Abstract
Aging is closely associated with imbalanced transcription. Regulated transcription in different organs is significantly different during aging, indicating that organ-specific transcriptomics is critical for understanding this process. Here we analyze the transcriptomics of the intestines of 3-, 15-, 30-, 40- and 50-days old female flies, which include young, middle-aged, and old flies. We find that the differential expression of protein-coding genes and lncRNAs is significant in aging, and fly age is characterized by well-separated gene expression trajectories. The highly clustered differentially expressed genes are connected to specific biological processes and signalling pathways. In particular, the Imd and Toll pathways are the top two immune signalling pathways that are highly regulated, and members with increased expression in the Imd pathway span all upstream activating events and include many ubiquitylation-associated factors and regulators of NF-κB factor Relish. Increased expression of Toll pathway members includes sensing mediators for all kinds of microorganisms and multiple proteases in the proteolytic processing cascade. Moreover, the expression of molecular markers of intestinal cells is greatly changed. Enterocyte markers are the most significantly influenced, and enteroendocrine markers AstA and NPF, as well as intestinal stem cell (ISC)/enteroblast (EB) markers Esg and Klu are expressed at low levels in young flies and much higher levels in aged flies. Furthermore, lncRNAs show similar expression trends and clustering patterns to those of protein-coding genes. Lastly, we find that ISC/EB-specific knock-down of 13 out of 19 genes that are highly differentially expressed reduces the lifespan of the fly. Together, the characterized transcriptomics and newly identified functional genes in aging will provide potential targets for preventing intestinal aging and associated disorders.
Keywords: Aging, Intestine, Transcriptomics, lncRNA, Lifespan, Drosophila
Subject terms: Cell biology, Immunology, Gastroenterology
Introduction
The aging process is highly complex and characterized by the physiological decline of multiple organs; it is closely associated with a progressive increase in disease susceptibility. The intestine is one of the most versatile tissues in the body and is responsible for many physiological processes, including nutrient absorption, interaction with commensal microbiota, and immune modulation. Moreover, the intestine plays critical roles as a physical and functional barrier to protect the body from chemical, physical and microbiota stimuli. The intestine is a fast turn-over tissue with a continuously renewing epithelium, and an impaired intestinal barrier is a prominent feature in aged organisms and tightly linked with declined health, increased microbiota load and chronic inflammation, reduced lifespan, and disease1,2.
Aging is regarded as the biggest risk factor for many human diseases. Due to complications in human aging research, animal models such as mice, zebrafish, flies, and nematodes are being used as valuable resources. Findings from animal models, such as regulations and signalling pathways, have been proven to be highly conserved during evolution and provide fundamental and insightful understandings of aging3,4. Even though comprehensive knowledge is being continuously accumulated, our understanding of aging is still limited.
Inspired by advances in high-throughput omics technologies, significant progress has been made in understanding the intrinsic mechanisms of aging, and a large amount of data from transcriptomics, epigenomics, proteomics, and metabolomics has been collected and brought together to understand aging5. Aging-related transcriptomic data from human and animal models have been pooled together, and gene databases and longevity databases have been established. Analysis of these databases suggests that differentially expressed genes during the aging process in different tissues are significantly different, with little overlap6. However, highly similar age-related expression changes were observed in the same tissues across mammalian species7. Recent studies have shown that aging is closely associated with imbalanced transcription8, transcriptional elongation speed9, transcriptional output10, and loss of transcriptional fidelity11.
Drosophila melanogaster has been used for aging studies for more than one hundred years, starting in 191612. With many benefits for research, Drosophila has a short lifespan, conserved genes and signalling pathways, powerful genetic techniques, abundant genetic resources, and low-cost maintenance13,14. Until now, aging-related transcriptomic analyses of the whole body or organs have been performed in flies. A significant collection of transcriptomic data from female flies was obtained using microarray, transcripts of the whole body were detected at 11 timepoints starting from day-6 post-eclosion to day-85 with 1-week interval, and 9 clusters of aging-associated differential gene expression were identified15,16 . Another microarray analysis using 7 tissues, including the nervous, muscular, digestive, renal, reproductive, and storage systems of adult males at multiple timepoints indicated that transcript levels changed significantly with aging, and aging-related genes showed tissue-specific expression patterns17. In addition, analysis of the head, thorax, and whole body of 3-day and 40-day-old male flies found that mitochondrial genes were greatly downregulated, and proteasome subunits were upregulated in the thorax of aged flies18. Analysis of the male thorax and abdomen of young, mid-aged, and old flies found that the transcript level changed with age19. Moreover, analysis of 10-day and 61-day old male flies found that Drosophila aging is associated with increased expression of innate immune response genes20. The analysis was also performed on female flies21. Analysis of 2-day and 45-day old female flies found that genes involved in muscle development and maintenance were down-regulated in older flies22. Furthermore, a cardiac-specific study using 1-week and 5-week fly adults suggested that the transcription factor Odd-skipped is a crucial regulator of cardiac aging23. Despite the analysis of aging-specific transcriptomics in the whole body or some organs of Drosophila, the expression characteristics in the fly intestine during the aging process are still unclear.
Here, we analyzed the transcription of protein-coding genes and lncRNAs in the female fly intestine during the aging process. We found that both protein-coding genes and lncRNAs showed well-regulated expression patterns during the aging process, and the young, mid-aged, and old flies were characterized by distinct and age-specific transcriptomics. The differentially expressed genes during the aging process can be grouped into 8 clusters, representing dynamic gene expression trends in the fly gut. Moreover, gene expression trends were closely connected to specific biological processes and signalling pathways. To be specific, upregulated genes were highly enriched in immune response and immune signalling pathways. Furthermore, many molecular marker genes for intestinal cells showed differential expression, which will help improve our understanding of the over-proliferation and aberrant differentiation of ISCs during the aging process. The expression patterns of lncRNAs were similar to those of protein-coding genes. Lastly, we identified 13 genes that might be required for lifespan maintenance and 4 genes that are required for maintenance of gut integrity.
Materials and methods
Drosophila genetics
All flies were maintained at 25 °C on standard corn medium unless otherwise noted. The flies used in this study were as follows: w 1118; esg-Gal4, UAS-GFP (esg); esg-Gal4, UAS-GFP; tubGal80 ts (esg ts); and RNAi fly lines (Table S1), which were obtained from the Bloomington Stock Center and the Vienna Drosophila Resource Center.
Samples for RNA-seq
Newly hatched flies were collected and aged at 25 °C. The intestines of aged female flies on day-3, -15, -30, -40, and -50 were dissected in cold PBS. Total RNA was extracted using Trizol with DNase treatment at Beijing Genomics Institute (BGI Co., Ltd., China). The integrity of the extracted RNA was assessed using Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). The mRNA sequencing libraries were constructed using Illumina TruSeq Stranded mRNA Sample Prep Kit (Illumina, Cat. No.RS-122-2101), and paired-end 50 bp sequencing was performed using the Illumina HiSeq 2000. All RNA-Seq experiments included three biological replicates with about 200 fly guts in each replicate.
RNA-seq data quality control, alignment and counting
The raw sequencing data were assessed using trim_galore (version 0.6.6) to remove adapter sequence, and low-quality data was filtered out with FastQC (version 0.11.9) (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/)24. A total of 40–50 million high-quality reads per sample with an approximate GC content of 50% were generated. Alignment to the Drosophila melanogaster reference genome (dmel_r6.41_FB2021_04) was conducted using hisat2 (version 2.2.1), and alignment rate was 60–90%. Gene annotations were assigned using Flybase (dmel-all-r6.55.gtf)25, and read counting was performed using featureCounts (v2.0.6)26. A protein-coding gene was considered expressed when it had 10 or more reads in at least one sample. A long non-coding RNA (lncRNA) was considered expressed when it was detected in all three samples at a given timepoint.
