans in Cancer Research (ACACR, Baltimore, MD, USA).
References
- 1.Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R.C., Croce C.M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adhikary S., Eilers M. Transcriptional regulation and transformation by Myc proteins. Nat Rev Mol Cell Biol. 2005;6(8):635–645. doi: 10.1038/nrm1703. [DOI] [PubMed] [Google Scholar]
- 3.Llombart V., Mansour M.R. Therapeutic targeting of "undruggable" MYC. EBioMedicine. 2022;75 doi: 10.1016/j.ebiom.2021.103756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelengaris S., Khan M. The many faces of c-MYC. Arch Biochem Biophys. 2003;416(2):129–136. doi: 10.1016/s0003-9861(03)00294-7. [DOI] [PubMed] [Google Scholar]
- 5.de la Cova C., Abril M., Bellosta P., Gallant P., Johnston L.A. Drosophila myc regulates organ size by inducing cell competition. Cell. 2004;117(1):107–116. doi: 10.1016/s0092-8674(04)00214-4. [DOI] [PubMed] [Google Scholar]
- 6.Bettess M.D., Dubois N., Murphy M.J., et al. c-Myc is required for the formation of intestinal crypts but dispensable for homeostasis of the adult intestinal epithelium. Mol Cell Biol. 2005;25(17):7868–7878. doi: 10.1128/MCB.25.17.7868-7878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dang C.V. A time for MYC: metabolism and therapy. Cold Spring Harbor Symp Quant Biol. 2016;81:79–83. doi: 10.1101/sqb.2016.81.031153. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu M.E., Castillo F., Soucek L. Structural and biophysical insights into the function of the intrinsically disordered Myc oncoprotein. Cells. 2020;9(4):1038. doi: 10.3390/cells9041038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll P.A., Freie B.W., Mathsyaraja H., Eisenman R.N. The MYC transcription factor network: balancing metabolism, proliferation and oncogenesis. Front Med. 2018;12(4):412–425. doi: 10.1007/s11684-018-0650-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juan L.S., Cagigal M.L., Fernandez-Flores A., Mayorga M., Gandarillas A. Protooncogene MYC drives human melanocyte melanogenesis and senescence. Cancer Gene Ther. 2022;29(8–9):1160–1167. doi: 10.1038/s41417-021-00424-3. [DOI] [PubMed] [Google Scholar]
- 11.Casey S.C., Tong L., Li Y., et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352(6282):227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan K.I., Zhang S., Li G., et al. MYC oncogene: a druggable target for treating cancers with natural products. Aging Dis. 2024;15(2):640–697. doi: 10.14336/AD.2023.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carabet L.A., Rennie P.S., Cherkasov A. Therapeutic inhibition of Myc in cancer. structural bases and computer-aided drug discovery approaches. Int J Mol Sci. 2018;20(1):120. doi: 10.3390/ijms20010120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X.N., Su X.X., Cheng S.Q., Sun Z.Y., Huang Z.S., Ou T.M. MYC modulators in cancer: a patent review. Expert Opin Ther Pat. 2019;29(5):353–367. doi: 10.1080/13543776.2019.1612878. [DOI] [PubMed] [Google Scholar]
- 15.Garralda E., Beaulieu M.E., Moreno V., et al. MYC targeting by OMO-103 in solid tumors: a phase 1 trial. Nat Med. 2024;30(3):762–771. doi: 10.1038/s41591-024-02805-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baluapuri A., Wolf E., Eilers M. Target gene-independent functions of MYC oncoproteins. Nat Rev Mol Cell Biol. 2020;21(5):255–267. doi: 10.1038/s41580-020-0215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang C., Zhang J., Yin J., et al. Alternative approaches to target Myc for cancer treatment. Signal Transduct Targeted Ther. 2021;6(1):117. doi: 10.1038/s41392-021-00500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conacci-Sorrell M., McFerrin L., Eisenman R.N. An overview of MYC and its interactome. Cold Spring Harb Perspect Med. 2014;4(1) doi: 10.1101/cshperspect.a014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalkat M., Resetca D., Lourenco C., et al. MYC protein interactome profiling reveals functionally distinct regions that cooperate to drive tumorigenesis. Mol Cell. 2018;72(5):836–848.e7. doi: 10.1016/j.molcel.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q., West-Osterfield K., Spears E., Li Z., Panaccione A., Hann S.R. MB0 and MBI are independent and distinct transactivation domains in MYC that are essential for transformation. Genes. 2017;8(5):134. doi: 10.3390/genes8050134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yada M., Hatakeyama S., Kamura T., et al. Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7. EMBO J. 2004;23(10):2116–2125. doi: 10.1038/sj.emboj.7600217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sears R., Nuckolls F., Haura E., Taya Y., Tamai K., Nevins J.R. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14(19):2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richards M.W., Burgess S.G., Poon E., et al. Structural basis of N-Myc binding by Aurora-A and its destabilization by kinase inhibitors. Proc Natl Acad Sci USA. 2016;113(48):13726–13731. doi: 10.1073/pnas.1610626113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMahon S.B., Van Buskirk H.A., Dugan K.A., Copeland T.D., Cole M.D. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell. 1998;94(3):363–374. doi: 10.1016/s0092-8674(00)81479-8. [DOI] [PubMed] [Google Scholar]
- 25.Kurland J.F., Tansey W.P. Myc-mediated transcriptional repression by recruitment of histone deacetylase. Cancer Res. 2008;68(10):3624–3629. doi: 10.1158/0008-5472.CAN-07-6552. [DOI] [PubMed] [Google Scholar]
