Abstract
Background
Calcitonin gene related peptide (CGRP) pathway targeting therapies have proven efficacy, safety and tolerability. However, CGRP is also involved in immune responses, and reports of an increased risk of infection have emerged. This meta-analysis aims to verify whether CGRP-targeting therapies show evidence of increasing infection risk.
Methods
A systematic review was conducted according to PRISMA-Harms guidelines. A PubMed and Embase search result selection and extraction was performed. Risk of bias, sensitivity analysis, and fixed/random effects network meta-analyses were conducted for incidence of infectious adverse events in the studied populations with subsequent effect size assessment. An additional infectious serious adverse event search was performed in double-blind and open-label studies.
Results
The search and selection process yielded 37 randomized placebo-controlled trials. 22,518 patients (77.3% women) treated with erenumab, fremanezumab, galcanezumab, eptinezumab, atogepant and rimegepant participated in these studies. Preventive CGRP-targeting therapies appear to increase the infection relative risk (RR = 1.08 [1.01; 1.14], p = 0.016, Number Needed to Harm [NNH] = 287). However, in individual analyses only galcanezumab and eptinezumab showed an increase in risk of infections: galcanezumab at clinically used doses (RR 1.13 [1.02; 1.25], p = 0.024, NNH = 77); eptinezumab at higher doses (RR 1.23 [1.04; 1.45], p = 0.015, NNH = 24). Fremanezumab was associated with fewest infectious SAEs (n = 3 in 3 studies), while erenumab showed the highest incidence of these events (n = 36 in 11 studies).
Conclusions
CGRP has multiple and often potentially opposing effects on the immune system. In effect, preventive CGRP pathway antagonists (especially eptinezumab and galcanezumab) possibly only mildly increase the risk of infections. However, it is unlikely to affect most migraine patients considering relatively high NNH, low effect size and few infectious SAEs reported so far. The result of CGRP-targeting therapies potentially depends on the type of pathogen and patient’s immune status. Consequently, in immunocompromised patients or at public health levels the increased infection risk may have more pronounced effect.
Supplementary Information
The online version contains supplementary material available at 10.1186/s10194-025-02040-0.
Keywords: Erenumab, Fremanezumab, Galcanezumab, Eptinezumab, Atogepant, Rimegepant, Migraine, Infection, CGRP
Background
Calcitonin gene related peptide (CGRP) pathway targeting therapies are becoming a staple in migraine management [1, 2]. It is a consequence of their confirmed efficacy in migraine prevention [3] and (in the case of some gepants) acute treatment [4]. Moreover, favourable tolerability and safety have been reported [5]. However, reports have also emerged of an increased infection risk for respiratory tract infections in the case of erenumab [6] and galcanezumab [7] as well as urinary tract infections for atogepant [8] and in some cases rimegepant [9]. Should these reports be confirmed, a significant impact on individual and public health could be expected considering the high prevalence of migraine [10] which may affect also people with immune system disorders [11].
CGRP plays various roles in humans that are not limited to headache aetiopathogenesis, but are also involved in immune response [12, 13]. CGRP potentially takes part in response to viral [14], bacterial [15], fungal [16] infections and parasitic infestations [17]. Therefore, investigating the association between the CGRP blockade and the risk of infection is of significant clinical importance. Despite that, infection rates have not been assessed in a systematic manner in people treated with CGRP-targeting medications. Consequently, it is unclear whether the increased risk of infections mentioned in the previous paragraph is a class effect in response to CGRP pathway blockade.
The purpose of this meta-analysis is to identify whether CGRP-targeting therapies show evidence of increasing infection. We hypothesized that CGRP-targeting medications may cause infections and that this effect may vary depending on the medication dose or infection type.
Methods
Eligibility criteria
The meta-analysis was performed according to the ‘PRISMA-Harms’ reporting guidelines [18]. The protocol was recorded in the international prospective register of systematic reviews (PROSPERO CRD42024588786). The following inclusion criteria were followed:
unlimited publication dates prior to database search.
full text available in English language.
trials in adult patients treated with registered CGRP-targeting monoclonal antibodies or CGRP-receptor antagonists for at least 12 weeks.
at least one infectious adverse event in a published manuscript and/or its supplementary materials.
studies considered for meta-analysis: randomised, double-blind, placebo-controlled, registered trials.
The selected studies were cross-referenced with the clinicaltrials.gov registry for AEs, as different approaches to AE reporting thresholds may have been used in these sources. The studied populations were not restricted in respect to diagnosis (i.e. disorders other than migraine were not excluded). Trials in children and adolescents were excluded as currently no CGRP-targeting medication is registered for people under the age of 18. Studies on acute treatment were excluded due to the fact that not all their participants took gepants daily or every other day. Unregistered therapies (i.e. 1st generation gepants) were excluded due to unacceptable safety profiles and limited generalizability to everyday practice. Active comparator trials without placebo group were excluded. Studies that did not provide data on the number of AEs or the number of patients who experienced AEs were also excluded. Duplicate records and post-hoc analyses were excluded unless they presented new data on infectious AEs.
Database search and data extraction
The database search was performed at one time-point (17 Sep. 2024) in PubMed and Embase. The search phrase was defined as: ((erenumab) AND (Placebo)) OR ((erenumab) AND (real world)) OR ((erenumab) AND (open-label)) OR ((AMG 334) AND (Placebo)) OR ((AMG 334) AND (real world)) OR ((AMG 334) AND (open-label)) OR ((fremanezumab) AND (Placebo)) OR ((fremanezumab) AND (real world)) OR ((fremanezumab) AND (open-label)) OR ((TEV-48125) AND (Placebo)) OR ((TEV-48125) AND (real world)) OR ((TEV-48125) AND (open-label)) OR ((LBR-101) AND (Placebo)) OR ((LBR-101) AND (real world)) OR ((LBR-101) AND (open-label)) OR ((galcanezumab) AND (Placebo)) OR ((galcanezumab) AND (real world)) OR ((galcanezumab) AND (open-label)) OR ((LY2951742) AND (Placebo)) OR ((LY2951742) AND (real world)) OR ((LY2951742) AND (open-label)) OR ((eptinezumab) AND (Placebo)) OR ((eptinezumab) AND (real world)) OR ((eptinezumab) AND (open-label)) OR ((ALD403) AND (Placebo)) OR ((ALD403) AND (real world)) OR ((ALD403) AND (open-label)) OR ((atogepant) AND (Placebo)) OR ((atogepant) AND (real world)) OR ((atogepant) AND (open-label)) OR ((AGN-241689) AND (Placebo)) OR ((AGN-241689) AND (real world)) OR ((AGN-241689) AND (open-label)) OR ((rimegepant) AND (Placebo)) OR ((rimegepant) AND (real world)) OR ((rimegepant) AND (open-label)) OR ((BMS-927711) AND (Placebo)) OR ((BMS-927711) AND (real world)) OR ((BMS-927711) AND (open-label)].
Two authors (DK and KM) independently assessed the results obtained in the database search. The first step involved title and abstract (PubMed and Embase) assessment for eligibility according to inclusion and exclusion criteria. In the second step, the manuscripts of publications selected in the first step were analysed. Any discrepancies between the authors were resolved by the third author (MS) separately, after the first and second step analysis.
Data extraction from selected manuscripts or their supplements and clinicaltrials.gov records was performed separately by DK and KM. If discrepancies were found between sources describing the same study (e.g. manuscript and clinicaltrials.gov database) then the higher number of AEs was included in the meta-analysis. This was due to the fact that manuscripts often underreport AEs if a given threshold of participants is not reached, whereas trial registries in some situations may present more data. The AEs were classified to one of the categories according to Table 1. When an AE was described with a specific aetiology different from that proposed in Table 1, it was classified accordingly (e.g. COVID-19 pneumonia was classified as viral rather than bacterial). Data excluded from the extraction involved inflammatory disorders of predominantly non-infectious aetiology or infections secondary to another event. Other excluded AEs involved: chronic sinusitis (unless during exacerbation), chronic obstructive pulmonary disease (unless during exacerbation), cholecystitis, appendicitis, gastritis (unless classified as infective), vector borne diseases (incl. Lyme disease and Denga), neuritis, uveitis and gingivitis (unless classified as infective).
Table 1.