Gene expression analysis
After gene expression data were normalized, Principal Component Analysis (PCA) was performed using the ‘prcomp’ function within the ‘stats’ package (version 4.3.2) in R. Data visualization was facilitated using the ‘ggplot2’ and ‘ggfortify’ packages. Cluster heatmaps were generated using the ‘pheatmap’ package. Differentially expressed genes (DEGs) were identified by comparing gene expression in samples on different days using the ‘DESeq2’ package (version 1.42.0)27, and a gene was considered differentially expressed when the absolute log2-fold change is ≥ 1 and the adjusted p value is < 0.05. Intersection analysis of upregulated or downregulated genes across 10 groups was performed and visualized using the ‘UpSetR’ package. Time-series expression patterns were analyzed using a fuzzy clustering method, and the ‘MFuzz’ package (version 2.62.0)28 was used for time-series clustering analysis.
Functional enrichment analysis
The most recent versions of the Gene Ontology (GO) database (http://www.geneontology.org/) and the KEGG pathway database (https://www.kegg.jp/) were used. The ‘clusterProfiler’ package (version 4.10.0)29 in R was employed to annotate differentially expressed genes and perform enrichment analysis, and genes were clustered based on expression patterns. Multiple hypothesis testing corrections were applied, and a gene with an adjusted p value < 0.05 was considered statistically significant. To visualize the significant findings from the enrichment analysis, the seven most significant GO terms or KEGG terms were generated using voronoiTreemap with the ‘WeightedTreemaps’ package (version 0.1.2) in R. Unless stated otherwise, all calculations, analyses, and data processing were performed using custom-written scripts in R30.
Lifespan assay
For the gene knockdown, the esg-Gal80ts system was used to achieve temporal control of RNAi induction. Parental flies were maintained at 18ºC during mating and egg laying to prevent RNAi activation. Newly eclosed adult progenies were transferred to 29ºC within 24 h of eclosion and maintained at this temperature throughout the lifespan assay. This temperature shift inactivates Gal80and enables Gal4-mediated RNAi expression specifically in adult stages. Male flies and female flies were separated and placed into vials with 15 flies in each vial to avoid overcrowding. Flies were transferred to new vials every 3 days. Surviving flies were counted at each transfer until all flies died. Two independent lifespan experiments were performed, each with 150 female and 150 male flies per genotypes, using "esg-Gal80ts/ + " as the control for comparisons with "esg-Gal80ts/RNAi.". Survival curves were plotted using the Kaplan–Meier estimates (GraphPad Prism 6). Pairwise comparisons between the experimental group and control were performed using the Log-rank (Mantel-Cox) test, with Chi-square values and p values reported in Supplementary Table S11. Lifespan assays were considered biologically significant only when log-rank p < 0.05 in two independent experiments.
Smurf assay
The smurf assay was performed as described to determine intestinal integrity31. Briefly, male flies and female flies were separated and transferred onto fresh medium containing FD&C blue dye #1 (2.5% w/v, Sigma) for 8 h. A fly was counted as a “smurf” when dye coloration was observed outside the digestive tract.
Statistics
All statistical comparisons were performed using data collected from three biological replicates. Hierarchical clustering techniques were utilized to explore the resemblance among samples, with Euclidean distances serving as the metric to gauge the proximity of data points within the collective datasets. To analyze differential gene expression, gene count data underwent an initial normalization process to rectify any systematic discrepancies. Subsequently, data were modeled to adhere to a negative binomial distribution through implementation of a generalized linear model, which is particularly adept at handling over-dispersed data. To ascertain the significance of variance in gene expression, Wald tests were employed. To mitigate the risk of false positives arising from multiple hypothesis testing, the Benjamini–Hochberg procedure was adopted. Functional enrichment analysis of differentially expressed genes was performed using the hypergeometric distribution test, commonly referred to as Fisher’s exact test, to provide insights into the biological significance of observed changes. The fuzzy c-means (FCM) clustering algorithm was harnessed to categorize gene expression patterns across various timepoints, offering a nuanced understanding of temporal expression dynamics. Survival time probability distributions were estimated using the Kaplan–Meier method, and the Log-rank test was applied to detect statistical discrepancies across different survival curves, providing a comparative view of survival outcomes.
Results
Clearcut gene expression profiles during aging process in fly gut
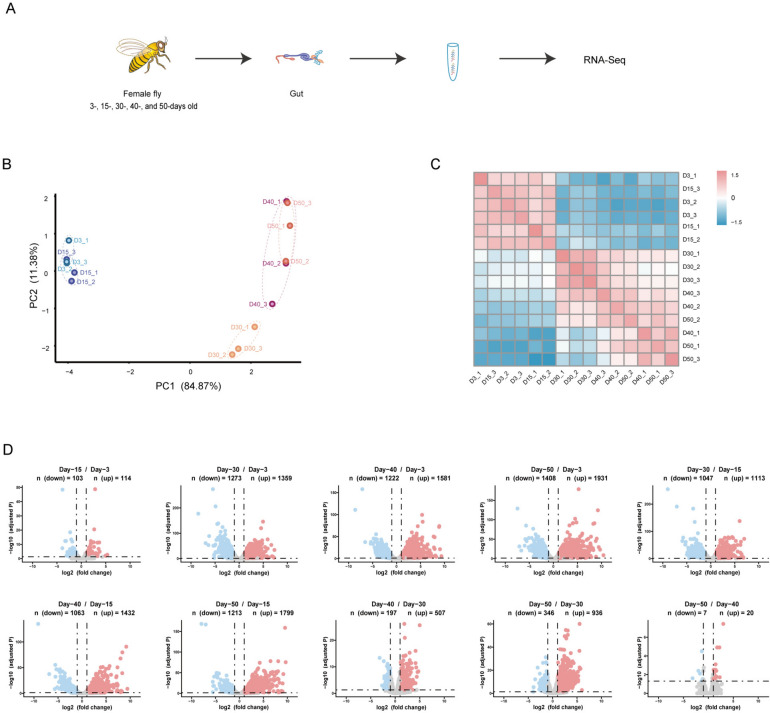
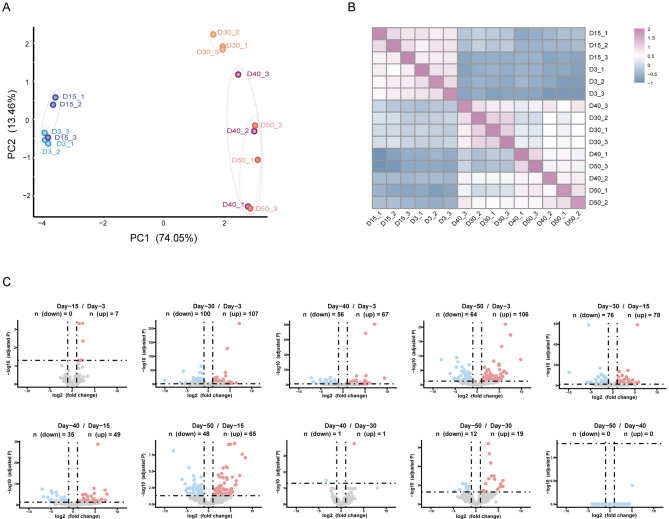
To profile transcriptomics in the fly gut during the aging process, newly hatched flies were collected and aged at 25 °C. The guts of female flies on day-3 (D3), -15 (D15), -30 (D30), -40 (D40), and -50 (D50) post-eclosion were dissected, and poly-A enriched RNA-seq was performed (Fig. 1A). The RNA-seq data were aligned to the Drosophila melanogaster BDGP6 (dmel_r6.41_FB2021_04) using hisat2 (v2.2.1) after quality control and normalization. We identified 10,941 genes with at least 10 reads using featureCounts (v2.0.6) (Table S2), which includes 10,074 protein-coding genes (Table S3). The principal component analysis (PCA) and hierarchical clustering results indicated that samples were clustered predominantly by the fly age. Samples on day-3 and day-15 were clustered together, while samples on day-30, -40, and -50 were distant from samples on day-3 and day-15, and samples on day-40 and -50 were clustered together and separated from day-30 samples (Fig. 1B, C). These results indicated that aging has a remarkable effect on gene expression. As flies on day-3 correspond to young age, day-30 as mid-age, and day-50 as old age16,32, the gene expression trajectories during the aging process suggested that age-specific transcriptomics are generated in the fly gut to characterize aging stages.