- 26.Chen T., Li K., Liu Z., et al. WDR5 facilitates EMT and metastasis of CCA by increasing HIF-1α accumulation in Myc-dependent and independent pathways. Mol Ther. 2021;29(6):2134–2150. doi: 10.1016/j.ymthe.2021.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas L.R., Foshage A.M., Weissmiller A.M., Tansey W.P. The MYC-WDR5 nexus and cancer. Cancer Res. 2015;75(19):4012–4015. doi: 10.1158/0008-5472.CAN-15-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cowling V.H., Chandriani S., Whitfield M.L., Cole M.D. A conserved Myc protein domain, MBIV, regulates DNA binding, apoptosis, transformation, and G2 arrest. Mol Cell Biol. 2006;26(11):4226–4239. doi: 10.1128/MCB.01959-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas L.R., Foshage A.M., Weissmiller A.M., et al. Interaction of MYC with host cell factor-1 is mediated by the evolutionarily conserved Myc box IV motif. Oncogene. 2016;35(27):3613–3618. doi: 10.1038/onc.2015.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelengaris S., Khan M., Evan G. c-MYC: more than just a matter of life and death. Nat Rev Cancer. 2002;2(10):764–776. doi: 10.1038/nrc904. [DOI] [PubMed] [Google Scholar]
- 31.Meyer N., Penn L.Z. Reflecting on 25 years with MYC. Nat Rev Cancer. 2008;8(12):976–990. doi: 10.1038/nrc2231. [DOI] [PubMed] [Google Scholar]
- 32.Das S.K., Lewis B.A., Levens D. MYC: a complex problem. Trends Cell Biol. 2023;33(3):235–246. doi: 10.1016/j.tcb.2022.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sammak S., Zinzalla G. Targeting protein-protein interactions (PPIs) of transcription factors: challenges of intrinsically disordered proteins (IDPs) and regions (IDRs) Prog Biophys Mol Biol. 2015;119(1):41–46. doi: 10.1016/j.pbiomolbio.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Hnisz D., Shrinivas K., Young R.A., Chakraborty A.K., Sharp P.A. A phase separation model for transcriptional control. Cell. 2017;169(1):13–23. doi: 10.1016/j.cell.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solvie D., Baluapuri A., Uhl L., et al. MYC multimers shield stalled replication Forks from RNA polymerase. Nature. 2022;612(7938):148–155. doi: 10.1038/s41586-022-05469-4. [DOI] [PubMed] [Google Scholar]
- 36.Schaub F.X., Dhankani V., Berger A.C., et al. Pan-cancer alterations of the MYC oncogene and its proximal network across the cancer genome atlas. Cell Syst. 2018;6(3):282–300.e2. doi: 10.1016/j.cels.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schick M., Habringer S., Nilsson J.A., Keller U. Pathogenesis and therapeutic targeting of aberrant MYC expression in haematological cancers. Br J Haematol. 2017;179(5):724–738. doi: 10.1111/bjh.14917. [DOI] [PubMed] [Google Scholar]
- 38.Adams J.M., Harris A.W., Pinkert C.A., et al. The c-myc oncogene driven by immunoglobulin enhancers induces lymphoid malignancy in transgenic mice. Nature. 1985;318(6046):533–538. doi: 10.1038/318533a0. [DOI] [PubMed] [Google Scholar]
- 39.Xu-Monette Z.Y., Deng Q., Manyam G.C., et al. Clinical and biologic significance of MYC genetic mutations in de novo diffuse large B-cell lymphoma. Clin Cancer Res. 2016;22(14):3593–3605. doi: 10.1158/1078-0432.CCR-15-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kalkat M., De Melo J., Hickman K.A., et al. MYC deregulation in primary human cancers. Genes. 2017;8(6):151. doi: 10.3390/genes8060151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beroukhim R., Mermel C.H., Porter D., et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayes T.K., Neel N.F., Hu C., et al. Long-term ERK inhibition in KRAS-mutant pancreatic cancer is associated with MYC degradation and senescence-like growth suppression. Cancer Cell. 2016;29(1):75–89. doi: 10.1016/j.ccell.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu P., Cheng H., Santiago S., et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nat Med. 2011;17(9):1116–1120. doi: 10.1038/nm.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weng A.P., Millholland J.M., Yashiro-Ohtani Y., et al. c-Myc is an important direct target of Notch 1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20(15):2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yochum G.S., Sherrick C.M., Macpartlin M., Goodman R.H. A beta-catenin/TCF-coordinated chromatin loop at MYC integrates 5' and 3' Wnt responsive enhancers. Proc Natl Acad Sci USA. 2010;107(1):145–150. doi: 10.1073/pnas.0912294107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J., Si J., Gan L., et al. Inhibition of Wnt signalling pathway by XAV939 enhances radiosensitivity in human cervical cancer HeLa cells. Artif Cells, Nanomed Biotechnol. 2020;48(1):479–487. doi: 10.1080/21691401.2020.1716779. [DOI] [PubMed] [Google Scholar]
- 47.Tsai W.B., Aiba I., Long Y., et al. Activation of Ras/PI3K/ERK pathway induces c-Myc stabilization to upregulate argininosuccinate synthetase, leading to arginine deiminase resistance in melanoma cells. Cancer Res. 2012;72(10):2622–2633. doi: 10.1158/0008-5472.CAN-11-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dang C.V. MYC on the path to cancer. Cell. 2012;149(1):22–35. doi: 10.1016/j.cell.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farrell A.S., Sears R.C. MYC degradation. Cold Spring Harb Perspect Med. 2014;4(3):a014365. doi: 10.1101/cshperspect.a014365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yumimoto K., Nakayama K.I. Recent insight into the role of FBXW7 as a tumor suppressor. Semin Cancer Biol. 2020;67(Pt 2):1–15. doi: 10.1016/j.semcancer.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 51.Wu H., Yang T.Y., Li Y., et al. Tumor necrosis factor receptor-associated factor 6 promotes hepatocarcinogenesis by interacting with histone deacetylase 3 to enhance c-Myc gene expression and protein stability. Hepatology. 2020;71(1):148–163. doi: 10.1002/hep.30801. [DOI] [PubMed] [Google Scholar]