Adverse events to be extracted from included studies. COVID-19– Coronavirus disease 2019
| Adverse event (synonym) | Predominant aetiology |
|---|---|
| Upper respiratory tract infection | Viral |
| Nasopharyngitis (rhinitis, pharyngitis, tonsillitis) | |
| Sinusitis (rhinosinusitis) | |
| Otitis media | |
| Herpes | |
| Laryngitis | |
| Tracheitis | |
| Bronchitis | |
| COVID-19 | |
| Gastroenterocolitis | |
| Meningitis | |
| Other viral | |
| Otitis externa | Bacterial |
| Mastoiditis | |
| Exacerbation of chronic obstructive pulmonary disease | |
| Pneumonia | |
| Tuberculosis | |
| Urinary tract infection (cystitis) | |
| Pyelonephritis | |
| Vagnitis (vulvovaginitis) | |
| Sepsis (urosepsis) | |
| Diverticulitis | |
| Cellulitis | |
| Folliculitis | |
| Hordeolum (chalazion) | |
| Abscess | |
| Other bacterial | |
| Unclassified or other | Other |
Apart from data for the meta-analysis, a separate extraction of infectious serious AEs (SAEs) was performed in randomized placebo-controlled and open-label (OL) trials, as well as published real-world evidence (RWE). In this case, only trials that reported at least one infectious SAE were included. Search and selection strategies were similar to those described above, but with adjustments regarding study type. Data from the entire selection and extraction process is available in the publicly available repository [19].
Statistical analysis and quality assessment
It was conducted using both fixed-effect and random-effect models. The statistical heterogeneity was assessed using Cochran’s Q test, I2 statistic and Pearson χ2 tests. The Biggerstaff and Jackson method was applied to determine the statistical significance of heterogeneity. Baujat plots were employed to identify studies contributing to the heterogeneity and influencing the overall result of the meta-analysis. If the heterogeneity tests indicated a low risk of heterogeneity (p > 0.001, I² < 25%), the fixed-effect model was applied for the meta-analysis. The Pearson χ2 test was used for the analysis of contingency tables to assess statistically significant differences between the expected (placebo) frequencies and the observed frequencies. When significant differences (p < 0.05) were found, the treatment-related relative risk (RR) of infection was calculated. In the case of contingency Table (2 × 2), the following classification of effect size was adopted: small (1.0 < RR < 1.5), medium (1.5 ≤ RR < 2.5) and large (RR ≥ 2.5). Furthermore, Number Needed to Harm (NNH) was calculated to assess the risk of a harmful side effects as 1/RD. Risk Difference (RD) was calculated as: RD = EER– CER. EER is the percentage of participants in the experimental group who experienced an adverse effect (infection), and CER is the percentage of participants in the control group who experienced the same adverse effect.
RR estimations were performed in subgroups depending on medication and dose (‘any dose’, ‘any clinically registered dose’ and ‘high dose’). The doses were considered high when they were equal to or above the highest registered dose (e.g. erenumab ≥ 140 mg monthly, fremanezumab ≥ 675 mg per 3 months, galcanezumab ≥ 240 mg monthly, eptinezumab ≥ 300 mg quarterly, atogepant ≥ 60 mg per day, rimegepant > 75 mg every second day). Alternatively, a dose associated with temporal exposition to higher medication doses (e.g. quarterly fremanezumab 675 mg). Meta-analysis was also performed for pooled data for all included particles at clinically registered dose and high doses. In case of infections the data was pooled for overall infections, viral infections, coronavirus disease 2019 (COVID-19), bacterial infections UTIs for each subanalysis. COVID-19 was selected due to its potentially lower risk of reporting bias compared to other respiratory tract infections, especially during the first years of pandemic. RR estimates were calculated for data pooled across all indications. Age and sex were chosen as they are associated with increased risk of some infections [20, 21]. The publication date was chosen as potential moderator to exclude risk related to unidentified drift in study methods.
The Cochrane tool for assessing risk of bias in randomised trials version 2 (RoB 2) was used. Publication bias was analysed in the symmetry of funnel plots, the ‘trim-and-fill’ method and the Egger’s test. The I-squared (I²) statistic was used to describe the percentage of the total variance in study results that is attributable to heterogeneity rather than chance. Although not a formal statistical test, I² helps in assessing the degree of heterogeneity (e.g., low, moderate, high). Studies with high bias risk were to be excluded from the analysis. A random-effect meta-regression of the extracted data concerning sex, age, and publication date was performed to estimate the effect of each moderator on the final outcome variable. Galbraith plots were used to assess the consistency of study results and identify outliers. The effect size of age, sex and publication date was calculated as Hedges’ g with corresponding 95% confidence intervals (95% CI).
Results
PubMed and Embase databases search yielded 1138 results in total. The selection process has been presented in Fig. 1. Altogether, 37 trials eligible for data extraction and meta-analysis were found. Studies included in the meta-analysis are listed in Table 2. These trials evaluated two gepants and four monoclonal antibodies currently registered for migraine prevention. A total of 22 518 participants (77.3% women) were randomised in the included trials. Dosing schemes included registered and unregistered regimens for different forms of migraine or chronic cluster headache. Risk of bias of individual studies was low (Supplement 8), although lack of systematic evaluation of infectious AEs (e.g. via validated questionnaires) and reporting thresholds may have led to missed infections. However, no signs of infection reporting bias was identified.
Fig. 1.
Flow chart of study selection
Table 2.
Table 2 studies included in the meta-analysis
| Active substance | Study registration number | Maximal single doses (mg) | Participants randomised (n) | Diagnosis | |
|---|---|---|---|---|---|
| Erenumab | Reuter et al. 2018 [22] | NCT03096834 | 140 | 243 | RM |
| Sun et al. 2016 [23] | NCT01952574 | 7, 21, 70 | 472 | EM | |
| Tepper et al. 2017 [24] | NCT02066415 | 70, 140 | 660 | CM | |
| Goadsby 2017 et al. [25] | NCT02456740 | 70, 140 | 952 | EM | |
| Sakai et al. 2019 [26] | NCT02630459 | 28, 70, 140 | 474 | EM | |
| Takeshima et al. 2021 [27] | NCT03812224 | 70 | 261 | RM | |
| Dodick et al. 2018 [28] | NCT02483585 | 70 | 572 | EM | |
| Wang et al. 2021 [29] | NCT03333109 | 70, 140 | 894 | EM | |
| Yu et al. 2022 [30] | NCT03867201 | 70 | 557 | CM | |
| Tepper et al. 2024 [31] | NCT03971071 | 70, 140 | 619 | CM + MO | |
| Fremanezumab | Dodick et al. 2018 [32] | NCT02629861 | 225, 675 | 875 | EM |
| Ferrari et al. 2019 [33] | NCT03308968 | 225, 675 | 838 | EM, CM | |
| Bigal et al. 2015 [34] | NCT02021773 | 225, 675, 900 | 263 | CM | |
| Silberstein et al. 2017 [35] | NCT02621931 | 225, 675 | 1130 | CM | |
| Sakai et al. 2021 [36] | NCT03303079 | 225, 675 | 569 | CM | |
| Bigal et al. 2015 (2) [37] | NCT02025556 | 225, 675 | 296 | HFEM | |
| Sakai et al. 2021 (2) [38] | NCT03303092 | 225, 675 | 356 | EM | |
| Galcanezumab | Skljarevski et al. 2018 [39] | NCT02163993 | 5, 50, 12, 300 | 410 | EM |
| Stauffer et al. 2018 [40] | NCT02614183 | 120, 240 | 858 | EM | |
| Dodick et al. 2014 [41] | NCT01625988 | 150 | 217 | Migraine | |
| Mulleners et al. 2020 [42] | NCT03559257 | 120 | 462 | RM | |
| Skljarevski et al. 2018 (2) [43] | NCT02614196 | 120, 240 | 915 | EM | |
| Dodick et al. 2020 [44] | NCT02438826 | 300 | 237 | CCH | |
| Hu et al. 2022 [45] | NCT03963232 | 120, 240 | 520 | EM | |
| Detke et al. 2018 [46] | NCT02614261 | 120, 240 | 1113 | CM | |
| Sakai et al. 2020 [47] | NCT02959177 | 120, 240 | 459 | EM | |
| Eptinezumab | Ashina et al. 2022 [48] | NCT04418765 | 100, 300 | 891 | RM |
| Dodick et al. 2014 (2) [49] | NCT01772524 | 1000 | 163 | HFEM | |
| Yu et al. 2023 [50] | NCT04772742 | 100 | 193 | CM + MO | |
| Dodick et al. 2019 [51] | NCT02275117 | 10, 30, 100, 300 | 617 | CM | |
| Ashina et al. 2020 [52] | NCT02559895 | 30, 100, 300 | 888 | EM | |
| Lipton et al. 2020 [53] | NCT02974153 | 100, 300 | 1072 | CM | |
| Atogepant | Ailani et al. 2021 [54] | NCT03777059 | 10, 30, 60 | 902 | EM |
| Pozo-Rosich et al. 2023 [55] | NCT03855137 | 30, 60 | 773 | CM | |
| Goadsby et al. 2020 [56] | NCT02848326 | 10, 30, 60 | 825 | EM | |
| Tassorelli et al., 2024 [57] | NCT04740827 | 60 | 313 | RM | |
| Rimegepant | Croop et al. 2021 [58] | NCT03732638 | 75 | 741 | Migraine |
EM– episodic migraine, CM– chronic migraine, RM– resistant migraine, MOH– medication overuse, CCH– chronic cluster headache, HFEM– high frequency episodic migraine
Erenumab
The meta-analysis included 10 studies with 5704 participants aged 41.9 (SD = 11.8). The details of each analysis have been presented in Supplement 1. The studies had low heterogeneity (Q = 7.59, df = 9, p = 0.576, I2 = 0.00%) and their publication bias was also low in funnel plot and Egger’s tests (t = -0.942, df = 8, p = 0.374). Additionally, the ‘trim-and-fil test’ did not identify biased trials. L’Abbe and Galbraith diagram analysis showed that particular study results followed the combined effect. Sensitivity analysis did not show that the exclusion of one study from the analysis led to a significant RR change.