Fig. 1.
Differential expression of protein-coding genes during aging process in fly gut. (A) Scheme of workflow. (B) Principal component analysis (PCA) plot of protein-coding genes expression. Samples from different aging timepoints were presented with unique colors. (C) Heatmap illustrating correlation of gene expression at various timepoints. (D) Volcano plot showing relative expression of protein-coding genes in comparisons between different timepoints. Number of differentially expressed genes (DEGs) were shown. The y-axis represents statistical significance as −log10 (adjusted p value), and the x-axis represents log2 fold change. Up-regulated genes were marked in red, down-regulated genes were marked in blue.
Distinct and well-regulated transcriptomics during aging process
To characterize the age-dependent gene expression patterns, we analyzed the differential expression of protein-coding genes among samples at different aging timepoints using DESeq2, and a gene expressed with a significant difference (fold change ≥ ± 2, adjusted p < 0.05) between samples in 3 replicates was regarded as differentially expressed. We generated 10 groups of differentially expressed genes (DEGs) in total comparisons (Table S4). Among all groups, the number of DEGs increased remarkably in older flies when compared with young flies, suggesting significant changes in transcriptomics during the aging process. The most significant difference appeared between the day-50 and day-3 with 3339 DEGs, while the smallest number of DEGs was between the day-50 and day-40 with only 27 genes, which included both up-regulated and down-regulated genes. Second to the smallest number of DEGs was between the day-15 and day-3 with 217 genes. And other comparisons had the number of DEGs in-between the day-50 versus day-3 and the day-50 versus day-40 groups (Fig. 1D). These data were consistent with the results of the PCA and hierarchical clustering (Fig. 1B, C), suggesting that gene expression is well-regulated during the aging process.
To characterize gene expression patterns during the aging process, we performed Upset analysis. We found that most differentially expressed genes were shared by different comparisons. In upregulated DEGs, the largest number appeared in comparisons between day-30, -40 and -50 versus day-3 and day-15 (n = 489), respectively. The second was DEGs unique in-between day-50 and day-30 (n = 151), the third was in comparisons between day-40 and -50 versus day-3, day-15 and day-30 (n = 129), respectively, and the fourth was between day-30 and day-3 (n = 106) (Figure S1A). As flies on day-3, day-30 and day-50 correspond to young age, mid-age, and old age, respectively, the shared DEGs or unique DEGs in the above comparisons suggested that there are significant changes of gene expression patterns during the aging process. In down-regulated DEGs, similar to up-regulated DEGs, the biggest number appeared in comparisons between day-30, -40 and -50 versus day-3 and day-15 (n = 431), respectively, and the second was in comparisons between day-30 versus day-3 and day-15 (n = 167), respectively. The third and fourth were in comparisons between day-30 versus day-3 (n = 146) and day-50 versus day-3 (n = 110) (Figure S1B). All these data suggested that the aging process is characterized by distinct and well-regulated transcriptomics.
Dynamic gene expression trends during aging process in fly gut
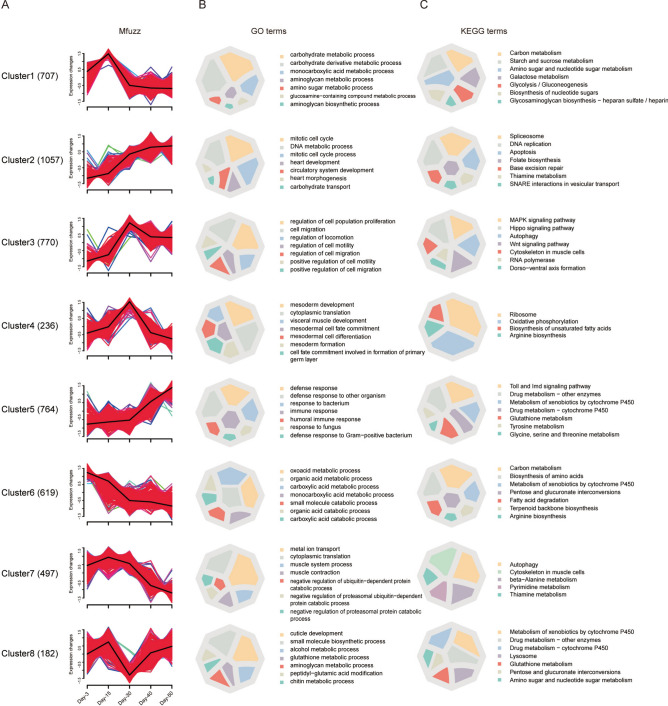
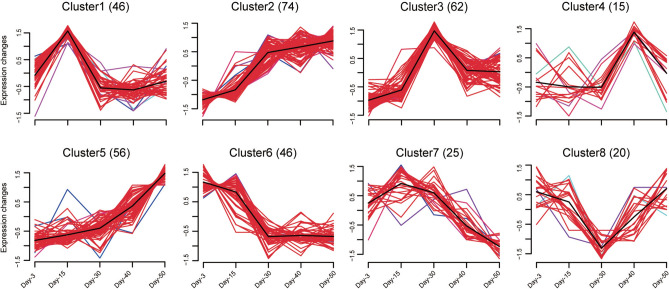
When differentially expressed genes in all comparisons were polled together and duplicated genes were removed, 4832 DEGs were generated (Table S5). We analyzed the expression pattern of these DEGs at five aging timepoints using R-Mfuzz. Based on expression trends, these DEGs can be clustered into 8 groups and show distinct expression characters. The cluster1 included 707 DEGs, these DEGs were expressed at medium level on day-3, reached the highest level on day-15, and decreased their expression soon after. The cluster2 included 1057 DEGs, had the lowest expression level on day-3, increased their expression gradually and reached the highest level on day-50. The cluster3 included 770 DEGs, had the lowest expression level on day-3, reached the highest level on day-30, and decreased their expression mildly soon after. The cluster4 included 236 DEGs, reached the highest level on day-30, and had similar and very low levels at other timepoints. The cluster5 included 764 DEGs, increased their expression slowly until day-30, then increased their expression sharply and reached the highest level on day-50. The cluster6 included 619 DEGs, had the highest level on day-3, decreased their expression sharply until day-30, then slightly decreased their expression and reached the lowest level on day-50. The cluster7 included 497 DEGs, had the mild-high expression level on day-3, increased their expression on day-15, then decreased expression and reached the lowest level on day-50. The cluster8 included 182 DEGs, had the lowest expression level on day-30, and similar and medium level at other timepoints (Fig. 2A). Moreover, the DEGs in some clusters showed similar expression trends, while had differences at specific aging timepoints. The cluster4 DEGs reached the highest expression level on day-30, while the cluster8 DEGs had the lowest expression level on day-30, and expression level at other timepoints was similar, suggesting that DEGs in cluster4 and cluster8 might play important regulatory roles in the intestine on day-30, the middle-aged flies. Furthermore, DEGs in cluster2 and cluster5 had the lowest expression level on day-3 and the highest level on day-50, but showed different expression trends in the aging process.