- 52.Patel J.H., Loboda A.P., Showe M.K., Showe L.C., McMahon S.B. Analysis of genomic targets reveals complex functions of MYC. Nat Rev Cancer. 2004;4(7):562–568. doi: 10.1038/nrc1393. [DOI] [PubMed] [Google Scholar]
- 53.Lin C.Y., Lovén J., Rahl P.B., et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151(1):56–67. doi: 10.1016/j.cell.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nie Z., Hu G., Wei G., et al. c-Myc is a universal amplifier of expressed genes in lymphocytes and embryonic stem cells. Cell. 2012;151(1):68–79. doi: 10.1016/j.cell.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Si W., Shen J., Du C., et al. A miR-20a/MAPK1/c-Myc regulatory feedback loop regulates breast carcinogenesis and chemoresistance. Cell Death Differ. 2018;25(2):406–420. doi: 10.1038/cdd.2017.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li G., Liu H., Feng R., et al. A bioactive ligand-conjugated iridium(III) metal-based complex as a Keap1-Nrf2 protein-protein interaction inhibitor against acetaminophen-induced acute liver injury. Redox Biol. 2021;48 doi: 10.1016/j.redox.2021.102129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mirzaei S., Gholami M.H., Hashemi F., et al. Advances in understanding the role of P-gp in doxorubicin resistance: molecular pathways, therapeutic strategies, and prospects. Drug Discov Today. 2022;27(2):436–455. doi: 10.1016/j.drudis.2021.09.020. [DOI] [PubMed] [Google Scholar]
- 58.Yang L., Shi P., Zhao G., et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Targeted Ther. 2020;5(1):8. doi: 10.1038/s41392-020-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee K.M., Giltnane J.M., Balko J.M., et al. MYC and MCL1 cooperatively promote chemotherapy-resistant breast cancer stem cells via regulation of mitochondrial oxidative phosphorylation. Cell Metabol. 2017;26(4):633–647.e7. doi: 10.1016/j.cmet.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bretones G., Delgado M.D., León J. Myc and cell cycle control. Biochim Biophys Acta. 2015;1849(5):506–516. doi: 10.1016/j.bbagrm.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Kaczmarek L., Hyland J.K., Watt R., Rosenberg M., Baserga R. Microinjected c-Myc as a competence factor. Science. 1985;228(4705):1313–1315. doi: 10.1126/science.4001943. [DOI] [PubMed] [Google Scholar]
- 62.Cerni C. Telomeres, telomerase, and myc. An update. Mutat Res. 2000;462(1):31–47. doi: 10.1016/s1383-5742(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 63.Mateyak M.K., Obaya A.J., Sedivy J.M. c-Myc regulates cyclin D-Cdk4 and-Cdk6 activity but affects cell cycle progression at multiple independent points. Mol Cell Biol. 1999;19(7):4672–4683. doi: 10.1128/mcb.19.7.4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wong J.V., Dong P., Nevins J.R., Mathey-Prevot B., You L. Network calisthenics: control of E2F dynamics in cell cycle entry. Cell Cycle. 2011;10(18):3086–3094. doi: 10.4161/cc.10.18.17350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amati B., Alevizopoulos K., Vlach J. Myc and the cell cycle. Front Biosci. 1998;3:d250–d268. doi: 10.2741/a239. [DOI] [PubMed] [Google Scholar]
- 66.Vlach J., Hennecke S., Alevizopoulos K., Conti D., Amati B. Growth arrest by the cyclin-dependent kinase inhibitor p27Kip1 is abrogated by c-Myc. EMBO J. 1996;15(23):6595–6604. [PMC free article] [PubMed] [Google Scholar]
- 67.Möröy T., Saba I., Kosan C. The role of the transcription factor Miz-1 in lymphocyte development and lymphomagenesis-Binding Myc makes the difference. Semin Immunol. 2011;23(5):379–387. doi: 10.1016/j.smim.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 68.Brenner C., Deplus R., Didelot C., et al. Myc represses transcription through recruitment of DNA methyltransferase corepressor. EMBO J. 2005;24(2):336–346. doi: 10.1038/sj.emboj.7600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsieh A.L., Walton Z.E., Altman B.J., Stine Z.E., Dang C.V. MYC and metabolism on the path to cancer. Semin Cell Dev Biol. 2015;43:11–21. doi: 10.1016/j.semcdb.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vaupel P., Multhoff G. Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol. 2021;599(6):1745–1757. doi: 10.1113/JP278810. [DOI] [PubMed] [Google Scholar]
- 71.Graves J.A., Wang Y., Sims-Lucas S., et al. Mitochondrial structure, function and dynamics are temporally controlled by c-Myc. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0037699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim J.W., Zeller K.I., Wang Y., et al. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol Cell Biol. 2004;24(13):5923–5936. doi: 10.1128/MCB.24.13.5923-5936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Osthus R.C., Shim H., Kim S., et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000;275(29):21797–21800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 74.Gan L., Xiu R., Ren P., et al. Metabolic targeting of oncogene MYC by selective activation of the proton-coupled monocarboxylate family of transporters. Oncogene. 2016;35(23):3037–3048. doi: 10.1038/onc.2015.360. [DOI] [PubMed] [Google Scholar]