Erenumab has not been shown to increase the risk of viral, bacterial, UTI and overall infection risk (Table 3). These results were consistent across ‘high’, ‘registered/clinical’ (Fig. 2) or ‘any’ dosing schemes. There was only one study assessing COVID-19 [31], so in that case a meta-analysis could not be performed. In this trial erenumab significantly increased the risk of COVID-19 infection. In this case the RR of COVID-19 was 2.07 [1.18, 3.61, p = 0.008, power of the test being calculated post-hoc was 1 - β = 0.84]. In other words, erenumab has a medium effect on COVID-19 infection risk. None of the four moderating variables included in the meta-regression (gender, age, sample size, and year of publication) had a significant impact on the overall effect of the meta-analysis.
Table 3.
Dose and infection-type dependent risk of infection in patients treated with erenumab vs. Placebo
| Erenumab dose | Statistics | Infection | ||||
|---|---|---|---|---|---|---|
| Any | Viral | Bacterial | COVID-19* | UTI | ||
| Any | p- value | 0.753 | 0.464 | 0.921 | 0.008 | 0.561 |
| RR | 1.04 | 1.04 | 1.03 | 2.07 | 0.77 | |
| Clinical | p-value | 0.397 | 0.447 | 0.615 | 0.008 | 0.720 |
| RR | 1.05 | 1.05 | 1.15 | 2.07 | 0.88 | |
| High | p-value | 0.140 | 0.300 | 0.597 | 0.005 | 0.827 |
| RR | 1.13 | 1.12 | 1.19 | 2.35 | 0.92 | |
COVID-19 - Coronavirus disease 2019; *Only one study assessed COVID-19 frequency, so the results regarding this infection represent risk reported
Fig. 2.
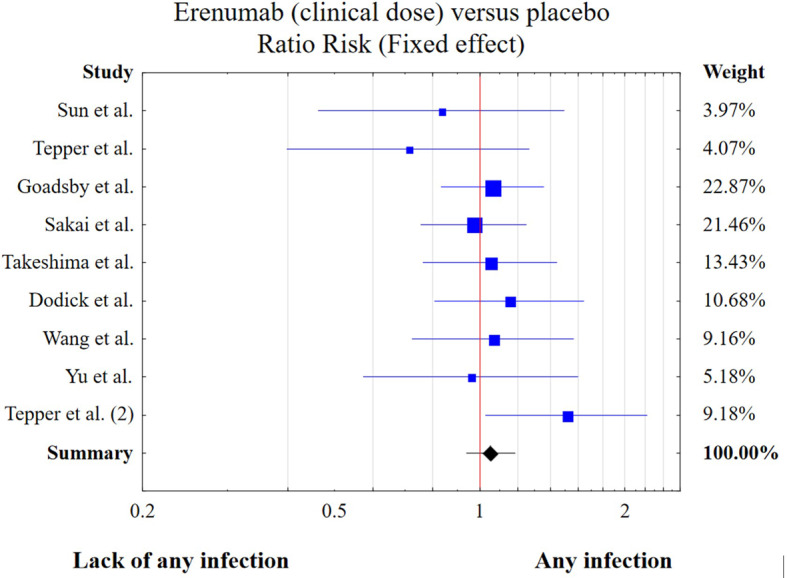
Risk of any infection in patients treated with clinical doses of erenumab vs. placebo
Fremanezumab
The meta-analysis included 7 studies with 4327 participants aged 42.7 years (SD = 11.4). The details of each analysis have been presented in Supplement 2. The studies had low heterogeneity (Q = 1.62, df = 6, p = 0.951, I2 = 0.00%) and their publication bias was also low in funnel plot and Egger’s tests (t = 0.411, df = 5, p = 0.698). Moreover, the ‘trim-and-fil test’ did not identify biased trials. L’Abbe and Galbraith diagram analysis showed that particular study results followed the combined effect. Sensitivity analysis did not show that the exclusion of one study from the analysis led to a significant RR change.
Fremanezumab has not been shown to increase the risk of viral, bacterial, UTI and overall infection risk (Table 4). The results were consistent across ‘high’, ‘registered/clinical’ (Fig. 3) or ‘any’ dosing schemes. None of the four moderating variables included in the meta-regression had a significant impact on the overall effect of the meta-analysis.
Table 4.
Dose and infection-type dependent risk of infection in patients treated with fremanezumab vs. placebo
| Fremanezumab dose | Statistics | Infection | ||||
|---|---|---|---|---|---|---|
| Any | Viral | Bacterial | COVID-19 | Urinary | ||
| Any | p- value | 0.900 | 0.894 | 0.960 | - | 0.812 |
| RR | 1.01 | 0.99 | 1.01 | 1.08 | ||
| Clinical | p-value | 0.457 | 0.429 | 0.625 | - | 0.646 |
| RR | 1.08 | 1.07 | 1.15 | 1.18 | ||
| High | p- value | 0.855 | 0.602 | 0.650 | - | 0.345 |
| RR | 0.99 | 0.95 | 1.12 | 1.37 | ||
COVID-19 - Coronavirus disease 2019; UTI– urinary tract infection; RR– risk ratio
Fig. 3.

Risk of any infection in patients treated with clinical doses of fremanezumab vs. placebo
Galcanezumab
The meta-analysis included 9 studies with 5191 participants aged 41.8 years (SD = 11.3). The details of each analysis have been presented in Supplement 3. The studies had low heterogeneity (Q = 6.94, df = 8, p = 0.543, I2 = 0.00%) and their publication bias was also low in funnel plot and Egger’s tests (t = 1.543, df = 7, p = 0.167). Furthermore, the ‘trim-and-fil test’ did not identify biased trials. L’Abbe and Galbraith diagram analysis showed that results followed the combined effect. Sensitivity analysis did not show that the exclusion of one study from the analysis led to a significant RR change. However, one exception was subanalysis of UTIs where excluding one publication (RCT5 [43]) significantly affected the result of the meta-analysis. The relative risk of UTIs after excluding this study will be RR = 1.74 [(1.00; 3.00), p = 0.049], which indicates result bordering on insignificance.
Galcanezumab increased the overall risk of any or viral infections at clinical doses (Table 5, Fig. 4). The number needed to harm (NNH) for any infection was 77 (E1 = 328, E0 = 1055, C1 = 517, C0 = 1790). In this case the RR of infection was 1.13 [1.00, 1.28, p = 0.024, power of the test calculated post-hoc was 1 - β = 0.81]. In other words, galcanezumab has a small effect on infection risk. No increase in infection risk was found for bacterial infections, COVID-19 or UTIs. The results were not significant for high or ‘any dose’s used. In patients receiving high doses of galcanezumab female sex was associated with lower risk of infections ( b = -0.010, Z = -2.003, p = 0.045). None of the other three moderating variables included in the meta-regression had a significant impact on the overall effect of the meta-analysis.