Fig. 2.
Distinct expression trends are connected with specific biological processes and signalling pathways. (A) Mfuzz of differentially expressed genes showing 8 clusters of expression trend. Number of DEGs were shown in bracket. The black line represents an average expression trend of all genes within a cluster, a shift of red region indicates contribution to the expression trend, and colored lines indicate the membership degree of individual gene within a cluster. (B) GO (Gene Ontology) and C. KEGG (Kyoto Encyclopedia of Genes and Genomes) enrichment analysis of DEGs. The top-seven significantly enriched categories were presented in color charts.
Distinct gene expression trends are closely connected with specific biological processes and signalling pathways
To systematically resolve transcriptional dynamics underlying intestinal aging in Drosophila melanogaster, we identified eight temporally distinct gene clusters through time-series analysis (Fig. 2A). Integrated Gene Ontology (GO) and KEGG pathway analyses revealed that these clusters were functionally associated with biological processes exhibiting phased activation or suppression during aging (p < 0.05; Tables S6-S7). Three interconnected functional phases emerged, each characterized by unique molecular signatures.
Early metabolic reprogramming supports post-eclosion maturation (D3-D15). Young adult flies (D3) exhibited pronounced activation of carbohydrate metabolism pathways, with Cluster1 genes enriched in glycolysis and starch/sucrose metabolism (Fig. 2B, C). These metabolic programs declined rapidly after D15, coinciding with the completion of gut maturation following eclosion. Concurrently, Cluster8 genes transiently peaked at D15, showing dual roles in structural remodeling and redox homeostasis (Fig. 2B, C). This coordinated metabolic-structural regulation aligns with known nutrient-dependent transitions in early Drosophila adulthood, where larval-derived resources fuel post-eclosion tissue specialization.
Midlife restructuring balances damage control with tissue maintenance (D15-D30). From D15 onward, transcriptional priorities shifted toward genomic stability and structural consolidation. Cluster2 genes were progressively upregulated, engaging in DNA repair mechanisms (Fig. 2B, C), while Cluster4 reached peak expression at D30 through ribosome biogenesis and oxidative phosphorylation, coupled with muscle differentiation programs (Fig. 2B, C). Notably, Cluster3 displayed a transient D30 activation of stress-responsive pathways (Fig. 2C), reflecting a time-limited adaptive response to accumulating cellular damage. This tripartite regulation-sustained DNA repair, bioenergetic investment, and transient stress buffering-mirrors transcriptional adaptations observed in aging Drosophila muscle stem cells.
Late-phase dysregulation heralds systemic functional decline (D30-D50). After D30, progressive metabolic decay and inflammatory escalation dominated the transcriptional landscape. Cluster6 genes, persistently downregulated from D30 onward, were linked to mitochondrial dysfunction (Fig. 2B, C). Conversely, Cluster5 exhibited chronic activation of innate immunity and detoxification systems, indicative of age-related inflammaging in the Drosophila gut. Neuromuscular deterioration was evidenced by Cluster7’s gradual decline in ion transport and β-alanine metabolism (Fig. 2B, C), consistent with sarcopenia phenotypes in aged flies. Intriguingly, Cluster8 partially recovered glutathione-associated expression by D50 (Fig. 2B), suggesting compensatory detoxification attempts despite failing to regain youthful activity levels.
Divergent regulatory strategies highlight aging checkpoint mechanisms. The contrast between transient (Cluster3) versus sustained (Cluster4) midlife transcriptional programs implies distinct survival strategies: short-term stress adaptation versus long-term structural investment. Furthermore, Cluster8’s biphasic trajectory-peaking at D15, collapsing at D30, then partially recovering-reveals dynamic redox regulation attempts across aging stages. These findings collectively map a hierarchical functional decline in the Drosophila gut: early metabolic priming establishes adult tissue integrity, midlife adaptations temporarily mitigate damage, and late-phase failures culminate in inflammatory-metabolic collapse.
Aging-associated immune signalling alterations in fly gut
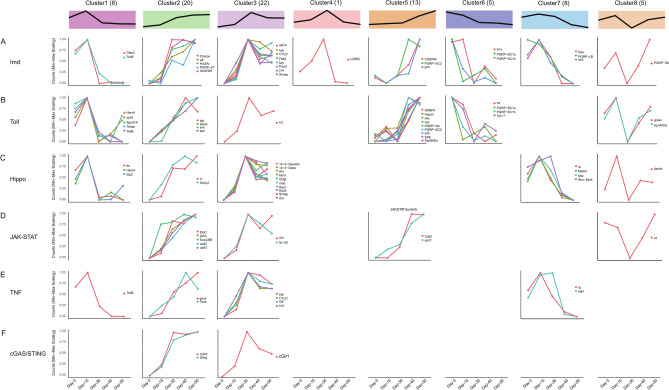
The intestine is the largest organ for immune response. Chronic inflammation is designated as a new hallmark of aging, and systemic inflammation is closely linked with aging-related alterations and named “inflammaging”33. Our data indicated that immune response was significantly up-regulated in the aging process (Fig. 2). To gain a better understanding of the aging-related status of immune response in fly gut, we manually curated genes in the immune-related signalling pathways of Imd, Toll, JAK-STAT, HIPPO, cGAS-STING and TNF using the up-to-date annotations34. We analyzed 264 genes in total in these signalling pathways, 240 of them were expressed in the female fly gut, and 82 genes were differentially expressed (Table S8). When these DEGs were classified based on their expression trends, we found that the Imd and Toll signalling pathways were the top 2 pathways significantly enriched during the aging process (Fig. 3, Table S8).
Fig. 3.
Gene expression trends of immune-related signalling pathways. (A–F). Distribution maps illustrating the expression patterns of DEGs across immune-related signalling pathways.
Activation of the Imd pathway involves multiple events, including phosphorylation of the key transcription factor Relish, and activation by the IKK complex, FADD/Dredd-dependent Relish processing, and the JNK signalling pathway35,36. We found that genes involved in these events had significantly increased expression during the aging process, including key (kenny), which encodes a scaffold protein in the IKK complex, fadd (Fas-associated death domain), which encodes an adaptor protein for FADD/Dredd-dependent Relish processing, and JNK encoding gene bsk (basket) and its negative feedback regulator puc (puckered) (Fig. 3A, cluster3)37. A previous study found that the JNK activity increases with age in fly gut38, which is consistent with our data. Moreover, post-translational ubiquitylation plays important regulatory roles in the Imd signalling39. We found that genes encoding ubiquitylation-related enzymes such as eff (effete), cyld, ubcE2M, and key had increased expression in the aging process (Fig. 3A, cluster2 and cluster3), while diap2 reached the highest expression level on day-15 and decreased its expression soon after (Fig. 3A, cluster1), and an E3 ligase for Kenny encoding by lubel, reached the highest expression level on day-30 (Fig. 3A, cluster4). In addition, genes encoding Relish regulators, such as charon, akirin, parp1, and slmap, also increased their expression during the aging process (Fig. 3A, cluster2 and cluster3). The transcription factor FOXO is a master regulator of stress response, and increased FOXO activity extends lifespan in model organisms, such as c. elegans and Drosophila40, its coding gene foxo reached the highest expression level on day-15 and decreased its expression soon after (Fig. 3A, cluster7).