- 75.David C.J., Chen M., Assanah M., Canoll P., Manley J.L. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature. 2010;463(7279):364–368. doi: 10.1038/nature08697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Z., Deng X., Liu Y., Liu Y., Sun L., Chen F. PKM2, function and expression and regulation. Cell Biosci. 2019;9:52. doi: 10.1186/s13578-019-0317-8. published correction appears in Cell Biosci. 2019;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yue M., Jiang J., Gao P., Liu H., Qing G. Oncogenic MYC activates a feedforward regulatory loop promoting essential amino acid metabolism and tumorigenesis. Cell Rep. 2017;21(13):3819–3832. doi: 10.1016/j.celrep.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 78.Wise D.R., DeBerardinis R.J., Mancuso A., et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci U S A. 2008;105(48):18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao P., Tchernyshyov I., Chang T.C., et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458(7239):762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang R., Dillon C.P., Shi L.Z., et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35(6):871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barfeld S.J., Fazli L., Persson M., et al. Myc-dependent purine biosynthesis affects nucleolar stress and therapy response in prostate cancer. Oncotarget. 2015;6(14):12587–12602. doi: 10.18632/oncotarget.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morrish F., Isern N., Sadilek M., Jeffrey M., Hockenbery D.M. c-Myc activates multiple metabolic networks to generate substrates for cell-cycle entry. Oncogene. 2009;28(27):2485–2491. doi: 10.1038/onc.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morrish F., Hockenbery D. MYC and mitochondrial biogenesis. Cold Spring Harb Perspect Med. 2014;4(5) doi: 10.1101/cshperspect.a014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fogal V., Richardson A.D., Karmali P.P., Scheffler I.E., Smith J.W., Ruoslahti E. Mitochondrial p32 protein is a critical regulator of tumor metabolism via maintenance of oxidative phosphorylation. Mol Cell Biol. 2010;30(6):1303–1318. doi: 10.1128/MCB.01101-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu L., Ulbrich J., Müller J., et al. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature. 2012;483(7391):608–612. doi: 10.1038/nature10927. [DOI] [PubMed] [Google Scholar]
- 86.Purhonen J., Klefström J., Kallijärvi J. MYC-an emerging player in mitochondrial diseases. Front Cell Dev Biol. 2023;11 doi: 10.3389/fcell.2023.1257651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grewal S.S., Li L., Orian A., Eisenman R.N., Edgar B.A. Myc-dependent regulation of ribosomal RNA synthesis during Drosophila development. Nat Cell Biol. 2005;7(3):295–302. doi: 10.1038/ncb1223. [DOI] [PubMed] [Google Scholar]
- 88.Schlosser I., Hölzel M., Mürnseer M., Burtscher H., Weidle U.H., Eick D. A role for c-Myc in the regulation of ribosomal RNA processing. Nucleic Acids Res. 2003;31(21):6148–6156. doi: 10.1093/nar/gkg794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gao F.Y., Li X.T., Xu K., Wang R.T., Guan X.X. c-MYC mediates the crosstalk between breast cancer cells and tumor microenvironment. Cell Commun Signal. 2023;21(1):28. doi: 10.1186/s12964-023-01043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen C., Cai S., Wang G., et al. c-Myc enhances colon cancer cell-mediated angiogenesis through the regulation of HIF-1α. Biochem Biophys Res Commun. 2013;430(2):505–511. doi: 10.1016/j.bbrc.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 91.Du X., Ou X., Song T., et al. Adenosine A2B receptor stimulates angiogenesis by inducing VEGF and eNOS in human microvascular endothelial cells. Exp Biol Med. 2015;240(11):1472–1479. doi: 10.1177/1535370215584939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen X., Yang F., Zhang T., et al. MiR-9 promotes tumorigenesis and angiogenesis and is activated by MYC and OCT4 in human glioma. J Exp Clin Cancer Res. 2019;38(1):99. doi: 10.1186/s13046-019-1078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Psathas J.N., Thomas-Tikhonenko A. MYC and the art of microRNA maintenance. Cold Spring Harb Perspect Med. 2014;4(8) doi: 10.1101/cshperspect.a014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hanahan D., Coussens L.M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 95.Reimann M., Lee S., Loddenkemper C., et al. Tumor stroma-derived TGF-beta limits myc-driven lymphomagenesis via Suv39h1-dependent senescence. Cancer Cell. 2010;17(3):262–272. doi: 10.1016/j.ccr.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 96.Swaminathan S., Hansen A.S., Heftdal L.D., et al. MYC functions as a switch for natural killer cell-mediated immune surveillance of lymphoid malignancies. Nat Commun. 2020;11(1):2860. doi: 10.1038/s41467-020-16447-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dhanasekaran R., Baylot V., Kim M., et al. MYC and Twist1 cooperate to drive metastasis by eliciting crosstalk between cancer and innate immunity. Elife. 2020;9 doi: 10.7554/eLife.50731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu C.H., van Riggelen J., Yetil A., Fan A.C., Bachireddy P., Felsher D.W. Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc Natl Acad Sci USA. 2007;104(32):13028–13033. doi: 10.1073/pnas.0701953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hydbring P., Larsson L.G. Cdk2: a key regulator of the senescence control function of Myc. Aging. 2010;2(4):244–250. doi: 10.18632/aging.100140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li B., Zhang G., Wang Z., et al. c-Myc-activated USP2-AS1 suppresses senescence and promotes tumor progression via stabilization of E2F1 mRNA. Cell Death Dis. 2021;12(11):1006. doi: 10.1038/s41419-021-04330-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bravo-Cordero J.J., Hodgson L., Condeelis J. Directed cell invasion and migration during metastasis. Curr Opin Cell Biol. 2012;24(2):277–283. doi: 10.1016/j.ceb.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chuan Y.C., Iglesias-Gato D., Fernandez-Perez L., et al. Ezrin mediates c-Myc actions in prostate cancer cell invasion. Oncogene. 2010;29(10):1531–1542. doi: 10.1038/onc.2009.442. [DOI] [PubMed] [Google Scholar]