Table 5.
Dose and infection-type dependent risk of infection in patients treated with galcanezumab vs. Placebo
| Galcanezumab dose | Statistics | Infection | ||||
|---|---|---|---|---|---|---|
| Any | Viral | Bacterial | COVID-19 | Urinary | ||
| Any | p- value | 0.426 | 0.632 | 0.228 | 0.315 | 0.226 |
| RR | 1.04 | 1.02 | 1.26 | 0.33 | 1.31 | |
| Clinical | p-value | 0.024 | 0.046 | 0.317 | 0.315 | 0.669 |
| RR | 1.13 | 1.13 | 1.25 | 0.33 | 1.13 | |
| High | p- value | 0.855 | 0.234 | 0.202 | - | 0.074 |
| RR | 0.98 | 0.92 | 1.35 | - | 1.61 | |
COVID-19 - Coronavirus disease 2019; UTI– urinary tract infection; RR– risk ratio
Fig. 4.

Risk of any infection in patients treated with clinical doses of galcanezumab vs. placebo
Eptinezumab
The meta-analysis included 6 studies with 3824 participants aged 40.4 years (SD = 10.7). The details of each analysis have been presented in Supplement 4. The studies had low heterogeneity (Q = 1.10, df = 5, p = 0.954, I2 = 0.00%) and their publication bias was also low in funnel plot and Egger’s tests (t = 0.484, df = 4, p = 0.654). Additionally, the ‘trim-and-fil test’ did not identify biased trials. L’Abbe and Galbraith diagram analysis showed that particular study results followed the combined effect. Sensitivity analysis did not show that the exclusion of one study from the analysis led to significant RR change.
Eptinezumab has been shown to increase the overall risk of any or viral infections at higher doses (Table 6, Fig. 5). NNH for any infection equals 24 (E1 = 239, E0 = 832, C1 = 197, C0 = 893). In this case, the RR of infection was 1.23 [1.08, 1.40, p = 0.015, power of the test calculated post-hoc was 1 - β = 0.82]. In other words, eptinezumab has a small effect on infection risk. No increased infection risk was found for bacterial infections, COVID-19 or UTIs. The results were not significant for registered clinical doses or ‘any dose’ used. None of the four moderating variables included in the meta-regression had a significant impact on the overall effect of the meta-analysis.
Table 6.
Dose and infection-type dependent risk of infection in patients treated with eptinezumab vs. Placebo
| Eptinezumab dose | Statistics | Infection | ||||
|---|---|---|---|---|---|---|
| Any | Viral | Bacterial | COVID-19 | Urinary | ||
| Any | p- value | 0.717 | 0.599 | 0.585 | 0.604 | 0.421 |
| RR | 1.03 | 1.04 | 0.87 | 1.16 | 0.81 | |
| Clinical | p-value | 0.472 | 0.411 | 0.699 | 0.604 | 0.760 |
| RR | 1.06 | 1.07 | 0.90 | 1.16 | 0.92 | |
| High | p- value | 0.015 | 0.038 | 0.246 | 0.827 | 0.439 |
| RR | 1.23 | 1.21 | 1.37 | 1.08 | 1.25 | |
COVID-19 - Coronavirus disease 2019; UTI– urinary tract infection; RR– risk ratio
Fig. 5.

Risk of any infection in patients treated with higher doses of eptinezumab vs. placebo
Atogepant and Rimegepant
Due to the limited number of studies for rimegepant, the meta-analysis was possible only for atogepant. It included 4 studies with 2813 participants aged 41.2 years (SD = 1.1). The details of each analysis are presented in Supplement 5. The studies had low heterogeneity (Q = 1.93, df = 3, p = 0.587, I2 = 0.00%) and their publication bias was also low in funnel plot and Egger’s tests (t = -0.152, df = 2, p = 0.893). Moreover, the ‘trim-and-fil test’ did not identify biased trials. L’Abbe and Galbraith diagrams analysis showed that particular studies results followed the combined effect. Sensitivity analysis did not show that the exclusion of one study from the analysis led to a significant RR change.
Atogepant has not been shown to increase the risk of viral, bacterial, UTIs and overall infection (Table 7). The results were consistent across ‘high’, ‘registered’ (Fig. 6) or ‘any’ dosing schemes. None of the four moderating variables included in the meta-regression had a significant impact on the overall effect of the meta-analysis.
Table 7.
Dose and infection-type dependent risk of infection in patients treated with Atogepant vs. Placebo
| Atogepant dose | Statistics | Infection | ||||
|---|---|---|---|---|---|---|
| Any | Viral | Bacterial | COVID-19 | Urinary | ||
| Any | p- value | 0.839 | 0.627 | 0.285 | 0.585 | 0.349 |
| RR | 1.02 | 0.94 | 1.33 | 0.85 | 1.28 | |
| Clinical | p-value | 0.866 | 0.655 | 0.278 | 0.585 | 0.344 |
| RR | 1.03 | 0.95 | 1.33 | 0.85 | 1.29 | |
| High | p- value | 0.866 | 0.597 | 0.259 | 0.585 | 0.303 |
| RR | 1.02 | 0.93 | 1.39 | 0.85 | 1.35 | |
COVID-19 - Coronavirus disease 2019; UTI– urinary tract infection; RR– risk ratio
Fig. 6.

Risk of any infection in patients treated with clinical doses of atogepant vs. placebo
Meta-analysis for all CGRP-targeting preventive therapeutics
Only two studies included in the meta-analysis showed significant increase of infection risk (RCT10 for erenumab [31] and RCT 25 for galcanezumab [47]) (Fig. 7). Despite that, the meta-analysis showed an increased infection risk in patients treated with CGRP-targeting therapeutics (p = 0.016, RR = 1.08 [1.01, 1.14]) with NNH = 287 (E1 = 1893, E0 = 8664, C1 = 1423, C0 = 6670) (Fig. 7). This result was also significant for viral infections (RR = 1.07 [1.00, 1.14]), but not for COVID-19 or bacterial infections or other dosing schemes. The details of each analysis are presented in Supplements 6 and 7. In the sensitivity analysis exclusion of one study from the meta-analysis did not significantly affect the results, but excluding two studies (RCT25 [46] and 26 [47]) lead to loss of significance for the whole meta-analysis (RR = 1.06 [0.99; 1.14]).
Fig. 7.
Risk of any infection in patients treated with clinical doses of CGRP-targeting medications. RR– relative risk, LL– lower limit, UL– upper limit)
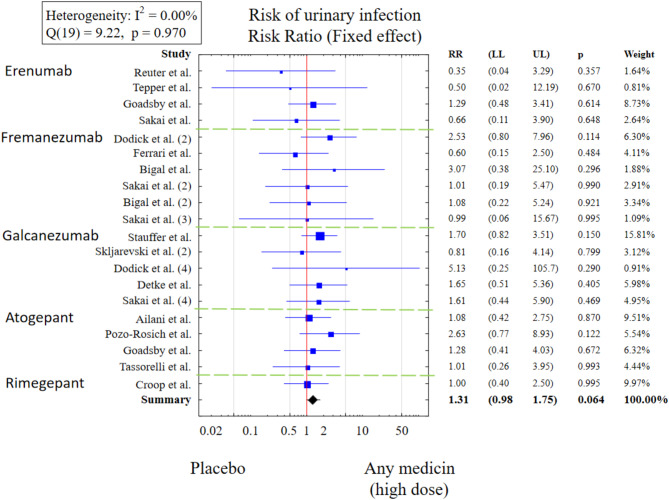
Risk of UTIs in people treated with high doses of CGRP-targeting medications was also not increased (RR = 1.31 [0.98, 1.75]) (Fig. 8). However, a sensitivity analysis indicates that excluding RCT1 (erenumab) [22] and RCT12 (fremanezumab) [33] from the meta-analysis significantly affects the results (p = 0.029). After excluding these two publications from the meta-analysis, the risk of urinary tract infections among patients taking any anti-CGRP drug is significantly higher compared to patients taking placebo (p = 0.029; RR = 1.39 [1.03, 1.87]). A negative, statistically significant relationship was observed between the logarithm of relative risk for UTIs and the age of patients taking anti-CGRP medications at clinical doses. An increase in the mean age in the study by one year was accompanied by a decrease in the logarithm of RR for UTIs by an average of 0.12.
Fig. 8.