Second to the Imd pathway, members of the Toll pathway had greatly increased expression during the aging process (Table S8), which included the sensing mediator PRGP-SA for detecting gram-positive bacterial peptidoglycan, glucan-binding protein family member GNBP3 for detecting fungi in pathogen-associated molecular patterns (PAMPs), and serine protease Psh (Persephone) and its protease inhibitor Nec (Necrotic) for recognition of danger signals to distinguish commensal and pathogenic microbes (Fig. 3B, cluster5)41. The proteolytic processing cascade is essential for Toll activation, and multiple genes encoding proteases in the cascade had increased expression, including spe (spätzle-processing enzyme) and its regulators seniu, snk (snake) and hayan, and protease inhibitor spn88Ea (Fig. 3B, cluster5 and cluster2). In addition, the Toll ligand cytokine spz (spätzle) had increased expression (Fig. 3B, cluster5). Interestingly, the Toll pathway members essential for dorsal/ventral pattern formation such as Snake, Easter and Pipe had increased expression too (Fig. 3B, cluster5)35,36,41.
Besides the Imd and Toll pathways, members of the other four immune-related signalling pathways also had increased expression in the aging process (Fig. 3C–F, Table S8). It is worth noting that the JAK/STAT signalling is essential for homeostasis and regeneration of fly intestine42,43. The ligands of JAK/STAT signalling, Upd1, Upd2, and Upd3, which are cytokine-like proteins and Drosophila homolog of IL-6, and suppressor of cytokine signalling (SOCS) protein SOCS36E, have continuously increased expression in the aging process (Fig. 3D, cluster2 and cluster5).
Differentially expressed molecular markers in fly gut during aging process
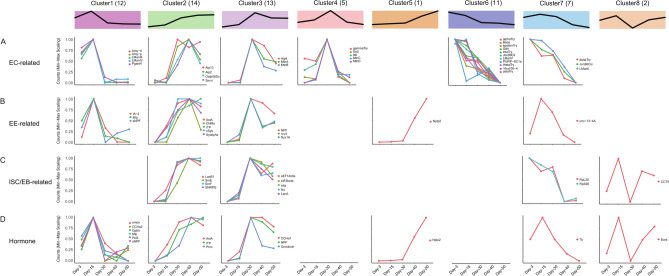
The fly gut shares many similarities of anatomic and physiological characteristics with the mammalian gut, it is composed of intestinal stem cells (ISCs), enteroblasts (EBs), enteroendocrine cells (EEs), and enterocytes (ECs). In aging flies, guts show epithelial dysplasia, characterized by ISCs over-proliferation and aberrant differentiation38,44. A recent study profiled gene expression in fly gut using single-cell RNA sequencing (scSEQ), and identified molecular markers of cells residing in the gut45. By comparison analysis, we found that EC markers were the most differentially expressed during the aging process, and DEGs for EC markers were highly enriched in cluster6, in which genes were expressed at the highest level on day-3 and then decreased their expression (Fig. 4A, Table S9). The enterocytes were divided into 15 clusters based on the expression of different trypsin genes45. Markers of most EC clusters were differentially expressed in the aging process, such as alphaTry and thetaTry (Fig. 4A, cluster6), and gammaTry (Fig. 4A, cluster4) for anterior enterocytes 1 to 3 (aEC1-3), Amy-p and Amy-d (Fig. 4A, cluster1) for aEC4, Lab transcription factor (Fig. 4A, cluster4) and Vha100-4 (Fig. 4A, cluster6) for middle ECs (mEC), metal ion binding proteins MtnA, MtnB (Fig. 4A, cluster3), MtnC and MtnD (Fig. 4A, cluster4) for copper and iron cells, PGRP-SC1a (Fig. 4A, cluster6) for large flat cells (LFC), zetaTry (Fig. 4A, cluster6) for posterior ECs 1 to 3 (pEC1-3), alkaline phosphatases Alp2 and Alp13 (Fig. 4A, cluster2), and Alp4 (Fig. 4A, cluster3) for differentiating ECs (dEC), transporters Smvt and Oatp58Dc (Fig. 4A, cluster2) for EC-like 1, Jon99Ciii (Fig. 4A, cluster7) and Bace (Fig. 4A, cluster6) for EC-like 2 and EC-like 3, and Pgant4 (Fig. 4A, cluster1) for cardia secretory cells. Second to ECs were markers of EEs and hormones secreted by EEs (Table S9)45,46. The EE cluster-specific marker AstA had lowest expression level on day-3 and increased its expression in aging (Fig. 4B, D, cluster2), another EE cluster-specific marker NPF had lowest expression level on day-3 and highest expression level on day-30 (Fig. 4B, D, cluster3), and other EE markers including IA-2 (Fig. 4B, cluster1), nrv3 (Fig. 4B, cluster3), unc-13-4A (Fig. 4B, cluster7), Sytalpha and nSyb (Fig. 4B, cluster2), and Syx1A (Fig. 4B, cluster3) involved in vesicle docking and secretion, and neuropeptides sNPF (Fig. 4B, D, cluster1), ion transporter peptide (ITP) (Fig. 4B, D, cluster2) and CNMa (Fig. 4B, cluster2), and neuropeptide-like precursor 2 (Nplp2) (Fig. 4B, D, cluster5) were also differentially expressed in the aging process. In ISCs and EBs, the typical marker Esg and newly identified EB marker Klumpfuss (Klu) were expressed at lowest level on day-3 and increased their expression in aging (Fig. 4C, cluster3), and genes expressed at higher level in ISCs than in EBs, including translation elongation factors eIF2beta and eEF1delta (Fig. 4C, cluster3), small ribonucleoprotein particles SmE, SmF and SNRPG (Fig. 4C, cluster2), and chaperone and heat-shock proteins CCT5 (Fig. 4C cluster8), were differentially expressed during the aging process. Together, these data provide expression information of molecular markers of intestinal cells in aging, and will be helpful in improve our understanding of ISCs over-proliferation and aberrant differentiation in aging.
Fig. 4.
Gene expression trends of molecular markers of intestinal cells and hormones. (A–D). Distribution maps illustrating the expression patterns of molecular markers and hormones in designated cells.
