ABSTRACT
This study aims to fill the knowledge gap in systematically mapping the evolution of omics-driven tumor immunotherapy research through a bibliometric lens. While omics technologies (genomics, transcriptomics, proteomics, metabolomics)provide multidimensional molecular profiling, their synergistic potential with immunotherapy remains underexplored in large-scale trend analyses. A comprehensive search was conducted using the Web of Science Core Collection for literature related to omics in tumor immunotherapy, up to August 2024. Bibliometric analyses, conducted using R version 4.3.3, VOSviewer 1.6.20, and Citespace 6.2, examined publication trends, country and institutional contributions, journal distributions, keyword co-occurrence, and citation bursts. This analysis of 9,494 publications demonstrates rapid growth in omics-driven tumor immunotherapy research since 2019, with China leading in output (63% of articles) yet exhibiting limited multinational collaboration (7.9% vs. the UK’s 61.8%). Keyword co-occurrence and citation burst analyses reveal evolving frontiers: early emphasis on “PD-1/CTLA-4 blockade” has transitioned toward “machine learning,” “multi-omics,” and “lncRNA,” reflecting a shift to predictive modeling and biomarker discovery. Multi-omics integration has facilitated the development of immune infiltration-based prognostic models, such as TIME subtypes, which have been validated across multiple tumor types, which inform clinical trial design (e.g. NCT06833723). Additionally, proteomic analysis of melanoma patients suggests that metabolic biomarkers, particularly oxidative phosphorylation and lipid metabolism, may stratify responders to PD-1 blockade therapy. Moreover, spatial omics has confirmed ENPP1 as a potential novel therapeutic target in Ewing sarcoma. Citation trends underscore clinical translation, particularly mutation-guided therapies. Omics technologies are transforming tumor immunotherapy by enhancing biomarker discovery and improving therapeutic predictions. Future advancements will necessitate longitudinal omics monitoring, AI-driven multi-omics integration, and international collaboration to accelerate clinical translation. This study presents a systematic framework for exploring emerging research frontiers and offers insights for optimizing precision-driven immunotherapy.
KEYWORDS: Omics, tumor immunotherapy, bibliometric analysis
Introduction
Immunotherapy is a treatment that harnesses the body’s own immune system to identify and eradicate cancer cells. These therapies markedly enhance both the survival rates and quality of life for patients by counteracting tumor immune evasion and immune suppression.1 Currently, several immunotherapies, including immune checkpoint inhibitors (ICIs), cancer vaccines, adoptive cell transfer (ACT), and oncolytic viral therapy (OVT), have demonstrated promising results.2 In particular, ICIs have been widely utilized for various tumor types, including lung cancer, melanoma, and gastric cancer.3 The discovery and implementation of ICIs represent a significant milestone in the field of cancer immunotherapy. Despite these advancements, challenges such as heterogeneous treatment responses and resistance mechanisms underscore the need for deeper molecular insights to optimize therapeutic strategies.4 For example, while programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) inhibitors have demonstrated clear efficacy in melanoma patients, a high proportion (60–70%) of advanced metastatic melanoma cases exhibit innate resistance.5 Therefore, a multi-omics approach is needed to decode tumor-immune interactions and identify predictive biomarkers.
Omics refers to a scientific methodology that employs high-throughput technology to comprehensively analyze various levels of molecular information in organisms, including genomics, transcriptomics, proteomics, and metabolomics. This approach offers a panoramic perspective for studying complex biological systems and disease mechanisms, and it plays a crucial role in fields such as personalized medicine, drug development, and tumor immunotherapy.6 Ongoing clinical trials, such as NCT03578185, utilize genomic analysis to identify predictive biomarkers for immunotherapy. Another study investigates the microenvironmental characteristics linked to primary resistance to anti-PD-1/PD-L1 and anti-angiogenesis therapy in gastric cancer by integrating spatial transcriptomics and plasma proteomics.7 Recent omics-based meta-analyses have investigated the application of omics within tumor immunotherapy and have elucidated associations between tumor immune microenvironment (TIME) characteristics and patient prognosis.8,9 These studies demonstrate the potential of omics approaches to identify predictive biomarkers and translate mechanistic insights into clinical applications. However, existing reviews predominantly focus on technical advancements or clinical applications, with limited integration of bibliometric insights to map the evolution of interdisciplinary collaborations, emerging themes, and regional contributions in this rapidly growing field.10,11
Bibliometric analysis is a powerful research methodology that employs quantitative techniques to systematically interpret complex research landscapes, uncovering scientific development trajectories, emerging hotspots, and thematic evolutions. This analytical framework enables a rigorous examination of publication patterns, collaboration networks, and keyword dynamics.12 By systematically analyzing data from academic documents, journals, and citation relationships, bibliometrics reveals the academic influence of various research areas. Previous bibliometric studies in oncology and precision medicine have successfully identified key contributors, interdisciplinary linkages, and shifts in research priority,13,14 yet none have specifically addressed the integration of omics technologies into tumor immunotherapy. This study provides the first systematic analysis of the application of omics in cancer immunotherapy research, highlighting emerging frontiers such as AI-driven multi-omics integration and spatial transcriptomics, thereby shedding light on future prospects and directions in this field. Specifically, within the context of multi-omics and cancer immunotherapy, bibliometrics can identify shifts in research focus, collaborations between disciplines, and contributions from regions or institutions. This approach not only complements traditional reviews by revealing macro-level patterns but also provides insights into the prioritization of high-impact research directions.
This approach typically involves indicators such as the number of published documents, citation counts, and collaboration networks, utilizing visualization technologies to depict the overall landscape and developmental dynamics of the academic field. In scientific research, bibliometrics aids researchers in organizing the knowledge structure while also providing crucial references for future research directions.15 Consequently, bibliometrics has been widely adopted across numerous fields, serving as a vital means to evaluate research frontiers, analyze scientific productivity, and assess academic impact. However, bibliometric methods have inherent limitations, including citation bias, where citations are predominantly skewed toward high-impact journals.16 To mitigate these, this study combines temporal trend analysis with multi-tool validation (CiteSpace, VOSviewer, Bibliometrix) to reduce biases. Furthermore, to enhance methodological transparency, this study primarily relies on the Web of Science Core Collection (WOSCC) as the main source of citation data, with supplementary validation from the Scopus database. This approach aims to reduce database-specific biases and ensure a comprehensive bibliometric evaluation.
This study represents the first bibliometric analysis of articles related to omics in tumor immunotherapy, aiming to (1) delineate the knowledge structure and evolution of this field, (2) identify underexplored areas such as multi-omics integration and spatial transcriptomics applications, and (3) uncover research hotspots and emerging trends to guide future investigations. By integrating temporal trend, collaboration networks, and keyword co-occurrence analysis, our work provides actionable insights for optimizing interdisciplinary synergies and prioritizing high-impact research directions.
Materials and methods
Data collection and data extraction
Methodologically, the Web of Science Core Collection (WOSCC) was prioritized due to its stringent journal selection criteria, comprehensive citation indexing, and extensive use in bibliometric analyses. Compared to other databases such as Scopus, Medline, and PubMed, Web of Science offers the most comprehensive and reliable bibliometric data, making it the most commonly used source for bibliometric analysis.17–21 To assess potential biases introduced by database-specific retrieval, we conducted a analysis using the same search strategy in the Scopus database and performed keyword and author co-occurrence analyses to help validate trend consistency across different platforms. A data search was conducted on August 12, 2024, encompassing all relevant literature regarding the application of omics in tumor immunotherapy from the inception of the database up to the search date. The search strategy was rigorously validated to ensure comprehensive coverage of relevant literature. First, we refined the search terms through multiple test queries and adjusted Boolean operators to optimize sensitivity. Second, we manually verified high-quality key articles in tumor immunotherapy and omics to confirm the completeness of the search.22–31 All target articles were successfully included in the final dataset, validating the effectiveness of the search strategy. The search formula utilized was TS=((Immunotherapy OR Immune Checkpoint Inhibitors OR immunotherapeutic OR immunotherapies OR immunotherapeutics) AND (Cancer OR Oncology OR Tumor OR Neoplasm OR Malignancy) AND (“Omic*” OR “Proteom*” OR “Transcriptom*” OR “Metabolom*” OR “Genome*” OR “Multi Omic*”)).Publications were filtered using the document type filter in Web of Science, excluding encompass book chapters, early access, proceeding paper, retracted publication, data paper and reviews. Complete records should be obtained from eligible articles and must include details such as the author, country, institution, journal, co-cited journals, citations, and keywords. These records should be saved in a plain text format.