Risk of urinary tract infections in patients treated with clinical doses of CGRP-targeting medications (RR– relative risk, LL– lower limit, UL– upper limit).
Infectious SAEs
Database search yielded 30 studies with gastrointestinal, respiratory and genitourinary infectious SAEs being the most often reported complaints (Table 8). Sepsis was reported in 5 instances, with 3 cases in the eptinezumab studies. Among all particles fremanezumab was associated with fewest infectious SAEs (n = 3), while erenumab showed highest incidence of these events (n = 36). However, it should be underlined that erenumab has far more studies assessing infectious SAEs than any other CGRP-targeting medication.
Table 8.
Serious infectious adverse events reported in blinded and open-label trials and real world evidence
| Gastrointestinal | Respiratory | Genitourinary | Skin & connective tissue | Sepsis | Other & unclassified | |
|---|---|---|---|---|---|---|
| Erenumab | ||||||
| NCT03096834 | 1 | 1 | 1 | 1 | ||
| NCT01952574 | 3 | 1 | 1 | 1 | ||
| NCT02456740 | 3 | 1 | 1 | 1 | ||
| NCT02630459 | 4 | 3 | ||||
| NCT03812224 | 1 | |||||
| NCT02483585 | 1 | 1 | 1 | 1 | ||
| NCT03333109 | 1 | |||||
| NCT03867201 | 1 | |||||
| NCT03971071 | 1 | |||||
| NCT02174861 | 1 | 1 | ||||
| BASEC ID 2018–02375 | 2 | 1 | ||||
| Fremanezumab | ||||||
| NCT03308968 | 1 | |||||
| NCT02021773 | 1 | |||||
| NCT02621931 | 1 | |||||
| Galcanezumab | ||||||
| NCT03559257 | 2 | |||||
| NCT02614196 | 1 | |||||
| NCT02438826 | 4 | |||||
| NCT03963232 | 1 | 1 | 1 | |||
| NCT02614261 | 1 | 1 | 2 | 1 | 1 | |
| NCT02959190 | 1 | |||||
| Eptinezumab | ||||||
| NCT04418765 | 5 | 5 | 1 | 1 | 3 | |
| NCT01772524 | 1 | |||||
| NCT02275117 | 1 | 1 | ||||
| NCT02974153 | 1 | |||||
| Rimegepant | ||||||
| NCT03266588 | 2 | 2 | 1 | 1 | ||
| NCT03732638 | 1 | |||||
| Atogepant | ||||||
| NCT03855137 | 1 | 2 | ||||
| NCT03700320 | 2 | 1 | 1 | |||
| NCT03939312 | 2 | 1 | 1 | |||
| NCT02848326 | 1 | |||||
| 36 | 29 | 10 | 7 | 5 | 7 | |
Gastrointestinal SAEs: gastroenterocolitis, gastrointestinal tuberculosis, herpes simplex hepatitis, diverticulitis, abdominal abscess, tooth abscess, rectal abscess, peritonitis, anal abscess, Clostridium difficile colitis; respiratory SAEs: pneumonia, mycoplasma infection, pharyngitis, tonsillitis, COVID-19, influenza; genitourinary SAEs: urinary tract infection, pyelonephritis, tubo-ovarian abscess, vaginal abscess; Skin and connective tissue SAEs: cellulitis, erysipelas, infected dermal cyst, pilonidal disease; Sepsis included 1 case of bacteraemia; other and unclassified SAEs: beta-haemolytic streptococcal infection, staphylococcal infection, pericarditis, meningitis, osteomyelitis, mastitis
Discussion
This meta-analysis pools data from randomised placebo-controlled trials and indicates that CGRP-targeting therapies may increase infection risk. However, the overall risk seems to be very low, with NNH as high as 287 and only two studies separately showing higher incidence of infections. In this light, the results should be interpreted carefully.
Only two separate studies showed statistically significant increased infection risk However, the advantage of the meta-analysis is to show weaker associations for which smaller studies are underpowered. To ensure that the observed result is not merely an effect of one positive trial we performed a sensitivity analyses. These showed that excluding any or both of the two of positive trials did not result in loss of significance. However, excluding two studies for galcanezumab (one positive and one negative) lead to that effect. Conversely, UTI risk seems not to be increased but sensitivity analysis indicated that excluding two studies changed the meta-analysis results from negative to positive. It should be than underlined that the sensitivity analysis does not dismiss the associations found in this meta-analysis but merely underlines that the effect found is small and requires further studies.
It seems that infection risk depends on particular medication, with eptinezumab and galcanezumab being the only drugs with risk increase in particle-specific meta-analysis. Despite negative results for other particles it should be remembered that also erenumab, atogepant and rimegepant have been implicated in increased risk of some infections by other authors [6, 8, 9]. The same has been reported for ubrogepant [59]. In this light, it seems probable that blocking the CGRP pathway may predispose to infections although overall clinical effect will be very small in otherwise healthy migraine patients. Considering small effect of CGRP-targeting medications on infection risk it is also unsurprising that only subthreshold signals were found that this risk depends on medication dosing or pathogen type.
Infection risk depends on a multitude of factors that could not be evaluated by this meta-analysis. For example, upper respiratory tract infection risk increases with social interactions, while migraine burden limits these interactions [60]. It is then possible that improvement with regard to migraine achieved by CGRP targeting therapies might have led to an increased exposure to viruses, which in turn could explain increased infection risk. Apart from that, CGRP-targeting therapies may cause constipation, which in turn is a risk factor of UTIs [61]– a phenomenon that could explain why atogepant was implicated to cause UTIs. Eptinezumab effects could also be related to intravenous administration of this drug and its higher risk of infections than subcutaneous or oral application.
The body’s response to an infection or parasitic invasion is inflammation, which may lead to elimination or neutralization of the pathogen. One such mechanism is the production and release of immunomodulatory neuropeptides like CGRP which can be produced by various cell types involved in the response to viruses and bacteria [12]. CGRP can promote anti-, as well as pro-inflammatory processes required for mounting an effective immune response. On one hand, it inhibits antigen presentation, the production of some crucial interleukins (e.g. IL-1β) and chemokines involved in innate immune response, as well as the targeted migration of inflammatory cells [62–66]. On the other hand, it increases the numbers of circulating granulocytes, monocytes and lymphocytes, stimulates mast cells degranulation, as well as promotes certain T-helper lymphocytes subtypes [67, 68]. In this light, CGRP(-R) antagonists may potentially alter these processes with difficult to predict clinical consequences.
In case of viral infections such as COVID-19, CGRP prevents viral replication, propagation and cross-reactivity [14, 69]; this has been especially proven by the Barbosa Bomfim et al. study, which gives biological evidence that blocking the CGRP pathway may promote severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The researchers showed that CGRP can directly prevent SARS-CoV-2 Omicron and Alpha variants from infecting bronchial epithelial cells. This effect was nullified by blocking CGRP receptors with olcegepant. Consequently, CGRP pathway antagonists may potentially increase the risk of COVID-19. This hypothesis has been recently supported by a randomized placebo-controlled trial which demonstrated a more than double risk of SARS-CoV-2 infection in people treated with erenumab [31]. Both of the above described studies indicate that increased COVID-19 risk in people undergoing CGRP-targeting therapies is biologically and clinically plausible. However, it should also be noted that several retrospective studies [70, 71] and our own meta-analysis did not find this association. This may be related to an overall small clinical effect or underreporting of mild cases and low risk of severe COVID-19.
In response to bacterial infection CGRP plays contradictory roles. On the one hand, it potentially contributes to bacterial infections by inhibiting innate immune response and reaction to Gram-negative microbes [15, 72–74]. On the other hand, CGRP shows antimicrobial activity and increases the production of protective mucous as well as elimination of the digested pathogenic bacteria [75, 76]. Furthermore, it may play a role in protecting tissue against damage during sepsis [77]. In this light, CGRP-pathway antagonists may influence mutually contradictory mechanisms and cause little net effect. This might be why no significant safety signals where identified by our meta-analysis.
Safety concerns for CGRP-targeting therapies have been pointed out by some authors [78, 79]. When interpreting the results of this meta-analysis readers should also take into account that it focuses mostly on otherwise healthy migraine patients. People with ‘any clinically significant hematologic, endocrine, pulmonary, renal, hepatic, gastrointestinal, or neurologic disease’ were barred from participation. In other words, some populations vulnerable to infection were underrepresented: elderly participants, people on immunosuppressive therapies, patients with acquired immunodeficiency, chronic disorders affecting susceptibility to infections like diabetes, chronic kidney disease, chronic obstructive pulmonary disease or severe cardiovascular disorders. However, the prevalence of migraine in these populations remains significant [80] and their increased use of CGRP-targeting therapies can be anticipated. Moreover, in most trials the placebo-controlled phase lasted 3 months. This timeframe could be insufficient to identify increased infection risk if CGRP(-R) antagonists cause immunosuppression developing over a longer period.