Multiple differentially expressed genes are essential for lifespan maintenance and gut integrity
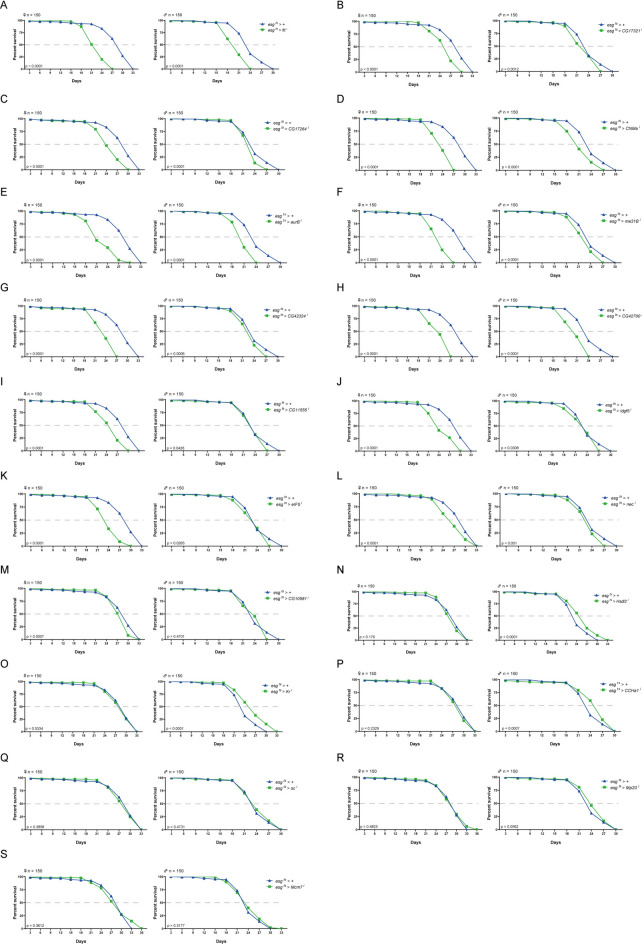
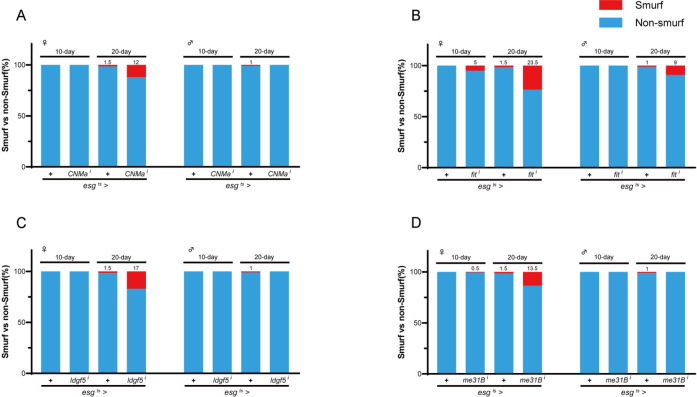
Can differential expression in aging be applied to define functional genes in lifespan? To explore this possibility, we performed a lifespan assay using esg-Gal4 combined with a temperature-sensitive GAL80 (GAL80ts) to knock-down the expression of selected DEGs specifically in the adult ISC and EB. Firstly, we compared the DEGs with the genes required for the maintenance of intestinal homeostasis identified by a previous study47. We collected RNAi fly lines of 19 genes (Table S1). Expression of these genes was significantly altered during the aging process (fold change ≥ ± 4, Figure S1C, Table S2), and knock-down of them in ISCs/EBs disrupted intestinal homeostasis47. We performed two paralleled experiments to analyze the lifespan of flies when these genes were knockdown, with 150 samples in each experiment. Compared with the control flies, ISC/EB-specific knock-down of 13 genes at adult stage decreased the lifespan of the fly (Fig. 5, Figure S2, Table S10 Table S11). Decreased lifespan could be observed in both female and male flies when expression of aur B (aurB i), CNMa (CNMa i), eIF5 (eIF5 i), fit (fit i), idgf5 (idgf5 i), me31B (me31B i), CG17264 (CG17264 i), CG11555 (CG11555 i), CG17321 (CG17321 i), CG42324 (CG42324 i), or CG42700 (CG42700 i) was reduced. Furthermore, we found that knock-down of CG10581 (CG10581 i) led to a significant lifespan reduction only in female flies (Fig. 5), while knockdown of nec reduced lifespan only male flies (Figure S2). Several of these newly identified lifespan-related genes have been shown to be potential aging-related markers, such as aur B48 functioning in chromatin organization and cell cycle, Nec49 and Idgf550 functioning in inflammatory and immune response, CNMa51,52, Fit53, and CG10581, the Drosophila homolog of nucleoside-triphosphate phosphatase (NTPCR), functioning in nutrient and metabolism, eIF554 and me31B55 functioning in protein synthesis and homeostasis. Moreover, the expression of aging-related genes showed cluster(s) tendency in aging, most of which were in cluster2 (8/13) and cluster5 (3/13), and expressed at the lowest level on day-3 and the highest level on day-50 (Figure S3). Next, we performed the Smurf assay to investigate whether these genes are required for the maintenance of the intestinal barrier integrity (Table S12). Our results showed that reduced expression of CNMa, fit, Idgf5 or me31B in adult ISC/EB disrupted the intestinal integrity (Fig. 6 and S4).
Fig. 5.
ISC/EB-specific knockdown of genes expression reduces lifespan. Survival curves of flies with indicated genotypes (n = 150 per group) following ISC/EB-specific knockdown driven by esg ts > Gal4. Survival curves of flies were shown as the following: fit (A), CG17321 (B), CG17264 (C), CNMa (D), aurB (E), me31B (F), CG42324 (G), CG42700 (H), CG11555 (I), Idgf5 (J), eIF5 (K), nec (L), CG10581 (M), Hsdl2 (N), Kr (O), CCHa1 (P), sc (Q), Mp20 (R) and Mcm7 (S).
Fig. 6.
Dysregulation of aging-related genes breaks intestinal integrity. Proportion of Smurf flies of indicated genotypes on specified days after birth, CNMa (A), fit (B), idgf5 (C), and me31B (D).
In our previous study, we have shown that Clbn localizes to the mitochondrial outer membrane of the enterocytes in fly gut, and clbn KO flies have shortened lifespan and broken gut integrity56. We found that clbn was differentially expressed during the aging process and had the highest expression level on day-30 (Figure S5 and Table S2), which provided more proof that differential expression information is helpful to define functional genes involved in lifespan maintenance. Till now, more than 200 genes in Drosophila had been shown to be associated with lifespan, and their information is being curated in the GenAge (https://www.genomics.senescence.info/genes/search.php?search=DROSOPHILA). We found that 175 of them were expressed in the fly gut, and 70 genes were differentially expressed in aging (Table S13, Figure S6). Moreover, the expression trends of these 70 DEGs were enriched in most clusters except cluster4 and cluster8 (Figure S7).
Distinct and well-regulated lncRNAs expression during aging process
Long non-coding RNAs (lncRNAs) play important regulatory roles across most of life, yet their functions in aging are less well known compared to protein-coding genes and microRNAs. Some lncRNAs have been suggested to playing roles in aging through their functions connected with aging hallmarks or aging-associated diseases57,58. In addition, lncRNAs are regarded as emerging players in the pathogenesis of intestinal disorders and are required to maintain intestinal homeostasis59. To investigate aging-related lncRNAs in the fly gut, we performed an analysis similar to that of protein-coding genes, and identified 979 lncRNAs that had at least 1 read in all 3 samples at any aging timepoint (Table S14). The PCA and hierarchical clustering results indicated that lncRNAs from day-3 and day-15 samples were clustered together, and samples of day-30, -40, and -50 were clustered together, which were similar to those of protein-coding genes (Fig. 7A, B). Moreover, 891 differentially expressed lncRNAs (DERs) (fold change ≥ ± 2, adjusted p < 0.05) were identified, and 10 groups of DERs were generated in total comparisons (Table S15). Among different comparisons, the largest number of DERs appeared between day-30 versus day-3 (n = 207), while the number of DERs was zero or very small in several comparisons, including day-15 versus day-3 (n = 7), day-40 versus day-30 (n = 2), and day-50 versus day-40 with no differentially expressed lncRNAs (n = 0) (Fig. 7C). Compared with differentially expressed genes, the DERs with no or minor differences appeared in comparisons between day-50 versus day-40 and day-15 versus day-3, which were similar. However, the largest number of DEGs appeared between day-50 versus day-3 (Fig. 1D), whereas in DERs, it was between day-30 versus day-3 (Fig. 7C), indicating a similar yet somewhat different response to aging in the fly gut between protein-coding genes and lncRNAs. Furthermore, the Upset analysis data showed that the top-3 DERs in up-regulated lncRNAs were appeared in comparisons between day-30 (n = 26), day-40 (n = 17) and day-50 (n = 15) versus day-3, respectively, and day-30 versus day-3 and day-15 (n = 15), whereas the top-3 DERs in down-regulated lncRNAs were in comparisons between day-30 versus day-3 (n = 36), a multiple comparison among day-30, -40 and -50 versus day-3 and -15 (n = 15), and day-30 versus day-15 (n = 13) (Figure S8A and S8B).
Fig. 7.