Visualized analysis
This study utilized R version 4.3.3,32 VOSviewer 1.6.20,33 and Citespace 6.234,35 for data analysis. The Bibliometrix36 R software package was employed to quantify both the publication volume and citation frequency of the literature, with the H-index primarily calculated using this tool. VOSviewer facilitated keyword analysis by evaluating keyword co-occurrence frequency and total link strength, enabling the construction of a keyword co-occurrence network. Additionally, this software was utilized for frequency analysis of collaboration between countries. Concurrently, Citespace 6.2 was applied to perform burst analysis on keywords and cited documents. Furthermore, Origin 2024 and SRplot37 were used to enhance the visual presentation of the results.
Result
Overview of publication status
As shown in Figure 1, a total of 9,494 articles on omics in the field of tumor immunotherapy were included following the screening process. Figure 2 presents a bar chart and line graph depicting the annual distribution and cumulative publication count of articles related to omics in tumor immunotherapy since the establishment of the database. Additionally, the heat map illustrates the number of publications over the past ten years from the five countries that produce the most articles. Since the first relevant article was published in 1977,38 this field remained relatively stagnant until 2004, with the number of articles not exceeding 100 until 2005 (103). However, beginning in 2019, the number of articles on omics in tumor immunotherapy has experienced rapid growth, reaching an impressive total of 9,494 articles by August 2024. The publication output from the five countries with the highest number of articles also exhibited a significant increase from 2019 to 2024, particularly in China.
Figure 1.

Flow-chart of the study.
Figure 2.

Number of publications per year and the cumulative number.
Figure 3 presents key historical milestones in immunotherapy, along with the timeline of major applications of omics technologies. The figure clearly illustrates the evolution of immunotherapy, with particular emphasis on the temporal relationship between key advancements in genomics, transcriptomics, proteomics, and other omics technologies.
Figure 3.

Key milestones and temporal relationship of omics and immunotherapy development.
Based on the temporal trends in publication volume of omics-related research within the field of tumor immunotherapy, along with key developments in omics technologies and clinical milestones in immunotherapy, the evolution of this discipline can be divided into three distinct phases: the Foundational Research Phase (1977–2004), the Technological Translation and Growth Phase (2005–2018), and the Rapid Development and Clinical Integration Phase (2019–2024).
Analysis of national publication volume and collaboration
An analysis of the number of publications by country revealed that a total of 67 countries and regions contributed relevant articles in this field. As illustrated in Figure 4, China leads with 5,972 articles, representing 63% of the total. The United States follows with 1,485 articles (15.7%), while Germany (n = 257, 2.7%), Japan (n = 179, 1.9%), and the United Kingdom (n = 170, 1.8%) complete the top five. Other countries published fewer than 170 articles. To further illustrate the quality of publications across countries, we present the top 10 countries with the highest total citation counts and average citations per publication in Table 1, reflecting their academic influence. The United States ranks highest in total citations with 106,575, followed by China with 61,280 and Germany with 6,942. Austria has the highest average citation count at 145.3, followed by the United States at 71.8 and Australia at 45.7.
Figure 4.

Map of country’s contribution based on the number of articles published.
Table 1.
Top 10 countries by total citations and average article citations.
| Country | Total Citations | Average Article Citations |
|---|---|---|
| USA | 106575 | 71.8 |
| CHINA | 61280 | 10.3 |
| GERMANY | 6942 | 27 |
| FRANCE | 6755 | 40.4 |
| UNITED KINGDOM | 6278 | 36.9 |
| CANADA | 4665 | 37.3 |
| AUSTRIA | 4505 | 145.3 |
| AUSTRALIA | 3975 | 45.7 |
| JAPAN | 3963 | 22.1 |
| ITALY | 3850 | 26.6 |
To address potential bias arising from disparities in national research capacity and to emphasize research specialization over absolute publication volume, we defined the O/T ratio (O/T%) as shown in Figure 4. This ratio quantifies the proportion of omics-related publications in tumor immunotherapy relative to the total number of medical publications from each country during the same period. Medical publication data were obtained from the WOSCC using the search query: TS = (Medicine OR Medical OR Health OR Healthcare OR Clinical). Our analysis indicates that China has the highest O/T% among the top 10 countries by publication volume, reaching 0.84%.
Multi-country joint publications (MCP) denote research collaborations that involve multiple countries. Despite China leading in the total number of published articles, its MCP ratio (=MCP/articles) stands at a mere 7.9%, the lowest among the top ten countries. The MCP ratio serves as a significant indicator for assessing the independence of research endeavors across different nations. In contrast, the United Kingdom boasts the highest MCP rate among these countries, reaching 61.8%. Supplementary Figure S1 and Supplementary Figure S2 illustrates the patterns of international cooperation, revealing that the most frequent collaboration occurs between the United States and China(365). This is followed by collaborations between the United States and Germany (142) and the United States and England (136). Notably, within the top ten countries exhibiting the highest frequencies of cooperation, only one partnership exists between China and Germany (91), while all other collaborations involve the United States.
Analysis of institutional output
A total of 7,492 institutions contributed to the publication of articles related to omics in tumor immunotherapy. Among these, Sun Yat-sen University ranked first with 393 articles, followed closely by Fudan University with 391 articles, Shanghai Jiao Tong University with 355 articles, Nanjing Medical University with 340 articles, and Central South University with 311 articles. The top 10 institutions with the most publications are presented in Figure 5, with nine of these institutions located in China.
Figure 5.

The top 10 institutions with the most publications.
An analysis of co-authorship by institutions revealed that 143 institutions published 30 or more articles related to this field. The clustering network is illustrated in Supplementary Figure S3. Supplementary Figure S3A presents the cluster network for the analysis of institutional co-authorship, where circles of the same color represent a cluster. The 143 institutions are categorized into four clusters. The largest cluster, Cluster 1 (red), comprises 62 institutions, including notable entities such as the MD Anderson Cancer Center, Harvard Medical School, and the National Cancer Institute of the United States. Supplementary Figure S3B incorporates a temporal element based on Supplementary Figure S3A, indicating that the average number of years of articles published by institutions in Cluster 1 is predominantly concentrated before 2021. In contrast, the average year of publication for articles from the other three clusters, which primarily consist of Chinese institutions, is 2022. This observation is largely consistent with the annual trends in article publication by various countries, as depicted in Figure 2.
Analysis of journals
The included articles encompass a total of 963 journals. Table 2 presents the top 10 journals ranked by publication volume along with their most recent impact factors. “FRONTIERS IN IMMUNOLOGY” holds the top position with 621 articles, followed by “FRONTIERS IN ONCOLOGY” with 469 articles. Notably, six of the ten journals are classified in the Journal Citation Report (JCR) quartile 1 (Q1). Among these journals, five are based in Switzerland, three in England, and two in the United States.
Table 2.
The top 10 journals in the field of omics in tumor immunotherapy.
| Rank | Sources | Articles | H_index | Country | IF | JCR-c |
|---|---|---|---|---|---|---|
| 1 | FRONTIERS IN IMMUNOLOGY | 621 | 35 | SWITZERLAND | 5.7 | Q1 |
| 2 | FRONTIERS IN ONCOLOGY | 469 | 28 | SWITZERLAND | 3.5 | Q2 |
| 3 | FRONTIERS IN GENETICS | 383 | 22 | SWITZERLAND | 2.8 | Q2 |
| 4 | JOURNAL FOR IMMUNOTHERAPY OF CANCER | 277 | 44 | ENGLAND | 10.3 | Q1 |
| 5 | SCIENTIFIC REPORTS | 225 | 21 | ENGLAND | 3.8 | Q1 |
| 6 | CANCERS | 219 | 23 | SWITZERLAND | 4.5 | Q1 |
| 7 | AGING-US | 188 | 21 | USA | 3.9 | Q2 |
| 8 | FRONTIERS IN CELL AND DEVELOPMENTAL BIOLOGY | 158 | 18 | SWITZERLAND | 4.6 | Q2 |
| 9 | NATURE COMMUNICATIONS | 151 | 51 | ENGLAND | 14.7 | Q1 |
| 10 | CLINICAL CANCER RESEARCH | 148 | 53 | USA | 10 | Q1 |
Analysis of authors
A total of 54,753 authors contributed to the publication of articles related to omics in tumor immunotherapy. The number of publications and the influence of the top 10 authors, ranked by the number of published articles, are presented in Table 3. ZHANG W ranks first with 67 published articles and an H-index of 22. The author with the highest H-index is WANG J, who has published 47 articles and has an H-index of 31.