- 103.Anderson S., Poudel K.R., Roh-Johnson M., et al. MYC-nick promotes cell migration by inducing fascin expression and Cdc42 activation. Proc Natl Acad Sci U S A. 2016;113(37):E5481–E5490. doi: 10.1073/pnas.1610994113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wolfer A., Ramaswamy S. MYC and metastasis. Cancer Res. 2011;71(6):2034–2037. doi: 10.1158/0008-5472.CAN-10-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ma X., Huang J., Tian Y., et al. Myc suppresses tumor invasion and cell migration by inhibiting JNK signaling. Oncogene. 2017;36(22):3159–3167. doi: 10.1038/onc.2016.463. [DOI] [PubMed] [Google Scholar]
- 106.Kanno Y. The uPA/uPAR system orchestrates the inflammatory response, vascular homeostasis, and immune system in fibrosis progression. Int J Mol Sci. 2023;24(2):1796. doi: 10.3390/ijms24021796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alfano D., Votta G., Schulze A., et al. Modulation of cellular migration and survival by c-Myc through the downregulation of urokinase (uPA) and uPA receptor. Mol Cell Biol. 2010;30(7):1838–1851. doi: 10.1128/MCB.01442-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McMahon S.B. MYC and the control of apoptosis. Cold Spring Harb Perspect Med. 2014;4(7) doi: 10.1101/cshperspect.a014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Muthalagu N., Junttila M.R., Wiese K.E., et al. BIM is the primary mediator of MYC-induced apoptosis in multiple solid tissues. Cell Rep. 2014;8(5):1347–1353. doi: 10.1016/j.celrep.2014.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Toh P.P.C., Luo S., Menzies F.M., Raskó T., Wanker E.E., Rubinsztein D.C. Myc inhibition impairs autophagosome formation. Hum Mol Genet. 2013;22(25):5237–5248. doi: 10.1093/hmg/ddt381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jahangiri L., Pucci P., Ishola T., et al. The contribution of autophagy and LncRNAs to MYC-driven gene regulatory networks in cancers. Int J Mol Sci. 2021;22(16):8527. doi: 10.3390/ijms22168527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gaikwad S.M., Phyo Z., Arteaga A.Q., et al. A small molecule stabilizer of the MYC G4-quadruplex induces endoplasmic reticulum stress, senescence and pyroptosis in multiple myeloma. Cancers. 2020;12(10):2952. doi: 10.3390/cancers12102952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang Y., Mao C., Yang R., et al. EGLN1/c-Myc induced lymphoid-specific helicase inhibits ferroptosis through lipid metabolic gene expression changes. Theranostics. 2017;7(13):3293–3305. doi: 10.7150/thno.19988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Robey R.W., Pluchino K.M., Hall M.D., Fojo A.T., Bates S.E., Gottesman M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18(7):452–464. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Velasco R.M., Sánchez P.J., García A.G., et al. Targeting BPTF sensitizes pancreatic ductal adenocarcinoma to chemotherapy by repressing ABC-transporters and impairing multidrug resistance (MDR) Cancers. 2022;14(6):1518. doi: 10.3390/cancers14061518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Foley S.A., Castell A., Kavanagh E., et al. MYC as a therapeutic target for the treatment of triple-negative breast cancer. J Clin Oncol. 2019;37(15_suppl l) [Google Scholar]
- 117.Xie X., Yu T., Li X., et al. Recent advances in targeting the "undruggable" proteins: from drug discovery to clinical trials. Signal Transduct Targeted Ther. 2023;8(1):335. doi: 10.1038/s41392-023-01589-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Boike L., Cioffi A.G., Majewski F.C., et al. Discovery of a functional covalent ligand targeting an intrinsically disordered cysteine within MYC. Cell Chem Biol. 2021;28(1):4–13.e17. doi: 10.1016/j.chembiol.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Du W., Du S., Dong X., et al. Biodegradable silica nanocapsules enable efficient nuclear-targeted delivery of native proteins for cancer therapy. Biomaterials. 2023;294 doi: 10.1016/j.biomaterials.2023.122000. [DOI] [PubMed] [Google Scholar]
- 120.Whitfield J.R., Soucek L. The long journey to bring a Myc inhibitor to the clinic. J Cell Biol. 2021;220(8) doi: 10.1083/jcb.202103090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shukla S., Fletcher S., Chauhan J., et al. 3JC48-3 (methyl 4'-methyl-5-(7-nitrobenzo[c][1, 2, 5]oxadiazol-4-yl)-[1, 1'-biphenyl]-3-carboxylate):a novel MYC/MAX dimerization inhibitor reduces prostate cancer growth. Cancer Gene Ther. 2022;29(11):1550–1557. doi: 10.1038/s41417-022-00455-4. [DOI] [PubMed] [Google Scholar]
- 122.Han H., Jain A.D., Truica M.I., et al. Small-molecule MYC inhibitors suppress tumor growth and enhance immunotherapy. Cancer Cell. 2019;36(5):483–497.e15. doi: 10.1016/j.ccell.2019.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hart J.R., Garner A.L., Yu J., et al. Inhibitor of MYC identified in a Kröhnke pyridine library. Proc Natl Acad Sci USA. 2014;111(34):12556–12561. doi: 10.1073/pnas.1319488111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Castell A., Yan Q., Fawkner K., et al. A selective high affinity MYC-binding compound inhibits MYC: max interaction and MYC-dependent tumor cell proliferation. Sci Rep. 2018;8(1) doi: 10.1038/s41598-018-28107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Struntz N.B., Chen A., Deutzmann A., et al. Stabilization of the max homodimer with a small molecule attenuates Myc-driven transcription. Cell Chem Biol. 2019;26(5):711–723.e14. doi: 10.1016/j.chembiol.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 126.Lustig L.C., Dingar D., Tu W.B., et al. Inhibiting MYC binding to the E-box DNA motif by ME47 decreases tumour xenograft growth. Oncogene. 2017;36(49):6830–6837. doi: 10.1038/onc.2017.275. [DOI] [PubMed] [Google Scholar]