Limitations
While the findings of this meta-analysis are based on high-quality evidence from randomized placebo-controlled trials, the limited number of studies and short duration of placebo-controlled phases should be taken into account when interpreting the results. It should also be noted that some particles (e.g. erenumab) have considerably more studies assessing infectious AEs than others (e.g. rimegepant). Therefore, it is impossible to compare the infection risk between included medications. Future research with more trials and diverse populations would help provide more robust and generalizable conclusions.
This meta-analysis assesses the infection risk in RCTs designed to test drug efficacy. In other words, the included trials were not specified to systematically evaluate infection incidence. Consequently, a bias of underreporting of infections should be expected, especially with regard to mild disorders self-treated by 78–95% patients [81]. Moreover, included studies differed with regard to recruited populations and diagnoses. It is therefore possible that unidentified factors contributed to the obtained results.
Conclusions
The wide systemic distribution of CGRP and its receptor, as well as the involvement of CGRP in many immunological processes, indicate the role of this neuropeptide in maintaining pathogen immunity against all major groups of infective agents. In this meta-analysis CGRP-targeting medications have a statistically significant but clinically weak effect on the increase in infection risk. These results should be interpreted carefully, considering that only few studies showed significant infection risk. Consequently, a CGRP pathway block may be insignificant to the majority of patients, and would rarely contribute to serious adverse reactions in populations without comorbidities affecting immunity. However, increased infection risk may prove to be important to healthcare systems when considering the high prevalence of migraine and increasing popularity of CGRP-targeting therapies. Further studies are needed to assess the safety of these medications, especially in immunocompromised patients (e.g. on immunosuppressive therapies).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- CGRP
Calcitonin gene-related peptide
- COVID-19
Coronavirus disease 2019
- EER
Percentage of participants in the experimental group who experienced an adverse effect (infection)
- NNH
Number Needed to Harm
- RD
Risk Difference
- RR
Relative risk
- SAE
Serious adverse event
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- UTI
Urinary tract infection
Author contributions
MS: project concept, project coordination, protocol draft and registration, database search, data extraction and analysis, risk of bias assessment, writing final manuscript. DK: studies selection and data extraction. KM: studies selection and data extraction, review of the meta-analysis and final manuscript. BB: statistical analysis coordination, review of the final manuscript. EKW: literature search and writing WK: literature search and writing. BM: review of statistical analysis project and review of the meta-analysis, review of the final manuscript. MWP: review of study concept and the final manuscript. AM: review of study concept and the final manuscript.
Funding
This study was supported by statutory financing from University of Warmia and Mazury, Olsztyn, Poland and Wroclaw Medical University, Wroclaw, Poland. The publication was funded by the Minister of Science under the'Regional Initiative of Excellence Program'.
Data availability
The datasets generated and/or analysed during the current study are available in the public repository: https://figshare.com/articles/dataset/CGRP_targeting_therapies_may_increase_infection_risk_a_meta-analysis_of_placebo-controlled_trials_and_a_narrative_review/28375523/1.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
MS reports personal fees from Pfizer, Teva, Bausch, AbbVie, Neuca and Novartis for speaker activities. DK reports no competing interests. KM reports no competing interests. BB reports no competing interests. EKW reports no competing interests. WK reports no competing interests. BM reports no competing interests. KPP reports no competing interests. MW-P is member of Editorial Board: The Journal of Headache and Pain; reports personalfees from Abb. Vie, Pfizer, Polpharma and Teva for speaker activities. AM reports personal fees from Novartis, Betapharm, TEVA and Ipsen for speaker activities or advisory boards.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Puledda F, Sacco S, Diener H-C et al (2024) International headache society global practice recommendations for preventive Pharmacological treatment of migraine. Cephalalgia 44:03331024241269735. 10.1177/03331024241269735 [DOI] [PubMed] [Google Scholar]
- 2.Waliszewska-Prosół M, Raffaelli B, Straburzyński M, Martelletti P (2024) Understanding the efficacy and tolerability of migraine treatment: a deep dive into CGRP antagonists. Expert Rev Clin Pharmacol 1–13. 10.1080/17512433.2024.2417655 [DOI] [PubMed]
- 3.Haghdoost F, Puledda F, Garcia-Azorin D et al (2023) Evaluating the efficacy of CGRP mAbs and gepants for the preventive treatment of migraine: A systematic review and network meta-analysis of phase 3 randomised controlled trials. Cephalalgia 43:033310242311593. 10.1177/03331024231159366 [DOI] [PubMed] [Google Scholar]
- 4.Karlsson WK, Ostinelli EG, Zhuang ZA et al (2024) Comparative effects of drug interventions for the acute management of migraine episodes in adults: systematic review and network meta-analysis. BMJ E 080107. 10.1136/bmj-2024-080107 [DOI] [PMC free article] [PubMed]
- 5.Messina R, Huessler E-M, Puledda F et al (2023) Safety and tolerability of monoclonal antibodies targeting the CGRP pathway and gepants in migraine prevention: A systematic review and network meta-analysis. Cephalalgia 43:033310242311521. 10.1177/03331024231152169 [DOI] [PubMed] [Google Scholar]
- 6.Pellesi L, De Icco R, Alawie HY et al (2021) A systematic review, meta-analysis and meta-regression evaluating the adverse reactions to erenumab in the preventive treatment of migraine. Exp Opin Drug Saf 20:467–474. 10.1080/14740338.2021.1866537 [DOI] [PubMed] [Google Scholar]
- 7.Gklinos P, Mitsikostas DD (2020) Galcanezumab in migraine prevention: a systematic review and meta-analysis of randomized controlled trials. Ther Adv Neurol Disord 13:1756286420918088. 10.1177/1756286420918088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou M, Luo X, He S et al (2024) Efficacy and safety of Atogepant, a small molecule CGRP receptor antagonist, for the preventive treatment of migraine: a systematic review and meta-analysis. J Headache Pain 25:116. 10.1186/s10194-024-01822-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong G, Kjærgaard NA, Shakibfar S, Sessa M (2023) Ubrogepant and Rimegepant: systematic review, meta-analysis, and meta-regression of clinical studies. Exp Opin Drug Saf 22:59–70. 10.1080/14740338.2023.2177270 [DOI] [PubMed] [Google Scholar]
- 10.on behalf of Lifting The Burden: the Global Campaign against Headache, Steiner TJ, Stovner LJ et al (2020) Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain 21:137, s10194-020-01208–0. 10.1186/s10194-020-01208-0 [DOI] [PMC free article] [PubMed]
- 11.Kirkland KE, Kirkland K, Many WJ Jr, Smitherman TA (2012) Headache among patients with HIV disease: prevalence, characteristics, and associations. Headache 52:455–466. 10.1111/j.1526-4610.2011.02025.x [DOI] [PubMed] [Google Scholar]
- 12.Holzmann B (2013) Antiinflammatory activities of CGRP modulating innate immune responses in health and disease. CPPS 14:268–274. 10.2174/13892037113149990046 [DOI] [PubMed] [Google Scholar]
- 13.Ray JC, Allen P, Bacsi A et al (2021) Inflammatory complications of CGRP monoclonal antibodies: a case series. J Headache Pain 22:121. 10.1186/s10194-021-01330-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barbosa Bomfim CC, Génin H, Cottoignies-Callamarte A et al (2024) CGRP inhibits SARS-CoV-2 infection of bronchial epithelial cells, and its pulmonary levels correlate with viral clearance in critical COVID-19 patients. J Virol 98:e00128–e00124. 10.1128/jvi.00128-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu F, Yu D, Qin X et al (2023) The neuropeptide CGRP enters the macrophage cytosol to suppress the NLRP3 inflammasome during pulmonary infection. Cell Mol Immunol 20:264–276. 10.1038/s41423-022-00968-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Fiscus RR, Yang L, Mathews HL (1995) Suppression of the functional activity of IL-2-Activated lymphocytes by CGRP. Cell Immunol 162:105–113. 10.1006/cimm.1995.1057 [DOI] [PubMed] [Google Scholar]