Table 3.
Top 10 authors in the field of omics in immunotherapy.
| Authors | Web of Science ResearcherID | Articles | H_index |
|---|---|---|---|
| ZHANG W | L-5761–2017 | 67 | 22 |
| ZHANG J | IZE-2454–2023 | 62 | 26 |
| WANG W | D-3652–2015 | 56 | 12 |
| WANG J | M-7813–2015 | 47 | 31 |
| ZHANG Y | HKV-8165–2023 | 44 | 25 |
| WANG Y | IXW-7812–2023 | 43 | 26 |
| LUO P | I-4790–2019 | 42 | 14 |
| ZHANG Y | GJV-3937–2022 | 42 | 25 |
| LI W | FGU-8322–2022 | 39 | 13 |
| LI L | ITX-6419–2023 | 38 | 18 |
Supplementary Figure S4A illustrates the collaborative relationships among authors. The 72 authors who published 20 or more articles were categorized into eight distinct clusters. The size of each circle represents the number of publications, while the color indicates the corresponding cluster. These eight clusters collectively form a substantial network, indicating that researchers engage in close collaboration in the field of omics in tumor immunotherapy. Supplementary Figure S4B incorporates a temporal dimension. WANG J is recognized as an earlier researcher in this domain, which partially explains his highest H-index. In contrast, the articles authored by ZHANG J and Li H are projected to be published on average in 2022, indicating that these authors are actively contributing to the field of omics in tumor immunotherapy.
Research hotspots
Most cited publications
Supplementary Table S1 presents the top 10 most frequently cited articles in this field, all of which have been cited over 1,500 times, underscoring their representativeness. These articles span the years 2014 to 2020. The most frequently cited article is “Mismatch Repair Deficiency Predicts Response of Solid Tumors to PD-1 Blockade,”39 published in Science in 2017, with 4,514 citations. This study assessed the efficacy of PD-1 blockade in patients with advanced MMR-deficient cancer across 12 different tumor types using whole-exome sequencing. Following this, “Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma,”40 published in the New England Journal of Medicine, has garnered 3,225 citations. In this study, Snyder et al. utilized whole-exome sequencing to investigate the genetic basis of melanoma’s clinical response to CTLA-4 blockade.
Citation burst analysis of references
Supplementary Figure S5 illustrates the top 25 most cited references. In this figure, the light blue line segment denotes the period from 1977 to 2024, while the dark blue line segment indicates the duration of citations. The red line marks the burst range of citation duration, where the minimum burst range is established at two years. The article with the strongest citation burst value is “Cancer immunology: Mutation landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer,”41 published in Science in 2015, with a strength of 123.49(Strength quantifies the significance of a citation burst, representing the magnitude of the sudden increase in citation frequency for a reference, author, or keyword during a specific time period.). This study conducted whole-exome sequencing of patients with non-small cell lung cancer (NSCLC) treated with pembrolizumab and demonstrated that the genomic landscape of lung cancer influences the response to anti-PD-1 therapy. The next highest citation burst value is attributed to “Robust enumeration of cell subsets from tissue expression profiles,”42 with an intensity of 113.53. Among these 25 articles, one is currently experiencing a citation explosion. This article, titled “Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response” (57.33),43 explores how the expression of genes within tumors interacts with the level of cytotoxic T lymphocyte infiltration to impact patient survival.
Frequency and co-occurrence analysis of keywords
After analyzing the literature included in this study, a total of 17,349 keywords were collected. Figure 6a displays the top 20 most frequent keywords, with “immunotherapy” appearing most frequently at 3,661 occurrences, followed by “expression” (n = 2,797), “cancer” (n = 2,276), and “prognosis” (n = 1,910). Among the top 20 keywords, “melanoma” is the only tumor type that appears, with 416 occurrences. China’s dominance in contributing 63% of publications in this field may reflect significant national investments in biotechnology and precision medicine initiatives, but it also raises concerns about regional biases. To address this, our analysis examines geographic collaboration patterns and compares thematic focuses across regions, aiming to distinguish globally relevant trends from region-specific priorities. Figure 6b illustrates the correlation and distribution of keywords across countries and institutions within this field. Regarding institutions, most have some coverage of each keyword; however, subtle differences exist. For instance, the University of California system shows minimal interest in “immune infiltration,” “hepatocellular carcinoma,” and “lung adenocarcinoma.” Conversely, Central South University exhibits limited association with “biomarker” but demonstrates significant interest in “immunotherapy” and “prognosis.” Harvard University pays little attention to “lung adenocarcinoma.” At the country level, China has made substantial contributions to all prominent keywords, particularly in “immunotherapy,” “prognosis,” and “tumor microenvironment.”
Figure 6.

Frequency and co-occurrence analysis of keywords: (a) the 20 most commonly used keywords; (b) A three-field plot of the keywords plus analysis (Left field: institutions; Middle field: keywords; Right field: countries); (c) Co-occurrence analysis of the top 100 most frequent keywords; and (d) Co-occurrence analysis of the top 100 most frequent keywords with time overlap.
Figure 6c presents a co-occurrence analysis of 143 keywords that each appear more than 100 times. The size of the nodes in the figure indicates the frequency of keyword usage, while the color denotes the keyword cluster. Additionally, the thickness of the connections reflects the strength of the relationship between pairs of keywords. These 143 keywords are categorized into four clusters. The first cluster (red) is the largest, encompassing 49 keywords. This cluster primarily focuses on various cancer types, including “bladder cancer,” “breast cancer,” “hepatocellular carcinoma,” and “lung adenocarcinoma,” as well as mechanisms involved in tumor immunotherapy, such as “angiogenesis,” “apoptosis,” “immune cell infiltration,” and “tumor immune microenvironment.” Cluster 2 (green) consists of 44 keywords, predominantly related to immunotherapy drugs, including “immune checkpoint inhibitors,” “pembrolizumab,” and “ipilimumab.” Cluster 3 comprises 33 keywords (blue), primarily related to various cells involved in tumor immunotherapy, such as “regulatory T-cells,” “lymphocytes,” “macrophages,” and “dendritic cells,” along with commonly employed omics methods like “proteomics.” Cluster 4 consists of 17 keywords (yellow) that pertain to key terms in omics research, including “gene expression,” “machine learning,” “methylation,” and “multi-omics.”
Figure 6d overlays the temporal aspect onto Figure 6c. The transition from purple to yellow indicates that the average activity time of keywords shifts from distant to more recent. Notably, the earliest average year for keywords with a frequency exceeding 100 is also within the past five years, further supporting the notion that significant advancements in this field are relatively recent.
Author keywords
Author keywords are selected by the authors to accurately reflect the document’s content. Figure 7a illustrates the heat map depicting the frequency of author keywords over the past decade (2014–2024). To enhance the presentation of the results, the frequencies have been standardized to a range of (0, 1). The color gradient from blue to red indicates low to high frequency. Notably, the author’s keyword “MACHINE LEARNING” has seen a significant increase over the past two years. As of August 12, 2024, the frequency of this keyword has surpassed that of 2023, based on the literature search. Additionally, there has been a growing focus on the keywords “MELANOMA” and “COLORECTAL CANCER” in 2024. Conversely, the keyword “TUMOR MUTATION BURDEN” has experienced a decline in frequency after reaching its peak in 2022. Due to the presence of missing keywords in some articles within the WOS database, this study employs R packages such as reshape2, tidyverse, and bibliometrix to conduct a terminology analysis of the literature from the past decade. Figure 7b illustrates the temporal changes in the terms extracted from the titles. The results of the terminology analysis exhibit slight variations when compared to the authors’ keywords. Notably, in 2024, terms such as “RESPONSE,” “IMMUNOTHERAPY,” and “ANALYSIS” have gained popularity. In contrast, the prominence of terms like “SIGNATURE,” “INFILTRATION,” “IMMUNE IDENTIFICATION,” and “CHECKPOINT” has declined relative to the years 2022 and 2023.
Figure 7.
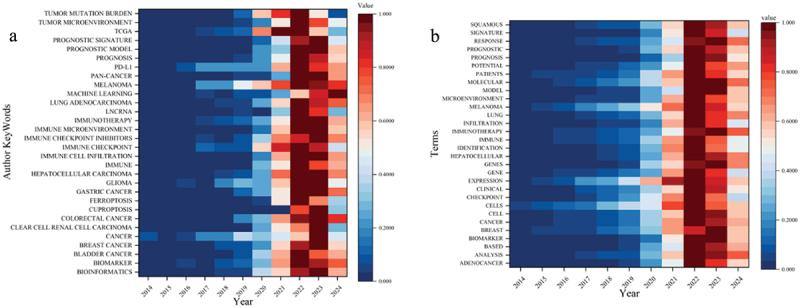
Heatmap of author keywords and terms.