- 127.Jeong K.C., Kim K.T., Seo H.H., et al. Intravesical instillation of c-MYC inhibitor KSI-3716 suppresses orthotopic bladder tumor growth. J Urol. 2014;191(2):510–518. doi: 10.1016/j.juro.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 128.Calabrese D.R., Chen X., Leon E.C., et al. Chemical and structural studies provide a mechanistic basis for recognition of the MYC G-quadruplex. Nat Commun. 2018;9(1):4229. doi: 10.1038/s41467-018-06315-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bahls B., Aljnadi I.M., Emídio R., Mendes E., Paulo A. G-quadruplexes in c-MYC promoter as targets for cancer therapy. Biomedicines. 2023;11(3):969. doi: 10.3390/biomedicines11030969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hilton J., Gelmon K., Bedard P.L., et al. Results of the phase I CCTG IND.231 trial of CX-5461 in patients with advanced solid tumors enriched for DNA-repair deficiencies. Nat Commun. 2022;13(1):3607. doi: 10.1038/s41467-022-31199-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hu M.H., Wang Y.Q., Yu Z.Y., et al. Discovery of a new four-leaf clover-like ligand as a potent c-MYC transcription inhibitor specifically targeting the promoter G-quadruplex. J Med Chem. 2018;61(6):2447–2459. doi: 10.1021/acs.jmedchem.7b01697. [DOI] [PubMed] [Google Scholar]
- 132.Hu M.H., Wu T.Y., Huang Q., Jin G. New substituted quinoxalines inhibit triple-negative breast cancer by specifically downregulating the c-MYC transcription. Nucleic Acids Res. 2019;47(20):10529–10542. doi: 10.1093/nar/gkz835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wu T.Y., Huang Q., Huang Z.S., Hu M.H., Tan J.H. A drug-like imidazole-benzothiazole conjugate inhibits malignant melanoma by stabilizing the c-MYC G-quadruplex. Bioorg Chem. 2020;99 doi: 10.1016/j.bioorg.2020.103866. [DOI] [PubMed] [Google Scholar]
- 134.Lancho O., Herranz D. The MYC enhancer-ome: long-range transcriptional regulation of MYC in cancer. Trends Cancer. 2018;4(12):810–822. doi: 10.1016/j.trecan.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen H., Liu H., Qing G. Targeting oncogenic Myc as a strategy for cancer treatment. Signal Transduct Targeted Ther. 2018;3:5. doi: 10.1038/s41392-018-0008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Devaiah B.N., Case-Borden C., Gegonne A., et al. BRD4 is a histone acetyltransferase that evicts nucleosomes from chromatin. Nat Struct Mol Biol. 2016;23(6):540–548. doi: 10.1038/nsmb.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Aggarwal R.R., Schweizer M.T., Nanus D.M., et al. A phase Ib/IIa study of the pan-BET inhibitor ZEN-3694 in combination with enzalutamide in patients with metastatic castration-resistant prostate cancer. Clin Cancer Res. 2020;26(20):5338–5347. doi: 10.1158/1078-0432.CCR-20-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bradbury R.H., Callis R., Carr G.R., et al. Optimization of a series of bivalent triazolopyridazine based bromodomain and extraterminal inhibitors: the discovery of (3R)-4-[2-[4-[1-(3-methoxy-[1, 2, 4]triazolo[4, 3-b]pyridazin-6-yl)-4-piperidyl]phenoxy]ethyl]-1, 3-dimethyl-piperazin-2-one (AZD5153) J Med Chem. 2016;59(17):7801–7817. doi: 10.1021/acs.jmedchem.6b00070. [DOI] [PubMed] [Google Scholar]
- 139.Hamilton E.P., Wang J.S., Oza A.M., et al. First-in-human study of AZD5153, a small-molecule inhibitor of bromodomain protein 4, in patients with relapsed/refractory malignant solid tumors and lymphoma. Mol Cancer Therapeut. 2023;22(10):1154–1165. doi: 10.1158/1535-7163.MCT-23-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Riveiro M.E., Astorgues-Xerri L., Vazquez R., et al. OTX015 (MK-8628), a novel BET inhibitor, exhibits antitumor activity in non-small cell and small cell lung cancer models harboring different oncogenic mutations. Oncotarget. 2016;7(51):84675–84687. doi: 10.18632/oncotarget.13181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cescon D.W., Hilton J., Morales Murilo S., et al. A phase I/II study of GSK525762 combined with fulvestrant in patients with hormone receptor-positive/HER2-negative advanced or metastatic breast cancer. Clin Cancer Res. 2024;30(2):334–343. doi: 10.1158/1078-0432.CCR-23-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Doroshow D.B., Eder J.P., LoRusso P.M. BET inhibitors: a novel epigenetic approach. Ann Oncol. 2017;28(8):1776–1787. doi: 10.1093/annonc/mdx157. [DOI] [PubMed] [Google Scholar]
- 143.Ebmeier C.C., Erickson B., Allen B.L., et al. Human TFIIH kinase CDK7 regulates transcription-associated chromatin modifications. Cell Rep. 2017;20(5):1173–1186. doi: 10.1016/j.celrep.2017.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Franco L.C., Morales F., Boffo S., Giordano A. CDK9: a key player in cancer and other diseases. J Cell Biochem. 2018;119(2):1273–1284. doi: 10.1002/jcb.26293. [DOI] [PubMed] [Google Scholar]
- 145.Heidemann M., Hintermair C., Voß K., Eick D. Dynamic phosphorylation patterns of RNA polymerase II CTD during transcription. Biochim Biophys Acta. 2013;1829(1):55–62. doi: 10.1016/j.bbagrm.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 146.Coombes R.C., Howell S., Lord S.R., et al. Dose escalation and expansion cohorts in patients with advanced breast cancer in a phase I study of the CDK7-inhibitor samuraciclib [published correction appears in Nat Commun. 2023;14(1):4741.] Nat Commun. 2023;14(1):4444. doi: 10.1038/s41467-023-40061-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sava G.P., Fan H., Coombes R.C., Buluwela L., Ali S. CDK7 inhibitors as anticancer drugs. Cancer Metastasis Rev. 2020;39(3):805–823. doi: 10.1007/s10555-020-09885-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Garralda E., Schram A.M., Bedard P.L., et al. A phase I dose-escalation study of LY3405105, a covalent inhibitor of cyclin-dependent kinase 7, administered to patients with advanced solid tumors. Oncol. 2024;29(1):e131–e140. doi: 10.1093/oncolo/oyad215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hu S., Marineau J.J., Rajagopal N., et al. Discovery and characterization of SY-1365, a selective, covalent inhibitor of CDK7. Cancer Res. 2019;79(13):3479–3491. doi: 10.1158/0008-5472.CAN-19-0119. [DOI] [PubMed] [Google Scholar]