- 17.Xu H, Ding J, Porter CBM et al (2019) Transcriptional atlas of intestinal immune cells reveals that neuropeptide α-CGRP modulates group 2 innate lymphoid cell responses. Immunity 51:696–708e9. 10.1016/j.immuni.2019.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zorzela L, Loke YK, Ioannidis JP et al (2016) PRISMA harms checklist: improving harms reporting in systematic reviews. 10.1136/bmj.i157. BMJ i157 [DOI] [PubMed]
- 19.Straburzyński M (2025) CGRP targeting therapies may increase infection risk– a meta-analysis of placebo-controlled trials and a narrative review
- 20.Martin-Loeches I, Torres A, Nagavci B et al (2023) ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Intensive Care Med 49:615–632. 10.1007/s00134-023-07033-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16:626–638. 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 22.Reuter U, Goadsby PJ, Lanteri-Minet M et al (2018) Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet 392:2280–2287. 10.1016/S0140-6736(18)32534-0 [DOI] [PubMed] [Google Scholar]
- 23.Sun H, Dodick DW, Silberstein S et al (2016) Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 15:382–390. 10.1016/S1474-4422(16)00019-3 [DOI] [PubMed] [Google Scholar]
- 24.Tepper S, Ashina M, Reuter U et al (2017) Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 16:425–434. 10.1016/S1474-4422(17)30083-2 [DOI] [PubMed] [Google Scholar]
- 25.Goadsby PJ, Reuter U, Hallström Y et al (2017) A controlled trial of erenumab for episodic migraine. N Engl J Med 377:2123–2132. 10.1056/NEJMoa1705848 [DOI] [PubMed] [Google Scholar]
- 26.Sakai F, Takeshima T, Tatsuoka Y et al (2019) A randomized phase 2 study of erenumab for the prevention of episodic migraine in Japanese adults. Headache 59:1731–1742. 10.1111/head.13652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeshima T, Sakai F, Hirata K et al (2021) Erenumab treatment for migraine prevention in Japanese patients: efficacy and safety results from a phase 3, randomized, double-blind, placebo‐controlled study. Headache 61:927–935. 10.1111/head.14138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dodick DW, Ashina M, Brandes JL et al (2018) ARISE: A phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia 38:1026–1037. 10.1177/0333102418759786 [DOI] [PubMed] [Google Scholar]
- 29.Wang S-J, Roxas AA, Saravia B et al (2021) Randomised, controlled trial of erenumab for the prevention of episodic migraine in patients from Asia, the middle East, and Latin America: the empower study. Cephalalgia 41:1285–1297. 10.1177/03331024211024160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu S, Kim B-K, Wang H et al (2022) A phase 3, randomised, placebo-controlled study of erenumab for the prevention of chronic migraine in patients from Asia: the DRAGON study. J Headache Pain 23:146. 10.1186/s10194-022-01514-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tepper SJ, Dodick DW, Lanteri-Minet M et al (2024) Efficacy and safety of erenumab for nonopioid medication overuse headache in chronic migraine: A phase 4, randomized, Placebo-Controlled trial. JAMA Neurol 81:1140. 10.1001/jamaneurol.2024.3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dodick DW, Silberstein SD, Bigal ME et al (2018) Effect of fremanezumab compared with placebo for prevention of episodic migraine: A randomized clinical trial. JAMA 319:1999–2008. 10.1001/jama.2018.4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrari MD, Diener HC, Ning X et al (2019) Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet 394:1030–1040. 10.1016/S0140-6736(19)31946-4 [DOI] [PubMed] [Google Scholar]
- 34.Bigal ME, Edvinsson L, Rapoport AM et al (2015) Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of chronic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 14:1091–1100. 10.1016/S1474-4422(15)00245-8 [DOI] [PubMed] [Google Scholar]
- 35.Silberstein SD, Dodick DW, Bigal ME et al (2017) Fremanezumab for the preventive treatment of chronic migraine. N Engl J Med 377:2113–2122. 10.1056/NEJMoa1709038 [DOI] [PubMed] [Google Scholar]
- 36.Sakai F, Suzuki N, Kim B et al (2021) Efficacy and safety of fremanezumab for chronic migraine prevention: multicenter, randomized, double-blind, placebo‐controlled, parallel‐group trial in Japanese and Korean patients. Headache 61:1092–1101. 10.1111/head.14169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bigal ME, Dodick DW, Rapoport AM et al (2015) Safety, tolerability, and efficacy of TEV-48125 for preventive treatment of high-frequency episodic migraine: a multicentre, randomised, double-blind, placebo-controlled, phase 2b study. Lancet Neurol 14:1081–1090. 10.1016/S1474-4422(15)00249-5 [DOI] [PubMed] [Google Scholar]
- 38.Sakai F, Suzuki N, Kim B et al (2021) Efficacy and safety of fremanezumab for episodic migraine prevention: multicenter, randomized, double-blind, placebo‐controlled, parallel‐group trial in Japanese and Korean patients. Headache 61:1102–1111. 10.1111/head.14178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skljarevski V, Oakes TM, Zhang Q et al (2018) Effect of different doses of galcanezumab vs placebo for episodic migraine prevention: A randomized clinical trial. JAMA Neurol 75:187. 10.1001/jamaneurol.2017.3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stauffer VL, Dodick DW, Zhang Q et al (2018) Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE-1 randomized clinical trial. JAMA Neurol 75:1080. 10.1001/jamaneurol.2018.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dodick DW, Goadsby PJ, Spierings ELH et al (2014) Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol 13:885–892. 10.1016/S1474-4422(14)70128-0 [DOI] [PubMed] [Google Scholar]
- 42.Mulleners WM, Kim B-K, Láinez MJA et al (2020) Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol 19:814–825. 10.1016/S1474-4422(20)30279-9 [DOI] [PubMed] [Google Scholar]
- 43.Skljarevski V, Matharu M, Millen BA et al (2018) Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE-2 phase 3 randomized controlled clinical trial. Cephalalgia 38:1442–1454. 10.1177/0333102418779543 [DOI] [PubMed] [Google Scholar]
- 44.Dodick DW, Goadsby PJ, Lucas C et al (2020) Phase 3 randomized, placebo-controlled study of galcanezumab in patients with chronic cluster headache: results from 3-month double-blind treatment. Cephalalgia 40:935–948. 10.1177/0333102420905321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu B, Li G, Li X et al (2022) Galcanezumab in episodic migraine: the phase 3, randomized, double-blind, placebo-controlled PERSIST study. J Headache Pain 23:90. 10.1186/s10194-022-01458-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Detke HC, Goadsby PJ, Wang S et al (2018) Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology 91. 10.1212/WNL.0000000000006640 [DOI] [PMC free article] [PubMed]
- 47.Sakai F, Ozeki A, Skljarevski V (2020) Efficacy and safety of galcanezumab for prevention of migraine headache in Japanese patients with episodic migraine: A phase 2 randomized controlled clinical trial. Cephalalgia Rep 3:2515816320932573. 10.1177/2515816320932573 [Google Scholar]
- 48.Ashina M, Lanteri-Minet M, Pozo-Rosich P et al (2022) Safety and efficacy of eptinezumab for migraine prevention in patients with two-to-four previous preventive treatment failures (DELIVER): a multi-arm, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol 21:597–607. 10.1016/S1474-4422(22)00185-5 [DOI] [PubMed] [Google Scholar]
- 49.Dodick DW, Goadsby PJ, Silberstein SD et al (2014) Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 13:1100–1107. 10.1016/S1474-4422(14)70209-1 [DOI] [PubMed] [Google Scholar]