Citation burst analysis of keywords
Figure 8 presents the top 25 keywords exhibiting the strongest citation bursts. The keyword “pd 1 blockade” demonstrates the highest intensity, recorded at 44.13. Attention to the keywords “melanoma,” “cancer immunotherapy,” “antigens,” and “metastatic melanoma” has persisted from 1991 to 2019, while “tumor infiltrating lymphocytes” and “gene therapy” have garnered attention from 1992 to 2020. This indicates a sustained interest in these keywords over a span of 28 years. In recent years, keywords such as “genome,” “ipilimumab,” “pd 1 blockade,” “ctla 4 blockade,” “checkpoint blockade,” and “safety” have gained increased attention.
Figure 8.

Top 25 keywords with the strongest citation bursts.
However, early citation patterns may be skewed due to lower publication volumes and selective indexing in databases such as WOSCC. For instance, articles published before 2000 might lack digital accessibility or fail to meet contemporary indexing standards, potentially resulting in an underestimation of their influence.
In addition, citation burst analysis tends to favor older papers with longer citation accumulation periods. To validate the emerging trends identified through keyword analysis, we cross-referenced entries from ClinicalTrials.gov. Using “genome” as an example, we conducted a search with the terms “cancer | genome | immunotherapy,” identifying 60 studies (as of February 2025) that were either recruiting or not yet recruiting. These studies explicitly link genome regulation with immunotherapy (e.g., NCT03578185, NCT06833723, NCT02299999), demonstrating the growing research potential and interest in this area.
Discussion
Research evolution and trends
This study employed bibliometric methods to investigate the research landscape surrounding omics and tumor immunotherapy from the inception of the WOS database until 2024. The earliest identified study, titled “Effect of xenogeneic immune RNA on normal human lymphocytes against human osteosarcoma cells in vitro,” was published in the “Journal of the National Cancer Institute” in 1977.38 Singh et al.‘s study38 was one of the early experiments exploring the regulation of lymphocyte anti-tumor activity using exogenous immune RNA (I-RNA), highlighting the critical role of RNA molecules in immune signaling. Although the concept of “omics” had not yet emerged at that time, techniques such as Sephadex G-200 column chromatography and sucrose density gradient centrifugation demonstrated early integration of molecular typing with functional genomics. Evidence from these early studies forms the empirical foundation for subsequent omics research in tumor immunotherapy. Since then, this field has experienced a relatively stagnant phase. Over time, concepts such as genomics, transcriptomics, proteomics, and metabolomics have emerged successively, leading to an increasing recognition among researchers of the significant role this technology plays in tumor immunotherapy. In the five-year period from 2019 to 2024, there has been a remarkable surge in related publications, with an increase of over 8,000 articles. Considering the historical context, this increase can be attributed to two primary factors. First, the rapid advancement of tumor immunotherapy has established it as a central pillar of cancer treatment. ICIs have demonstrated significant clinical efficacy across multiple cancer types; however, response rates vary considerably. Consequently, researchers urgently seek to elucidate resistance mechanisms and identify biomarkers through multi-omics analysis. Second, omics technologies have progressed rapidly, particularly with the emergence of single-cell RNA sequencing (scRNA-seq)44,45 and spatial omics,46,47 which offer novel methodologies for immunotherapy research. The integration of artificial intelligence (AI) algorithms with multi-omics data has introduced a new paradigm, facilitating the prediction of immunotherapy responses and the discovery of novel therapeutic targets.
Geographical and institutional contributions
In terms of countries/regions, and institutions, China undeniably holds a dominant position, accounting for 63% of the total publication output. Notably, nine of the top ten institutions are located in China. The United States, meanwhile, plays a significant role in international collaboration; among the top ten cooperative relationships between countries/regions, the number of articles coauthored with the United States reached 1,157, representing 12.2%. These findings are derived from the bibliometric analysis and thus, to some extent, represent robust and evidence-based conclusions. Analyzing the changes in publication numbers by country over time reveals that the United States had the highest publication count prior to 2019, indicating its leadership in the fields of omics in tumor immunotherapy, which facilitated the emergence of this area of research. However, post-2019, China has established a dominant position in terms of published articles, reflecting the advancements in China’s economic strength over the past five years and its increased investment in medical research. In addition, Korea, Germany, Japan, France, and the United Kingdom have also published a significant number of studies in this field.
Although the impressive productivity of certain national research systems is evident, it is essential to account for potential systemic biases when comparing countries. In some regions, research evaluation frameworks that prioritize publication quantity as a primary metric for institutional funding allocation may inadvertently encourage bulk submissions to international journals rather than supporting high-risk, high-reward research. Empirical studies indicate that these quantity-driven incentives can skew scholarly outputs, favoring incremental research topics that align with rapid publication cycles.48 Such systemic factors complicate direct comparisons of scientific productivity between nations with differing research governance models. These hypotheses represent tentative interpretations derived from the available evidence. The discrepancy between publication count, total citations, and average citations per article highlights this perspective.
Journal distribution and publication patterns
The number of journal publications serves as a valuable resource for researchers seeking potential journals for submission. Among the top 10 journals in the field of omics in tumor immunotherapy, all are classified within the JCR divisions Q1 and Q2, each publishing over 100 articles. Notably, the journal “FRONTIERS IN IMMUNOLOGY” has achieved an impressive total of 621 publications, highlighting the significant attention these leading journals devote to this dynamic field. While China leads in the volume of publications, no Asian journals are represented in the top 10. This underscores the necessity of establishing a prominent publication in Asia within this area of research.
Based on bibliometric data analysis, we hypothesize that the absence of Asian journals may be attributed to three key factors: (1) mainstream journal indexing systems (such as Web of Science) systematically undervalue non-English journals; (2) many Asian institutions prioritize publishing in Western journals; and (3) the “Matthew effect” in citation patterns creates cumulative advantages for established Western journals. These systemic biases may disproportionately direct high-quality Asian research toward Western publication platforms, while limiting the development of regional journals.
Temporal evolution of high-frequency keywords across phases
Upon comparing the time gradient in Figure 6d with the three developmental phases in this field, significant shifts in research focus become apparent:
Foundational Research Phase (1977–2004): Keywords did not appear more than 100 times, likely due to two main factors. First, technological limitations: omics technologies, such as genomics and proteomics, were in their early stages and had not yet been widely applied, limiting the generation of large-scale datasets. Second, gaps in terminology and data archiving: early studies often employed non-standardized terms or lacked digital accessibility, resulting in limited representation in modern databases.
Technological Translation and Growth Phase (2005–2018): Similarly, no keywords surpassed the 100-frequency threshold. Despite advancements in omics technologies (e.g., the rise of next-generation sequencing), research remained fragmented, focusing more on exploratory applications than on unified themes.
Rapid Development and Clinical Integration Phase (2019–2024): High-frequency keywords are predominantly concentrated in this phase, where some terms show clear transitions. Early-stage keywords (represented by purple) include terms such as “melanoma,” “lymphocytes,” “T-cells,” “gene-expression,” and “tumor-infiltrating lymphocytes.” With the rise of clinical trials, keywords shifted toward blue-green, reflecting terms like “immunotherapy,” “biomarkers,” “pembrolizumab,” and “multicenter,” aligning with the broad clinical application of these therapies. The yellow keywords, such as “multi-omics,” “lncRNA,” “ferroptosis,” and “prognosis,” reflect advancements in omics technologies, exploration of tumor immunotherapy mechanisms, and the application of omics in predicting patient prognosis. However, recent terms may be exaggerated due to publication bias (e.g., journals focusing specifically on omics).
It should be noted that while the keyword frequency data are evidence-based, the categorization into phases involves interpretative judgment which remains partly exploratory.