- 150.Hu S.H., Marineau J., Hamman K., et al. SY-5609, an orally available selective CDK7 inhibitor demonstrates broad anti-tumor activity. Cancer Res. 2019;79(13) [Google Scholar]
- 151.Freeman D.B., Hopkins T.D., Mikochik P.J., et al. Discovery of KB-0742, a potent, selective, orally bioavailable small molecule inhibitor of CDK9 for MYC-dependent cancers. J Med Chem. 2023;66(23):15629–15647. doi: 10.1021/acs.jmedchem.3c01233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Huang Z., Wang T., Wang C., Fan Y. CDK9 inhibitors in cancer research. RSC Med Chem. 2022;13(6):688–710. doi: 10.1039/d2md00040g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Frame S., Saladino C., MacKay C., et al. Fadraciclib (CYC065), a novel CDK inhibitor, targets key pro-survival and oncogenic pathways in cancer. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Paruch K., Dwyer M.P., Alvarez C., et al. Discovery of dinaciclib (SCH 727965): a potent and selective inhibitor of cyclin-dependent kinases. ACS Med Chem Lett. 2010;1(5):204–208. doi: 10.1021/ml100051d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mao B., Zhang Q., Ma L., Zhao D.S., Zhao P., Yan P. Overview of research into mTOR inhibitors. Molecules. 2022;27(16):5295. doi: 10.3390/molecules27165295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Graham L., Banda K., Torres A., et al. A phase II study of the dual mTOR inhibitor MLN0128 in patients with metastatic castration resistant prostate cancer. Invest N Drugs. 2018;36(3):458–467. doi: 10.1007/s10637-018-0578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wiegering A., Uthe F.W., Jamieson T., et al. Targeting translation initiation bypasses signaling crosstalk mechanisms that maintain high MYC levels in colorectal cancer. Cancer Discov. 2015;5(7):768–781. doi: 10.1158/2159-8290.CD-14-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Thompson P.A., Eam B., Young N.P., et al. Preclinical evaluation of eFT226, a novel, potent and selective eIF4A inhibitor with anti-tumor activity in B-cell malignancies. Blood. 2017;130:1530. [Google Scholar]
- 159.Allen-Petersen B.L., Sears R.C. Mission possible: advances in MYC therapeutic targeting in cancer. BioDrugs. 2019;33(5):539–553. doi: 10.1007/s40259-019-00370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sun X.X., He X., Yin L., Komada M., Sears R.C., Dai M.S. The nucleolar ubiquitin-specific protease USP36 deubiquitinates and stabilizes c-Myc. Proc Natl Acad Sci U S A. 2015;112(12):3734–3739. doi: 10.1073/pnas.1411713112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li M., Yu J., Ju L., et al. USP43 stabilizes c-Myc to promote glycolysis and metastasis in bladder cancer. Cell Death Dis. 2024;15(1):44. doi: 10.1038/s41419-024-06446-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Schauer N.J., Liu X., Magin R.S., et al. Selective USP7 inhibition elicits cancer cell killing through a p53-dependent mechanism. Sci Rep. 2020;10(1):5324. doi: 10.1038/s41598-020-62076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kategaya L., Di Lello P., Rougé L., et al. USP7 small-molecule inhibitors interfere with ubiquitin binding. Nature. 2017;550(7677):534–538. doi: 10.1038/nature24006. [DOI] [PubMed] [Google Scholar]
- 164.Turnbull A.P., Ioannidis S., Krajewski W.W., et al. Molecular basis of USP7 inhibition by selective small-molecule inhibitors. Nature. 2017;550(7677):481–486. doi: 10.1038/nature24451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hu Y., Luo M. Cinobufotalin regulates the USP36/c-Myc axis to suppress malignant phenotypes of colon cancer cells in vitro and in vivo. Aging. 2024;16(6):5526–5544. doi: 10.18632/aging.205661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Westermarck J., Hahn W.C. Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol Med. 2008;14(4):152–160. doi: 10.1016/j.molmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 167.Janghorban M., Farrell A.S., Allen-Petersen B.L., et al. Targeting c-MYC by antagonizing PP2A inhibitors in breast cancer. Proc Natl Acad Sci USA. 2014;111(25):9157–9162. doi: 10.1073/pnas.1317630111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sangodkar J., Perl A., Tohme R., et al. Activation of tumor suppressor protein PP2A inhibits KRAS-driven tumor growth. J Clin Invest. 2017;127(6):2081–2090. doi: 10.1172/JCI89548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Allen-Petersen B.L., Risom T., Feng Z., et al. Activation of PP2A and inhibition of mTOR synergistically reduce MYC signaling and decrease tumor growth in pancreatic ductal adenocarcinoma. Cancer Res. 2019;79(1):209–219. doi: 10.1158/0008-5472.CAN-18-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang D., Pierce A., Veo B., et al. A regulatory loop of FBXW7-MYC-PLK1 controls tumorigenesis of MYC-driven medulloblastoma. Cancers. 2021;13(3):387. doi: 10.3390/cancers13030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ellis P.M., Leighl N.B., Hirsh V., et al. A randomized, open-label phase II trial of volasertib as monotherapy and in combination with standard-dose pemetrexed compared with pemetrexed monotherapy in second-line treatment for non-small-cell lung cancer. Clin Lung Cancer. 2015;16(6):457–465. doi: 10.1016/j.cllc.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 172.Frost A., Mross K., Steinbild S., et al. Phase I study of the Plk1 inhibitor BI 2536 administered intravenously on three consecutive days in advanced solid tumours. Curr Oncol. 2012;19(1):e28–e35. doi: 10.3747/co.19.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Weiss G.J., Jameson G., Von Hoff D.D., et al. Phase I dose escalation study of NMS-1286937, an orally available polo-like kinase 1 inhibitor, in patients with advanced or metastatic solid tumors. Invest N Drugs. 2018;36(1):85–95. doi: 10.1007/s10637-017-0491-7. [DOI] [PubMed] [Google Scholar]