- 50.Yu S, Zhou J, Luo G et al (2023) Efficacy and safety of eptinezumab in patients with chronic migraine and medication-overuse headache: a randomized, double-blind, placebo-controlled study. BMC Neurol 23:441. 10.1186/s12883-023-03477-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dodick DW, Lipton RB, Silberstein S et al (2019) Eptinezumab for prevention of chronic migraine: A randomized phase 2b clinical trial. Cephalalgia 39:1075–1085. 10.1177/0333102419858355 [DOI] [PubMed] [Google Scholar]
- 52.Ashina M, Saper J, Cady R et al (2020) Eptinezumab in episodic migraine: A randomized, double-blind, placebo-controlled study (PROMISE-1). Cephalalgia 40:241–254. 10.1177/0333102420905132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lipton RB, Goadsby PJ, Smith J et al (2020) Efficacy and safety of eptinezumab in patients with chronic migraine: PROMISE-2. 10.1212/WNL.0000000000009169. Neurology 94: [DOI] [PMC free article] [PubMed]
- 54.Ailani J, Lipton RB, Goadsby PJ et al (2021) Atogepant for the preventive treatment of migraine. N Engl J Med 385:695–706. 10.1056/NEJMoa2035908 [DOI] [PubMed] [Google Scholar]
- 55.Pozo-Rosich P, Ailani J, Ashina M et al (2023) Atogepant for the preventive treatment of chronic migraine (PROGRESS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 402:775–785. 10.1016/S0140-6736(23)01049-8 [DOI] [PubMed] [Google Scholar]
- 56.Goadsby PJ, Dodick DW, Ailani J et al (2020) Safety, tolerability, and efficacy of orally administered Atogepant for the prevention of episodic migraine in adults: a double-blind, randomised phase 2b/3 trial. Lancet Neurol 19:727–737. 10.1016/S1474-4422(20)30234-9 [DOI] [PubMed] [Google Scholar]
- 57.Tassorelli C, Nagy K, Pozo-Rosich P et al (2024) Safety and efficacy of Atogepant for the preventive treatment of episodic migraine in adults for whom conventional oral preventive treatments have failed (ELEVATE): a randomised, placebo-controlled, phase 3b trial. Lancet Neurol 23:382–392. 10.1016/S1474-4422(24)00025-5 [DOI] [PubMed] [Google Scholar]
- 58.Croop R, Lipton RB, Kudrow D et al (2021) Oral Rimegepant for preventive treatment of migraine: a phase 2/3, randomised, double-blind, placebo-controlled trial. Lancet 397:51–60. 10.1016/S0140-6736(20)32544-7 [DOI] [PubMed] [Google Scholar]
- 59.Deng X, Zhou L, Liang C et al (2024) Comparison of effectiveness and safety of Lasmiditan and CGRP-antagonists for the acute treatment of migraine in adults: systematic review and network meta-analysis of randomised trials. J Headache Pain 25:16. 10.1186/s10194-024-01723-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Waliszewska-Prosół M, Straburzyński M, Czapińska-Ciepiela EK et al (2023) Migraine symptoms, healthcare resources utilization and disease burden in a large Polish migraine cohort: results from ‘migraine in Poland’—a nationwide cross-sectional survey. J Headache Pain 24:40. 10.1186/s10194-023-01575-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Axelgaard S, Kristensen R, Kamperis K et al (2023) Functional constipation as a risk factor for pyelonephritis and recurrent urinary tract infection in children. Acta Paediatr 112:543–549. 10.1111/apa.16608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hosoi J, Murphy GF, Egan CL et al (1993) Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature 363:159–163. 10.1038/363159a0 [DOI] [PubMed] [Google Scholar]
- 63.Mikami N, Matsushita H, Kato T et al (2011) Calcitonin Gene-Related peptide is an important regulator of cutaneous immunity: effect on dendritic cell and T cell functions. J Immunol 186:6886–6893. 10.4049/jimmunol.1100028 [DOI] [PubMed] [Google Scholar]
- 64.Assas MB, Wakid MH, Zakai HA et al (2016) Transient receptor potential vanilloid 1 expression and function in Splenic dendritic cells: a potential role in immune homeostasis. Immunology 147:292–304. 10.1111/imm.12562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fox FE, Kubin M, Cassin M et al (1997) Calcitonin Gene-Related peptide inhibits proliferation and antigen presentation by human peripheral blood mononuclear cells: effects on B7, Interleukin 10, and Interleukin12. J Invest Dermatology 108:43–48. 10.1111/1523-1747.ep12285627 [DOI] [PubMed] [Google Scholar]
- 66.Ding W, Stohl LL, Wagner JA, Granstein RD (2008) Calcitonin Gene-Related peptide biases Langerhans cells toward Th2-Type immunity. J Immunol 181:6020–6026. 10.4049/jimmunol.181.9.6020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fernandez S, Knopf MA, McGillis JP (2000) Calcitonin-gene related peptide (CGRP) inhibits interleukin-7-induced pre-B cell colony formation. J Leukoc Biol 67:669–676. 10.1002/jlb.67.5.669 [DOI] [PubMed] [Google Scholar]
- 68.Schlomer JJ, Storey BB, Ciornei R-T, McGillis JP (2007) Calcitonin gene-related peptide inhibits early B cell development in vivo. J Leukoc Biol 81:802–808. 10.1189/jlb.0306229 [DOI] [PubMed] [Google Scholar]
- 69.Robertson CE (2020) Could CGRP antagonists be helpful in the fight against COVID-19? headache: the journal of head and face pain head.13853. 10.1111/head.13853 [DOI] [PMC free article] [PubMed]
- 70.Wang K, Fenton BT, Deng Y et al (2023) Calcitonin Gene–Related peptide monoclonal antibodies and risk of SARS-CoV-2 infection and severe COVID-19 outcomes among veterans with migraine disorder. JAMA Netw Open 6:e2326371. 10.1001/jamanetworkopen.2023.26371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caronna E, José Gallardo V, Alpuente A et al (2021) Safety of anti-CGRP monoclonal antibodies in patients with migraine during the COVID-19 pandemic: present and future implications. Neurología (English Edition) 36:611–617. 10.1016/j.nrleng.2021.03.005 [DOI] [PubMed] [Google Scholar]
- 72.Bekdas M, Saygi B, Kilinc YB, Kilinc E (2024) Plasma levels of neurogenic inflammation related neuropeptides in pediatric patients with community-acquired pneumonia and their potential diagnostic value in distinguishing viral and bacterial pneumonia. Eur J Pediatr 183:1619–1627. 10.1007/s00431-023-05417-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feng Y, Tang Y, Guo J, Wang X (1997) Inhibition of LPS-induced TNF-α production by calcitonin gene-related peptide (CGRP) in cultured mouse peritoneal macrophages. Life Sci 61:PL281–PL287. 10.1016/S0024-3205(97)00866-7 [DOI] [PubMed] [Google Scholar]
- 74.Tang Y, Feng Y, Wang X (1998) Calcitonin gene-related peptide potentiates LPS-induced IL-6 release from mouse peritoneal macrophages. J Neuroimmunol 84:207–212. 10.1016/S0165-5728(97)00257-9 [DOI] [PubMed] [Google Scholar]
- 75.El Karim IA, Linden GJ, Orr DF, Lundy FT (2008) Antimicrobial activity of neuropeptides against a range of micro-organisms from skin, oral, respiratory and Gastrointestinal tract sites. J Neuroimmunol 200:11–16. 10.1016/j.jneuroim.2008.05.014 [DOI] [PubMed] [Google Scholar]
- 76.Benguettat O, Jneid R, Soltys J et al (2018) The DH31/CGRP enteroendocrine peptide triggers intestinal contractions favoring the elimination of opportunistic bacteria. PLoS Pathog 14:e1007279. 10.1371/journal.ppat.1007279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jusek G, Reim D, Tsujikawa K, Holzmann B (2012) Deficiency of the CGRP receptor component RAMP1 attenuates immunosuppression during the early phase of septic peritonitis. Immunobiology 217:761–767. 10.1016/j.imbio.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 78.Tana C, Cipollone F, Giamberardino MA, Martelletti P (2023) New drugs targeting calcitonin gene-related peptide for the management of migraines. Expert Opin Emerg Drugs 28:233–240. 10.1080/14728214.2023.2288334 [DOI] [PubMed] [Google Scholar]
- 79.Tana C, Cipollone F, Giamberardino MA (2023) New therapeutic options for migraine. CPD 29:1964–1966. 10.2174/1381612829666230821092238 [DOI] [PubMed] [Google Scholar]
- 80.Wang S-J (2010) Comorbidities of migraine. Front Neur 4. 10.3389/fneur.2010.00016
- 81.Melbye H, Joensen L, Risør MB, Halvorsen PA (2012) Symptoms of respiratory tract infection and associated care-seeking in subjects with and without obstructive lung disease; the Tromsø study: Tromsø 6. BMC Pulm Med 12:51. 10.1186/1471-2466-12-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the public repository: https://figshare.com/articles/dataset/CGRP_targeting_therapies_may_increase_infection_risk_a_meta-analysis_of_placebo-controlled_trials_and_a_narrative_review/28375523/1.