Differential expression of lncRNAs during aging process in fly gut. (A) Principal component analysis (PCA) plot of lncRNAs expression. Samples from different aging timepoints were presented with unique color. (B) Heatmap illustrating correlation of lncRNAs expression at various timepoints. (C) Volcano plot showing relative expression of lncRNAs in comparisons between different timepoints. Number of differentially expressed lncRNAs (DERs) were shown. The y-axis represents statistical significance as −log10 (adjusted p value), and the x-axis represents log2 fold change. Up-regulated lncRNAs were marked in red, down-regulated lncRNAs were marked in blue.
Next, we polled differentially expressed lncRNAs in all comparisons together, removed duplicated lncRNAs, and generated 334 DERs for further analysis (Table S16). Similar to expression patterns of DEGs at five aging timepoints, the DERs can also be clustered into 8 groups. Except for cluster4, which included 15 DERs, all other clusters showed similar expression pattern to those of DEGs (Fig. 8), suggesting that lncRNAs might perform similar functions to DEGs in the same cluster during aging. Moreover, lncRNAs in cluster4 achieved the highest expression level on day-40, while the number of protein-coding genes with the highest expression level on day-40 was small and could not be clustered separately, suggesting that these lncRNAs might play important roles on day-40 during the aging process.
Fig. 8.
Expression trends of differentially expressed lncRNAs. Mfuzz of DERs showing 8 clusters of expression trend. The number of DERs were shown in bracket.
Until recently, only a few Drosophila lncRNAs had been studied. We identified several differentially expressed lncRNAs that were proven functional in flies, such as bxd, flamenco (flam), hsromega (hsrω), irar, noe, sphinx, roX1, and roX2 (Figure S8C, Table S16). Expression trends analysis indicated that lncRNAs hsrω, sphinx, and irar were in cluster2, and roX1, noe, flam, and bxd were in cluster5. They were all expressed at lowest level on day-3 and highest level on day-50, while roX2 was in cluster4 and had the highest expression level on day-40 (Fig. 8). The lncRNAs roX1 and roX2 play central roles in dosage compensation, a conserved mechanism in most insects, worms, and mammals that is used to equalize expression of the X-linked genes in males and females60,61. The lncRNA sphinx might act as a negative regulator of courtship behavior in Drosophila62. Interestingly, neuron-specific knock-down of the lncRNA hsrω impairs locomotion and shortens lifespan63, and hsrω was suggested as one of the most informative genes in aging because its expression shows aging dynamics in the brain10.
Discussion
We performed a transcriptomic analysis of fly intestine during aging, which included protein-coding genes and lncRNAs and spans five aging timepoints including young, middle-aged, and old adult flies, and investigated the functions of aging-related genes in lifespan and maintenance of intestinal integrity. Firstly, we found that the differential expression of protein-coding genes and lncRNAs was significant in aging, 4,832 in 10,074 protein-coding genes and 334 out of 979 lncRNAs differentially expressed. The fly age was characterized by specific gene expression profiles showing clear-cut and well-separated patterns at different timepoints. Secondly, differentially expressed genes during the aging process were highly clustered, and genes in the same cluster were associated with specific biological processes and signalling pathways. In particular, many genes for immune response showed significantly increased expression in aging, and the Imd and Toll signalling pathways were the top 2 pathways highly regulated. Thirdly, the expression of molecular markers for intestinal cells was greatly changed in aging, and enterocyte markers were the most significantly influenced. Fourthly, lncRNAs expression showed similar expression trends and clustered patterns to those of protein-coding genes, suggesting a similar regulation of lncRNAs as protein-coding genes in aging. It should be noted that our RNA-seq libraries were prepared using poly-A enrichment approach, which can only detect the polyadenylated lncRNAs. Future studies employing nonpolyadenylated RNA sequencing may reveal the functions of non-polyadenylated lncRNAs in intestinal aging. Lastly, we identified new genes that are required for maintenance of lifespan and gut integrity.
The aging studies being performed in model organisms disclose the resemblance of physiological changes among animal species and tissue-specific changes, which strongly suggest that tissue-specific studies is critical to understand the intrinsic regulations of aging. A recent multi-omics study in the non-human primate Macaca fascicularis identified potential regulators of gut aging, and explored their roles in intestinal function and lifespan in c. elegans64. A worm study profiled transcriptomics across five somatic tissues, including the intestine, and observed significant transcriptomic changes during aging and a striking diversity among tissues65. The Drosophila undergoes similar physiological changes in aging as other animal species and is used for aging studies at multiple levels, including the organismal, tissue, cellular, and molecular level, Systemic approaches, such as omics studies, are used to understand and define the aging66. The RNA-seq study on purified fly intestinal stem cells found that genes from young flies clustered separately from mid-aged and geriatric flies, even though the number of differentially expressed genes is small67, consistent with our data (Fig. 1B, C).
Aging and immunity work as two sides of a coin. On one hand, aging contributes to the dysregulation of the immune functions. On the other hand, excessive activation of immune system accelerates aging. In Drosophila, intruding microorganisms induce the expression of immune effector molecules, antimicrobial peptides (AMPs), which are regulated by the Imd and Toll pathways68,69. In the intestine, the major entry point for pathogens, immune response regulated by the Imd and Toll pathways is highly regionalized. The Imd signalling is active all along the gut, but mostly in midgut, whereas the Toll signalling is active in foregut and hindgut68. In immune-related signalling pathways, including Imd, Toll, HIPPO, JAK-STAT, cGAS-STING and TNF, the Imd and Toll pathways are the top-2 significantly enhanced signalling pathways in aging in our study (Fig. 3). We found that increased expression of Imd pathway members in the aging process shows 3 characteristics: 1. All upstream activating events were involved; 2. Many ubiquitylation-associated factors, a crucial event for the Imd activation, were affected; 3. A lot of regulators of transcription factor Relish were upregulated. Similarly, increased expression of Toll pathway members shows the following characteristics: 1. Sensing mediators of all kinds of microorganisms recognized by the Toll pathway; 2. Multiple proteases in proteolytic processing cascade essential for Toll activation. However, we did not observe significant change in AMPs expression, which is different from previous studies20,70. We analyzed the fly intestine, which is different from previous studies that analyzed whole body of the fly, and we used more stringent criteria to choose differentially expressed genes (fold change ≥ ± 2, adjusted p < 0.05).
Aging is characterized by physiological decline in all organs and susceptibility to multi-organ diseases. A feature of aging-associated changes in regenerative tissues is increased cell heterogeneity. The fate and behavior of somatic stem cells become abnormal, losing their ability to produce differentiated cells. During aging, specific aging-associated transcriptomic, epigenetic, proteomic, metabolic, and microbiomic signatures can be generated. The changed profiles in omics may reflect the status of aging and aging markers71. The aging in fly intestine is characterized by epithelial dysplasia, showing hyper-proliferation of intestinal stem cells, aberrant differentiation, and breakdown of barrier integrity72. As the enterocyte is the largest cell population in intestine, ISCs/EBs and enteroendocrine cell each takes about 8% of intestinal cells45. We found that enterocyte markers were the significantly influenced in intestine, and the expression of molecular markers of almost all 15 enterocyte clusters were changed. Moreover, enteroendocrine-specific markers AstA and NPF and ISCs/EBs-specific markers Esg and Klu had the lowest expression level on day-3 and increased expression in aging (Fig. 4), indicating aging-associated regulation of intestinal cells.
Our study identified 13 lifespan-related functional genes from 19 genes, most of them had significantly increased expression during the aging process and were conserved in evolution, such as aurB, eIF5, me31B, nec, and CNMa signalling pathway. They were highly enriched in cluster2 and cluster5 (Figure S2). Moreover, their functions are closely connected to aging markers. In particular, previous studies indicated that cardiac tissue-specific knock-down of eIF5 by TinCD4 > Gal4 reduced lifespan73, and nec mutated flies were short lived49. Our study identified new functional genes required for lifespan in flies and provided experimental evidence that differential expression information during the aging process is helpful in gaining a better understand of aging and lifespan. Taking together, this study characterized transcriptomics of the fly gut in aging, and will be helpful to study intrinsic mechanisms of aging-related diseases.