Research hotspots and directions
The analysis of research hotspots encompasses the examination of references and keywords, which typically reveal the focal points and directions of inquiry within a specific field. By integrating various analytical approaches, omics can be broadly categorized into two prominent directions in the realm of tumor immunotherapy. The first direction pertains to the clinical application of omics in tumor immunotherapy, encompassing conditions such as melanoma, lung adenocarcinoma, breast cancer, and hepatocellular carcinoma. Presently, there is a growing emphasis among researchers on the aspect of ‘prognosis’ within this context. This trend highlights an important translational need: prognostic models based on multi-omics data can facilitate personalized risk stratification and dynamic monitoring of treatment efficacy. However, the clinical implementation of these models will necessitate thorough validation through prospective trials, alongside the standardization of omics workflows to guarantee reproducibility across institutions. The prognostic significance of multi-omics data is strongly supported by our bibliometric analysis and existing literature; however, the clinical implementation of these models involves exploratory elements that require further validation. For instance, Gong et al.49 employed metabolomics methodologies to investigate the metabolic dysregulation associated with triple-negative breast cancer (TNBC), examining the relationship between metabolic heterogeneity and prognosis. Similarly, Bao et al.50 conducted a multi-omics analysis of intrahepatic cholangiocarcinoma (ICC) utilizing proteomics, whole exome sequencing (WES), and scRNA-seq, leading to the identification of three molecular subtypes – chromatin remodeling, metabolism, and chronic inflammation – each associated with distinct prognostic outcomes in ICC. These studies illustrate how omics can enhance therapeutic decision-making. However, challenges persist in translating subtype-specific insights into actionable clinical protocols, particularly for rare cancers with limited multi-omics data.
Another aspect involves applying omics to investigate the underlying mechanisms of tumor immunotherapy, such as angiogenesis, apoptosis, immune cell infiltration, and the tumor immune microenvironment. Xiao et al.51 employed a multi-omics approach to uncover the distinctive microenvironmental characteristics of TNBC and proposed a mechanism for its immune evasion. They suggest that immune checkpoint inhibitors may be effective for the “immune-inflammation” cluster, while for the “immune-desert” and “innate immune inactivation” clusters, strategies for converting “cold tumors” into “hot tumors” should be considered. This underscores a critical research gap: although omics can stratify tumors into immune phenotypes, the development of targeted interventions to remodel these phenotypes is still in its early stages. Future studies should focus on combinatorial therapies (e.g., checkpoint inhibitors combined with metabolic modulators or epigenetic drugs) informed by omics-defined resistance mechanisms. McGrail et al.29 developed a comprehensive multi-omics framework and identified the DNA damage response protein ATM as a key driver of cytokine production. This finding suggests that the mechanisms underlying immune cell infiltration vary across different cancer lineages, which may have implications for enhancing immunotherapy in various malignancies and for clinical adaptation in oncology. Importantly, the lineage-specific variability in immune infiltration mechanisms highlights the limitations of “one-size-fits-all” immunotherapy approaches and emphasizes the need for lineage-targeted biomarker discovery using pan-cancer multi-omics datasets.
Emerging keywords such as “lncRNA” point to novel mechanistic frontiers. For example, lncRNA may serve as a potential predictor of therapeutic efficacy. In a study, researchers developed and validated a proliferative lncRNA signature based on 50 marker gene sets, which independently and accurately assessed overall survival (OS) and recurrence-free survival (RFS) in hepatocellular carcinoma (HCC) patients.52 On the other hand, lncRNAs may function as immune cell-specific gene expression regulators, and due to their impact on tumor angiogenesis, metastasis, apoptosis, and growth, could serve as potential therapeutic targets.53,54 Zhang et al.55 discovered that the lnc RNA LINC01132 enhances immune suppression and drug resistance in HCC through the NRF1/DPP4 axis. They performed a comprehensive screening using multiple omics data from HCC and validated their findings with qRT-PCR. Additionally, they conducted experiments in cell models, in vivo mouse models, and patient-derived xenograft models. The systematic integration of non-coding RNA omics with functional validation is crucial for identifying therapeutic targets. However, these areas remain underexplored in clinical contexts. While these results are supported by experimental data, the broader clinical applicability of lncRNA-targeted interventions remains a speculative area requiring further investigation.
Advances in omics technology applications
The extensive literature in this field clearly demonstrates that omics technology offers distinct advantages in tumor immunotherapy research. This is largely due to omics’ ability to provide multi-dimensional molecular information, systematically revealing the complexity and heterogeneity of tumors and their immune microenvironment.26,56 Through comprehensive analyses of genes, transcription, proteins, and metabolism, omics technology aids in the identification of potential therapeutic targets and the prediction of biomarkers.10,57,58
The application of transcriptomics in cancer immunotherapy, particularly scRNA-seq, enables the investigation of immune checkpoints, cytokine signaling pathways, and the dynamic alterations in immune cells. This approach enhances our understanding of the tumor microenvironment and its immune responses.59 It also offers new perspectives for identifying key factors and cell subpopulations that either promote tumor progression or increase tumor susceptibility to immunotherapy. Bagaev et al.60 utilized transcriptomics to classify four distinct tumor microenvironment subtypes that are conserved across 20 different cancers, detailing the tumor framework, mutation load, immune composition, anti-tumor immunity, and mechanisms of immune escape. Similarly, Kirschenbaum et al.31 employed time-resolved scRNA-seq to examine the cellular states and molecular trajectories within the dysfunctional immune microenvironment of glioblastoma.
In the field of proteomics, identifying the roles of immune-related proteins and signaling pathways enhances our understanding of the mechanisms underlying immunotherapy and facilitates the exploration of potential therapeutic targets. Harel et al.30 utilized proteomics to analyze clinical samples from advanced melanoma patients treated with programmed death 1 (PD-1) immunotherapy, quantifying over 10,300 proteins. Their findings indicated that responders exhibited higher levels of oxidative phosphorylation and lipid metabolism compared to non-responders. Similarly, Mooney et al.61 employed surfaceome and global proteome analyses to investigate proteins associated with Ewing sarcoma, revealing that ENPP1 was significantly expressed in Ewing sarcoma tumors relative to other childhood sarcomas and normal tissues, highlighting its potential as a target for immunotherapy.
Metabolomics elucidates the mechanisms by which tumor cells evade immune surveillance through metabolic remodeling by analyzing alterations in cellular metabolites, thereby offering potential biomarkers for personalized immunotherapy strategies. The metabolomic analysis conducted by Yuan et al.62 underscored the co-expression relationships among alanine, aspartate, and glutamate with glycolysis/gluconeogenic metabolism and HER2 levels in gastric cancer. Additionally, consensus clustering was employed to categorize gastric cancer patients into four subtypes exhibiting distinct metabolic characteristics, with the quiescent and AAG subtypes showing a higher likelihood of benefiting from immunotherapy. Furthermore, Zhang et al.63 discovered that specific metabolites facilitate communication between intestinal microorganisms and tumor immunity, proposing that modulation of the intestinal microbiota may serve as an effective strategy to enhance the response of colorectal cancer patients to immunotherapy.
Multi-omics technologies integrate data from various omics layers, including genomics, transcriptomics, proteomics, and metabolomics, providing a comprehensive understanding of the biological mechanisms of diseases and playing a role in predicting the efficacy of immunotherapy. For instance, Parra et al.64 conducted a multi-omics analysis on tissue and longitudinal blood samples from patients with metastatic squamous non-small cell lung cancer receiving nivolumab monotherapy or nivolumab plus ipilimumab. This analysis, which included multiplex immunofluorescence, gene expression profiling, whole-exome sequencing, and Olink proteomics, revealed immune features associated with the benefits of immunotherapy.
Simultaneously, omics technology shows significant promise in addressing immunotherapy-related adverse reactions (irAEs). Utilizing a multi-omics approach, Jing et al.24 present a method for identifying potential biomarkers of irAEs in cancer immunotherapy by integrating pharmacovigilance data with multi-omics data. Additionally, Nuñez et al.65 tracked changes in immune cell populations and cytokine levels over time in melanoma and NSCLC patients receiving ICIs, successfully identifying potential predictive biomarkers for future autoimmune toxicity.