- 174.Olmos D., Barker D., Sharma R., et al. Phase I study of GSK461364, a specific and competitive Polo-like kinase 1 inhibitor, in patients with advanced solid malignancies. Clin Cancer Res. 2011;17(10):3420–3430. doi: 10.1158/1078-0432.CCR-10-2946. [DOI] [PubMed] [Google Scholar]
- 175.Chiappa M., Petrella S., Damia G., Broggini M., Guffanti F., Ricci F. Present and future perspective on PLK1 inhibition in cancer treatment. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.903016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cohn G.M., Liefwalker D.F., Langer E.M., Sears R.C. PIN1 provides dynamic control of MYC in response to extrinsic signals. Front Cell Dev Biol. 2020;8:224. doi: 10.3389/fcell.2020.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Dubiella C., Pinch B.J., Koikawa K., et al. Sulfopin is a covalent inhibitor of Pin1 that blocks Myc-driven tumors in vivo. Nat Chem Biol. 2021;17(9):954–963. doi: 10.1038/s41589-021-00786-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Békés M., Langley D.R., Crews C.M. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21(3):181–200. doi: 10.1038/s41573-021-00371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li X., Zhang Z., Gao F., et al. c-Myc-targeting PROTAC based on a TNA-DNA bivalent binder for combination therapy of triple-negative breast cancer. J Am Chem Soc. 2023;145(16):9334–9342. doi: 10.1021/jacs.3c02619. [DOI] [PubMed] [Google Scholar]
- 180.Wang Y., Yang G., Zhang X., et al. Antitumor effect of anti-c-Myc aptamer-based PROTAC for degradation of the c-Myc protein. Adv Sci. 2024;11(26) doi: 10.1002/advs.202309639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Geng X., Wang X., Zhu D., Ying S. Synthetic lethal interactions in cancer therapy. Curr Cancer Drug Targets. 2017;17(4):304–310. doi: 10.2174/1568009616666160426122736. [DOI] [PubMed] [Google Scholar]
- 182.Thng D.K.H., Toh T.B., Chow E.K.H. Capitalizing on synthetic lethality of MYC to treat cancer in the digital age. Trends Pharmacol Sci. 2021;42(3):166–182. doi: 10.1016/j.tips.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 183.Goga A., Yang D., Tward A.D., Morgan D.O., Bishop J.M. Inhibition of CDK1 as a potential therapy for tumors over-expressing MYC. Nat Med. 2007;13(7):820–827. doi: 10.1038/nm1606. [DOI] [PubMed] [Google Scholar]
- 184.Huang Y., Fan Y., Zhao Z., et al. Inhibition of CDK1 by RO-3306 exhibits anti-tumorigenic effects in ovarian cancer cells and a transgenic mouse model of ovarian cancer. Int J Mol Sci. 2023;24(15) doi: 10.3390/ijms241512375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cottini F., Hideshima T., Suzuki R., et al. Synthetic lethal approaches exploiting DNA damage in aggressive myeloma. Cancer Discov. 2015;5(9):972–987. doi: 10.1158/2159-8290.CD-14-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cole K.A., Huggins J., Laquaglia M., et al. RNAi screen of the protein kinome identifies checkpoint kinase 1 (CHK1) as a therapeutic target in neuroblastoma. Proc Natl Acad Sci U S A. 2011;108(8):3336–3341. doi: 10.1073/pnas.1012351108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Krug L.M., Wozniak A.J., Kindler H.L., et al. Randomized phase II trial of pemetrexed/cisplatin with or without CBP501 in patients with advanced malignant pleural mesothelioma. Lung Cancer. 2014;85(3):429–434. doi: 10.1016/j.lungcan.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 188.Scagliotti G., Kang J.H., Smith D., et al. Phase II evaluation of LY2603618, a first-generation CHK1 inhibitor, in combination with pemetrexed in patients with advanced or metastatic non-small cell lung cancer. Invest N Drugs. 2016;34(5):625–635. doi: 10.1007/s10637-016-0368-1. [DOI] [PubMed] [Google Scholar]
- 189.Brooks K., Oakes V., Edwards B., et al. A potent Chk1 inhibitor is selectively cytotoxic in melanomas with high levels of replicative stress. Oncogene. 2013;32(6):788–796. doi: 10.1038/onc.2012.72. [DOI] [PubMed] [Google Scholar]
- 190.van Bussel M.T.J., Awada A., de Jonge M.J.A., et al. A first-in-man phase 1 study of the DNA-dependent protein kinase inhibitor peposertib (formerly M3814) in patients with advanced solid tumours. Br J Cancer. 2021;124(4):728–735. doi: 10.1038/s41416-020-01151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yap T.A., Fontana E., Lee E.K., et al. Camonsertib in DNA damage response-deficient advanced solid tumors: phase 1 trial results. Nat Med. 2023;29(6):1400–1411. doi: 10.1038/s41591-023-02399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dunham E.C., Leske A., Shifflett K., et al. Lifecycle modelling systems support inosine monophosphate dehydrogenase (IMPDH) as a pro-viral factor and antiviral target for New World arenaviruses. Antivir Res. 2018;157:140–150. doi: 10.1016/j.antiviral.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wang H., Lu J., Stevens T., et al. Premature aging and reduced cancer incidence associated with near-complete body-wide Myc inactivation. Cell Rep. 2023;42(8) doi: 10.1016/j.celrep.2023.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]