Future directions
While omics technologies have achieved significant advancements and widespread application in tumor immunotherapy research, their future development holds even greater potential. First, multi-omics integration will emerge as a central research focus, combining genomic, transcriptomic, proteomic, metabolomic, and microbiome data to enable comprehensive characterization of the TIME for personalized therapeutic guidance. To effectively integrate multi-omics into clinical practice, several key steps must be undertaken: the development of standardized protocols for data collection, analysis, and interpretation, as well as the establishment of a global data-sharing platform. This will enable cross-disciplinary validation and meta-analysis, ensuring reproducibility and consistency across studies. Additionally, substantial emphasis must be placed on validating biomarkers in large-scale clinical cohorts to confirm the clinical applicability of multi-omics signatures in predicting treatment outcomes. Second, the rapid evolution of spatial omics techniques provides unprecedented capability to resolve spatial heterogeneity within the TIME, potentially identifying critical geographical niches and cellular populations governing immunotherapy responsiveness. Although spatially resolved multi-omics can redefine therapeutic targets by mapping immune-tumor interactions at subcellular resolution, overcoming critical technological barriers, such as low throughput and high costs, is essential for achieving clinical scalability. Third, artificial intelligence (AI) and machine learning algorithms will enhance omics data mining efficiency, accelerating discovery of novel therapeutic targets and biomarkers. To bridge the gap between omics-based discoveries and clinical applications, AI-driven analysis should focus on developing robust predictive models that incorporate multi-omics data to guide patient stratification. Such AI models must be trained on large, diverse datasets to enhance accuracy and generalizability. However, the application of AI models in clinical settings still faces significant challenges, with interpretability being a major hurdle. To facilitate the clinical application of AI models, the next step should focus on developing interpretable AI frameworks that link omics features with underlying mechanistic pathways. This will ensure that these models are trusted by clinicians and comply with regulatory requirements.
Clinically, future efforts should focus on bridging omics discoveries with practical implementation through predictive models incorporating omics signatures, thereby improving patient stratification accuracy and reducing immune-related adverse events. For instance, Prelaj et al.10 utilized machine learning approaches to predict the efficacy of ICIs in cancer patients, focusing on genomics (including genomics, transcriptomics, and epigenomics), radiomics, pathology genomics, as well as real-world and multimodal data. Their work demonstrates the clinical application of multi-omics and machine learning. However, most predictive models remain retrospective, highlighting the urgent need for prospective validation in randomized controlled trials, such as incorporating omics-guided arms, to demonstrate their clinical utility. Concurrently, leveraging omics approaches for real-time monitoring of treatment response dynamics and resistance mechanisms will optimize therapeutic strategies and improve long-term outcomes. Collectively, these technological advancements will continue driving innovation in tumor immunotherapy and strengthen the foundation of precision oncology.
However, it should be noted that interpretations of future directions are primarily speculative, intended to guide subsequent research rather than conclusions directly derived from current bibliometric data.
Limitations
As a bibliometric analysis, this study inevitably has certain limitations. The literature is sourced from WOSCC, which, while encompassing the vast majority of relevant studies, may overlook some high-quality research that has not garnered sufficient citations, potentially due to a brief publication timeline. Future bibliometric studies may consider incorporating the Altmetric score as an evaluation metric. Additionally, we conducted a search using the same keywords in Scopus and included the co-occurrence and temporal co-occurrence diagrams of the top 100 most frequent keywords in Supplementary Figure S6 for further reference. Supplementary Figure S7 presents the author co-occurrence analysis for researchers with more than 30 publications retrieved from Scopus, along with a time-overlay visualization. This study’s reliance on English-language publications may introduce selection biases, as non-English research from regions with distinct priorities (e.g., regional innovations) could be underrepresented. Future work should integrate multilingual databases to better capture global trends. Due to limitations in the tools used, there are certain constraints in the analysis. Specifically, while bibliometric analysis using VOSviewer effectively identifies high-output authors within a field, it cannot automatically resolve issues related to authors with identical names. This limitation may lead to the incorrect merging or splitting of an author’s contributions. This challenge reflects a broader issue within bibliometric tools, as traditional methods relying on author names and institutional information struggle to fully address author disambiguation. Future studies could improve analytical accuracy by integrating manual verification with algorithmic enhancements, such as incorporating unique identifiers for author retrieval.
This study has certain limitations in terms of statistical validation. Although the bibliometric tools used are effective in identifying trends and knowledge network structures within the field, their analytical framework primarily focuses on descriptive visualization rather than statistical inference. Future research could enhance the robustness of conclusions by incorporating methods such as time series analysis and network correlation tests, combined with a more systematic statistical validation framework.
Conclusion
Overall, omics technology presents significant application prospects in the field of immunotherapy, facilitating the advancement of personalized treatment and precision medicine. By employing genomics, proteomics, metabolomics, and other technologies, we can aid in the development of biomarkers associated with immunotherapy response, thereby optimizing individualized treatment strategies. In the future, real-time dynamic monitoring of molecular changes in patients may enable accurate efficacy evaluations, adverse reaction risk predictions, and protocol adjustments. To ensure the success of clinical implementation, validation through large-scale prospective clinical trials will be essential. Thus, omics technology is poised to play a crucial role in enhancing the efficacy and safety of tumor immunotherapy.
Supplementary Material
AI Tool Acknowledgment
During the preparation of this work, the authors utilized the following generative AI tools for language refinement: ChatGPT-4.0 (OpenAI) and deepseek-R1 (DeepSeek Inc.). These tools were employed to enhance grammatical accuracy, restructure sentences, and improve paragraph coherence in the initial draft. Their use was strictly limited to optimizing academic expression and readability. The authors critically reviewed and edited all AI-generated content, retaining full responsibility for the scholarly validity, data interpretation, and conclusions presented in this article.
Biography
Huijuan Cui is a professor and doctoral supervisor at Beijing University of Chinese Medicine. Since graduating in 1985, she has specialized in integrative oncology, focusing on lung cancer and improving cancer patients’ quality of life through Traditional Chinese Medicine (TCM). Her research addresses the use of TCM to alleviate the side effects of chemotherapy, radiotherapy, and immunotherapy. She developed key formulations such as “Zhi Yang Ping Fu Decoction” and “Zi Zao Yang Rong Decoction,” which have benefited many patients. Professor Cui holds leadership roles in several national medical societies and has received five second-class scientific awards from the Chinese Association of Traditional Chinese Medicine. She has led multiple research projects, including National Natural Science Foundation grants, and holds three patents. With over 90 publications in core Chinese journals and 27 SCI papers, she has mentored more than 30 master’s and 8 doctoral students.
Funding Statement
This work was funded by National High Level Hospital Clinical Research Funding [2022-NHLHCRF-LX-02-0111], and China-Japan Friendship Hospital.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Author contribution
DH and CH conceived and designed the study. DH and WX wrote the manuscript, while DH, ZY, and LJ performed data analysis and interpretation. LZ and SY were responsible for the enhancement of figures and tables, and WA and WD participated in revising the manuscript. All authors contributed to the article and reviewed the submitted version.
Ethical approval
No ethical approval and patient consent were required for all analyses were based on literature research.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21645515.2025.2493539
References
- 1.Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK.. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rui R, Zhou L, He S. Cancer immunotherapies: advances and bottlenecks. Front Immunol. 2023;14:1212476. doi: 10.3389/fimmu.2023.1212476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emens LA, Romero PJ, Anderson AC, Bruno TC, Capitini CM, Collyar D, Gulley JL, Hwu P, Posey AD, Silk AW, et al. Challenges and opportunities in cancer immunotherapy: a society for immunotherapy of cancer (SITC) strategic vision. J Immunother Cancer. 2024;12(6):e009063. doi: 10.1136/jitc-2024-009063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li S, Zhang Z, Lai WF, Cui L, Zhu X. How to overcome the side effects of tumor immunotherapy. Biomed & Pharmacother. 2020;130:110639. doi: 10.1016/j.biopha.2020.110639. [DOI] [PubMed] [Google Scholar]
- 5.Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, Berent-Maoz B, Pang J, Chmielowski B, Cherry G, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35–15. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C, Wang J, Pan D, Wang X, Xu Y, Yan J, Wang L, Yang X, Yang M, Liu G-P. Applications of multi-omics analysis in human diseases. Med Comm. 2023;4(4):e315. doi: 10.1002/mco2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cousin S, Guégan JP, Shitara K, Palmieri LJ, Metges JP, Pernot S, Fukuoka S, Koyama S, Nishikawa H, Bellera CA, et al. Identification of microenvironment features associated with primary resistance to anti-PD-1/PD-L1 + antiangiogenesis in gastric cancer through spatial transcriptomics and plasma proteomics. Mol Cancer. 2024;23(1):197. doi: 10.1186/s12943-024-02092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yousuf S, Qiu M, Voith von Voithenberg L, Hulkkonen J, Macinkovic I, Schulz AR, Hartmann D, Mueller F, Mijatovic M, Ibberson D, et al. Spatially resolved multi-omics single-cell analyses inform mechanisms of immune dysfunction in pancreatic cancer. Gastroenterology. 2023;165(4):891–908.e14. doi: 10.1053/j.gastro.2023.05.036. [DOI] [PubMed] [Google Scholar]
- 9.Jaiswal A, Gautam P, Pietilä EA, Timonen S, Nordström N, Akimov Y, Sipari N, Tanoli Z, Fleischer T, Lehti K, et al. Multi-modal meta-analysis of cancer cell line omics profiles identifies ECHDC1 as a novel breast tumor suppressor. Mol Syst Biol. 2021;17(3):e9526. doi: 10.15252/msb.20209526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prelaj A, Miskovic V, Zanitti M, Trovo F, Genova C, Viscardi G, Rebuzzi SE, Mazzeo L, Provenzano L, Kosta S, et al. Artificial intelligence for predictive biomarker discovery in immuno-oncology: a systematic review. Ann Oncol Off J Eur Soc Med Oncol. 2024;35(1):29–65. doi: 10.1016/j.annonc.2023.10.125. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Z, Wang J, Wang J, Yang S, Wang R, Zhang G, Li Z, Shi R, Wang Z, Lu Q. Deciphering the tumor immune microenvironment from a multidimensional omics perspective: insight into next-generation CAR-T cell immunotherapy and beyond. Mol Cancer. 2024;23(1):131. doi: 10.1186/s12943-024-02047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hassan W, Duarte AE. Bibliometric analysis: a few suggestions. Curr Probl Cardiol. 2024;49(8):102640. doi: 10.1016/j.cpcardiol.2024.102640. [DOI] [PubMed] [Google Scholar]
- 13.Jiang S, Liu Y, Zheng H, Zhang L, Zhao H, Sang X, Xu Y, Lu X. Evolutionary patterns and research frontiers in neoadjuvant immunotherapy: a bibliometric analysis. Int J Surg (London, England). 2023;109(9):2774–2783. doi: 10.1097/JS9.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Zhuang Y, Yuan W, Gu Y, Dai X, Li M, Chen H, Zhou H. Radiomics in precision medicine for colorectal cancer: a bibliometric analysis (2013–2023). Front Oncol. 2024;14:1464104. doi: 10.3389/fonc.2024.1464104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan Z, He Q, Feng S. The collision of ChatGPT and traditional medicine: a perspective from bibliometric analysis. Int J Surg (London, England). 2023;109(11). doi: 10.1097/JS9.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, Zhang Y, Zhang B, Guo R, He M, Liu Z-L, Yang L, Wang H. Bibliometric study of immunotherapy for hepatocellular carcinoma. Front Immunol. 2023;14:1210802. doi: 10.3389/fimmu.2023.1210802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Ni Y, Ji H, Liu H, Liu S. Research trends of omics in ulcerative colitis: a bibliometric analysis. Front Med. 2023;10:1115240. doi: 10.3389/fmed.2023.1115240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu R, Zhao F, Xu Z, Zhang G, Du B, Shu Q. Current status and future of cancer vaccines: a bibliographic study. Heliyon. 2024;10(2):e24404. doi: 10.1016/j.heliyon.2024.e24404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan Y, R ND, Cao Y, Ke C, Pan J, Shi WY, Zhang W. Trends in the application of “omics” to Alzheimer’s disease: a bibliometric and visualized study. Neurol Sci. 2024;45(2):401–416. doi: 10.1007/s10072-023-07079-y. [DOI] [PubMed] [Google Scholar]
- 20.Pei Z, Chen S, Ding L, Liu J, Cui X, Li F, Qiu F. Current perspectives and trend of nanomedicine in cancer: a review and bibliometric analysis. J Control Release. 2022;352:211–241. doi: 10.1016/j.jconrel.2022.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Falagas ME, Pitsouni EI, Malietzis GA, Pappas G. Comparison of PubMed, Scopus, web of science, and Google Scholar: strengths and weaknesses. FASEB J Off Publ FedAm Soc Exp Biol. 2008;22(2):338–342. doi: 10.1096/fj.07-9492LSF. [DOI] [PubMed] [Google Scholar]
- 22.Zeng D, Wu J, Luo H, Li Y, Xiao J, Peng J, Ye Z, Zhou R, Yu Y, Wang G, et al. Tumor microenvironment evaluation promotes precise checkpoint immunotherapy of advanced gastric cancer. J Immunother Cancer. 2021;9(8):e002467. doi: 10.1136/jitc-2021-002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JB, Gao YX, Ye YH, Zheng Q-L, Luo H-Y, Wang S-H, Zhang T, Jin Q-W, Zheng C-H, Li P, et al. Comprehensive multi-omics analysis of pyroptosis for optimizing neoadjuvant immunotherapy in patients with gastric cancer. Theranostics. 2024;14(7):2915–2933. doi: 10.7150/thno.93124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing Y, Liu J, Ye Y, Pan L, Deng H, Wang Y, Yang Y, Diao L, Lin SH, Mills GB, et al. Multi-omics prediction of immune-related adverse events during checkpoint immunotherapy. Nat Commun. 2020;11(1):4946. doi: 10.1038/s41467-020-18742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Liu Y, Dai Y, Tang X, Yin T, Wang C, Wang T, Dong L, Shi M, Qin J, et al. Single-cell RNA-seq analysis reveals BHLHE40-driven pro-tumour neutrophils with hyperactivated glycolysis in pancreatic tumour microenvironment. Gut. 2023;72(5):958–971. doi: 10.1136/gutjnl-2021-326070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song X, Xiong A, Wu F, Li X, Wang J, Jiang T, Chen P, Zhang X, Zhao Z, Liu H, et al. Spatial multi-omics revealed the impact of tumor ecosystem heterogeneity on immunotherapy efficacy in patients with advanced non-small cell lung cancer treated with bispecific antibody. J Immunother Cancer. 2023;11(2):e006234. doi: 10.1136/jitc-2022-006234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Y, Wu L, Zhong Y, Zhou K, Hou Y, Wang Z, Zhang Z, Xie J, Wang C, Chen D, et al. Single-cell landscape of the ecosystem in early-relapse hepatocellular carcinoma. Cell. 2021;184(2):404–421.e16. doi: 10.1016/j.cell.2020.11.041. [DOI] [PubMed] [Google Scholar]
- 28.Pozniak J, Pedri D, Landeloos E, Van Herck Y, Antoranz A, Vanwynsberghe L, Nowosad A, Roda N, Makhzami S, Bervoets G, et al. A TCF4-dependent gene regulatory network confers resistance to immunotherapy in melanoma. Cell. 2024;187(1):166–183.e25. doi: 10.1016/j.cell.2023.11.037. [DOI] [PubMed] [Google Scholar]
- 29.McGrail DJ, Federico L, Li Y, Dai H, Lu Y, Mills GB, Yi S, Lin S-Y, Sahni N. Multi-omics analysis reveals neoantigen-independent immune cell infiltration in copy-number driven cancers. Nat Commun. 2018;9(1):1317. doi: 10.1038/s41467-018-03730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harel M, Ortenberg R, Varanasi SK, Mangalhara KC, Mardamshina M, Markovits E, Baruch EN, Tripple V, Arama-Chayoth M, Greenberg E, et al. Proteomics of melanoma response to immunotherapy reveals mitochondrial dependence. Cell. 2019;179(1):236–250.e18. doi: 10.1016/j.cell.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirschenbaum D, Xie K, Ingelfinger F, Katzenelenbogen Y, Abadie K, Look T, Sheban F, Phan TS, Li B, Zwicky P, et al. Time-resolved single-cell transcriptomics defines immune trajectories in glioblastoma. Cell. 2024;187(1):149–165.e23. doi: 10.1016/j.cell.2023.11.032. [DOI] [PubMed] [Google Scholar]
- 32.R: The R Project for Statistical Computing[EB/OL] . [2025 Feb 27]. https://www.r-project.org/.
- 33.Van Eck N, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technology. 2006;57(3):359–377. doi: 10.1002/asi.20317. [DOI] [Google Scholar]
- 35.Chen C, Hu Z, Liu S, Tseng H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther. 2012;12(5):593–608. doi: 10.1517/14712598.2012.674507. [DOI] [PubMed] [Google Scholar]
- 36.Aria M, Cuccurullo C. Bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetrics. 2017;11(4):959–975. doi: 10.1016/j.joi.2017.08.007. [DOI] [Google Scholar]
- 37.T D, C M, H X, Zhang G, Zeng L, Zhang G, Wu S, Wang Y. Srplot: a free online platform for data visualization and graphing. PLOS ONE. 2023;18(11):e0294236. doi: 10.1371/journal.pone.0294236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh I, Tsang KY, Blakemore WS. Effect of xenogeneic immune RNA on normal human lymphocytes against human osteosarcoma cells in vitro. J Natl Cancer Inst. 1977;58(3):505–510. doi: 10.1093/jnci/58.3.505. [DOI] [PubMed] [Google Scholar]
- 39.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (NY). 2017;357(6349):409–413. doi: 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science (NY). 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nat Methods. 2015;12(5):453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X, Li Z, Traugh N, Bu X, Li B, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24(10):1550–1558. doi: 10.1038/s41591-018-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, Kang B, Hu R, Huang JY, Zhang Q, et al. Landscape of infiltrating T cells in liver cancer revealed by single-cell sequencing. Cell. 2017;169(7):1342–1356.e16. doi: 10.1016/j.cell.2017.05.035. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Yu Q, Song T, Wang Z, Song L, Yang Y, Shao J, Li J, Ni Y, Chao N, et al. The heterogeneous immune landscape between lung adenocarcinoma and squamous carcinoma revealed by single-cell RNA sequencing. Signal Transduct Targeted Ther. 2022;7(1):289. doi: 10.1038/s41392-022-01130-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ou Z, Lin S, Qiu J, Ding W, Ren P, Chen D, Wang J, Tong Y, Wu D, Chen A, et al. Single-nucleus RNA sequencing and spatial transcriptomics reveal the immunological microenvironment of cervical squamous cell carcinoma. Adv Sci (Weinheim, Baden-Wurttemberg, Germany). 2022;9(29):e2203040. doi: 10.1002/advs.202203040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Che G, Yin J, Wang W, Luo Y, Chen Y, Yu X, Wang H, Liu X, Chen Z, Wang X, et al. Circumventing drug resistance in gastric cancer: a spatial multi-omics exploration of chemo and immuno-therapeutic response dynamics. Drug Resist Update Rev Commentaries Antimicrob Anticancer Chemother. 2024;74:101080. doi: 10.1016/j.drup.2024.101080. [DOI] [PubMed] [Google Scholar]
- 48.Hicks D, Wouters P, Waltman L, de Rijcke S, Rafols I. Bibliometrics: the leiden manifesto for research metrics. Nature. 2015;520(7548):429–431. doi: 10.1038/520429a. [DOI] [PubMed] [Google Scholar]
- 49.Gong Y, Ji P, Yang YS, Xie S, Yu T-J, Xiao Y, Jin M-L, Ma D, Guo L-W, Pei Y-C, et al. Metabolic-pathway-based subtyping of triple-negative breast cancer reveals potential therapeutic targets. Cell Metab. 2021;33(1):51–64.e9. doi: 10.1016/j.cmet.2020.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Bao X, Li Q, Chen J, Chen D, Ye C, Dai X, Wang Y, Li X, Rong X, Cheng F, et al. Molecular subgroups of intrahepatic cholangiocarcinoma discovered by single-cell RNA sequencing–assisted multiomics analysis. Cancer Immunol Res. 2022;10(7):811–828. doi: 10.1158/2326-6066.CIR-21-1101. [DOI] [PubMed] [Google Scholar]
- 51.Xiao Y, Ma D, Zhao S, Suo C, Shi J, Xue M-Z, Ruan M, Wang H, Zhao J, Li Q, et al. Multi-omics profiling reveals distinct microenvironment characterization and suggests immune escape mechanisms of triple-negative breast cancer. Clin Cancer Res An Off J Am Assoc Cancer Res. 2019;25(16):5002–5014. doi: 10.1158/1078-0432.CCR-18-3524. [DOI] [PubMed] [Google Scholar]
- 52.Liu C, Gao J, Yang D, Yu Q, Zhang S. Multi-omics and immune landscape of proliferative LncRNA signatures: implications for risk stratification and immunotherapy in hepatocellular carcinoma. Front Pharmacol. 2022;13:907433. doi: 10.3389/fphar.2022.907433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun C, Ye Y, Tan Z, Liu Y, Li Y, Hu W, Liang K, Egranov SD, Huang LA, Zhang Z, et al. Tumor-associated nonmyelinating Schwann cell–expressed PVT1 promotes pancreatic cancer kynurenine pathway and tumor immune exclusion. Sci Adv. 2023;9(5):eadd6995. doi: 10.1126/sciadv.add6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arshi A, Mahmoudi E, Raeisi F, Dehghan Tezerjani M, Bahramian E, Ahmed Y, Peng C. Exploring potential roles of long non-coding RNAs in cancer immunotherapy: a comprehensive review. Front Immunol. 2024;15:1446937. doi: 10.3389/fimmu.2024.1446937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Pan T, Zhou W, Zhang Y, Xu G, Xu Q, Li S, Gao Y, Wang Z, Xu J, et al. Long noncoding RNA LINC01132 enhances immunosuppression and therapy resistance via NRF1/DPP4 axis in hepatocellular carcinoma. J Appl Psychol Experimental Clinical Cancer Research: CR. 2022;41(1):270. doi: 10.1186/s13046-022-02478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Z, Chen L, Chen H, Zhao J, Li K, Sun J, Zhou M. Pan-cancer landscape of T-cell exhaustion heterogeneity within the tumor microenvironment revealed a progressive roadmap of hierarchical dysfunction associated with prognosis and therapeutic efficacy. EBioMedicine. 2022;83:104207. doi: 10.1016/j.ebiom.2022.104207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen A, Ye Y, Chen F, Xu Y, Zhang Z, Zhao Q, Zeng Z-L. Integrated multi-omics analysis identifies CD73 as a prognostic biomarker and immunotherapy response predictor in head and neck squamous cell carcinoma. Front Immunol. 2022;13:969034. doi: 10.3389/fimmu.2022.969034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li S, Yu W, Xie F, Luo H, Liu Z, Lv W, Shi D, Yu D, Gao P, Chen C, et al. Neoadjuvant therapy with immune checkpoint blockade, antiangiogenesis, and chemotherapy for locally advanced gastric cancer. Nat Commun. 2023;14(1):8. doi: 10.1038/s41467-022-35431-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guruprasad P, Lee YG, Kim KH, Ruella M. The current landscape of single-cell transcriptomics for cancer immunotherapy. J Exp Med. 2021;218(1):e20201574. doi: 10.1084/jem.20201574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bagaev A, Kotlov N, Nomie K, Svekolkin V, Gafurov A, Isaeva O, Osokin N, Kozlov I, Frenkel F, Gancharova O, et al. Conserved pan-cancer microenvironment subtypes predict response to immunotherapy. Cancer Cell. 2021;39(6):845–865.e7. doi: 10.1016/j.ccell.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Mooney B, Negri GL, Shyp T, Delaidelli A, Zhang H-F, Spencer Miko SE, Weiner AK, Radaoui AB, Shraim R, Lizardo MM, et al. Surface and global proteome analyses identify ENPP1 and other surface proteins as actionable immunotherapeutic targets in Ewing Sarcoma. Clin Cancer Res An Off J Am Assoc Cancer Res. 2024;30(5):1022–1037. doi: 10.1158/1078-0432.CCR-23-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuan Q, Deng D, Pan C, Ren J, Wei T, Wu Z, Zhang B, Li S, Yin P, Shang D. Integration of transcriptomics, proteomics, and metabolomics data to reveal HER2-associated metabolic heterogeneity in gastric cancer with response to immunotherapy and neoadjuvant chemotherapy. Front Immunol. 2022;13:951137. doi: 10.3389/fimmu.2022.951137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang SL, Cheng LS, Zhang ZY, Sun H-T, Li J-J. Untangling determinants of gut microbiota and tumor immunologic status through a multi-omics approach in colorectal cancer. Pharmacol Res. 2023;188:106633. doi: 10.1016/j.phrs.2022.106633. [DOI] [PubMed] [Google Scholar]
- 64.Parra ER, Zhang J, Duose DY, Gonzalez-Kozlova E, Redman MW, Chen H, Manyam GC, Kumar G, Zhang J, Song X, et al. Multi-omics analysis reveals immune features associated with immunotherapy benefit in patients with squamous cell lung cancer from phase III lung-MAP S1400I trial. Clin Cancer Res An Off J Am Assoc Cancer Res. 2024;30(8):1655–1668. doi: 10.1158/1078-0432.CCR-23-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nuñez NG, Berner F, Friebel E, Unger S, Wyss N, Gomez JM, Purde M-T, Niederer R, Porsch M, Lichtensteiger C, et al. Immune signatures predict development of autoimmune toxicity in patients with cancer treated with immune checkpoint inhibitors. Med. 2023;4(2):113–129.e7. doi: 10.1016/j.medj.2022.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