Supplementary Information
Acknowledgements
We thank Bloomington Stock Center and Vienna Drosophila Resource Center (VDRC) for fly stocks, members of Bi laboratory for advice and discussions.
Author contributions
Conceptualization: X.B. Methodology: Y.S., X.N., L.W., and X.B. Investigation: Y.S., X.N., Q.X., L.W., Y.Y., and L.Z. Formal analysis: Y.S., and X.B. Visualization: Y.S. Supervision: X.B. Funding acquisition: X.B. Project administration: X.B. Writing—original draft: X.B.
Funding
This work was supported by grants from National Natural Science Foundation of China Grant No. 32270596 and No. 31970605 to X.B.
Data availability
The RNA-seq data supporting the findings of this study are available in the NCBI BioProject database under accession number PRJNA1156936 and can be accessed at https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1156936.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yan Sang and Xiufan Ning contribute equally to this work.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-98888-y.
References
- 1.Salazar, A. M., Aparicio, R., Clark, R. I., Rera, M. & Walker, D. W. Intestinal barrier dysfunction: An evolutionarily conserved hallmark of aging. Dis. Model. Mech.16(4) (2023). [DOI] [PMC free article] [PubMed]
- 2.Funk, M. C., Zhou, J. & Boutros, M. Ageing, metabolism and the intestine. EMBO Rep.21(7), e50047 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell, S. J., Scheibye-Knudsen, M., Longo, D. L. & de Cabo, R. Animal models of aging research: Implications for human aging and age-related diseases. Annu. Rev. Anim. Biosci.3, 283–303 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Neves, J., Demaria, M., Campisi, J. & Jasper, H. Of flies, mice, and men: Evolutionarily conserved tissue damage responses and aging. Dev. Cell32(1), 9–18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutledge, J., Oh, H. & Wyss-Coray, T. Measuring biological age using omics data. Nat. Rev. Genet.23(12), 715–727 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palmer, D., Fabris, F., Doherty, A., Freitas, A. A. & de Magalhaes, J. P. Ageing transcriptome meta-analysis reveals similarities and differences between key mammalian tissues. Aging (Albany N. Y.)13(3), 3313–3341 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyshkovskiy, A. et al. Distinct longevity mechanisms across and within species and their association with aging. Cell186(13), 2929–49.e20 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoeger, T. et al. Aging is associated with a systemic length-associated transcriptome imbalance. Nature Aging2(12), 1191–1206 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debes, C. et al. Ageing-associated changes in transcriptional elongation influence longevity. Nature616(7958), 814–821 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davie, K. et al. A single-cell transcriptome atlas of the aging Drosophila brain. Cell174(4), 982–998 e20 (2018). [DOI] [PMC free article] [PubMed]
- 11.Sen, P. et al. Spurious intragenic transcription is a feature of mammalian cellular senescence and tissue aging. Nat. Aging3(4), 402–417 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loeb, J. & Northrop, J. H. Is there a temperature coefficient for the duration of life? Proc. Natl. Acad. Sci. U.S.A.2(8), 456–457 (1916). [DOI] [PMC free article] [PubMed]
- 13.Helfand, S. L. & Rogina, B. Genetics of aging in the fruit fly, Drosophila melanogaster. Annu. Rev. Genet.37, 329–348 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Grotewiel, M. S., Martin, I., Bhandari, P. & Cook-Wiens, E. Functional senescence in Drosophila melanogaster. Ageing Res. Rev.4(3), 372–397 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Carlson, K. A., Zhang, C. & Harshman, L. G. A dataset for assessing temporal changes in gene expression during the aging process of adult Drosophila melanogaster. Data Brief7, 1652–1657 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson, K. A. et al. Genome-wide gene expression in relation to age in large laboratory cohorts of Drosophila melanogaster. Genet. Res. Int.2015, 835624 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhan, M. et al. Temporal and spatial transcriptional profiles of aging in Drosophila melanogaster. Genome Res.17(8), 1236–1243 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Girardot, F., Lasbleiz, C., Monnier, V. & Tricoire, H. Specific age-related signatures in Drosophila body parts transcriptome. BMC Genom.7, 69 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou, S., Meadows, S., Sharp, L., Jan, L. Y. & Jan, Y. N. Genome-wide study of aging and oxidative stress response in Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A.97(25), 13726–13731 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landis, G. N. et al. Similar gene expression patterns characterize aging and oxidative stress in Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A.101(20), 7663–7668 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pletcher, S. D. et al. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Curr. Biol.12(9), 712–723 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Bordet, G., Lodhi, N., Kossenkov, A. & Tulin, A. Age-related changes of gene expression profiles in Drosophila. Genes (Basel)12(12), 1982 (2021). [DOI] [PMC free article] [PubMed]
- 23.Cannon, L. et al. Expression patterns of cardiac aging in Drosophila. Aging Cell16(1), 82–92 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics29(1), 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thurmond, J. et al. FlyBase 20: The next generation. Nucleic Acids Res.47(D1), D759–D765 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao, Y., Smyth, G. K. & Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics30(7), 923–930 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol.15(12), 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar, L. M. E. F. Mfuzz: A software package for soft clustering of microarray data. Bioinformation2(1), 5–7 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu, T. et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb.)2(3), 100141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res.28(1), 27–30 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michael, R., Masoud, J. A. & David, W. W. Organ-specific mediation of lifespan extension: More than a gut feeling? Ageing Res. Rev.12(1), 436–444 (2013). [DOI] [PMC free article] [PubMed]
- 32.Tzu-Chiao, L. et al. Aging Fly Cell Atlas identifies exhaustive aging features at cellular resolution. Science380(6650), eadg0934 (2023). [DOI] [PMC free article] [PubMed]
- 33.Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M. & Kroemer, G. Hallmarks of aging: An expanding universe. Cell186(2), 243–278 (2023). [DOI] [PubMed] [Google Scholar]
- 34.Attrill, H. et al. A new experimental evidence-weighted signaling pathway resource in FlyBase. Development151(3), dev202255 (2024). [DOI] [PMC free article] [PubMed]
- 35.Tanji, T. & Ip, Y. T. Regulators of the Toll and Imd pathways in the Drosophila innate immune response. Trends Immunol.26(4), 193–198 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Ferrandon, D., Imler, J. L., Hetru, C. & Hoffmann, J. A. The Drosophila systemic immune response: Sensing and signalling during bacterial and fungal infections. Nat. Rev. Immunol.7(11), 862–874 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Gan, T., et al. JNK signaling in Drosophila aging and longevity. Int. J. Mol. Sci.22(17), 9649 (2021). [DOI] [PMC free article] [PubMed]
- 38.Biteau, B., Hochmuth, C. E. & Jasper, H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell3(4), 442–455 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aalto, A. L., Luukkonen, V. & Meinander, A. Ubiquitin signalling in Drosophila innate immune responses. FEBS J. 291(20), 4397–4413(2024). [DOI] [PubMed]
- 40.Gui, T. & Burgering, B. M. T. FOXOs: Masters of the equilibrium. FEBS J.289(24), 7918–7939 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindsay, S. A. & Wasserman, S. A. Conventional and non-conventional Drosophila Toll signaling. Dev. Comp. Immunol.42(1), 16–24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herrera, S. C., Bach, E. A. JAK/STAT signaling in stem cells and regeneration: From Drosophila to vertebrates. Development146(2), dev167643 (2019). [DOI] [PMC free article] [PubMed]
- 43.Rachael, L. P., et al. The JAK-STAT pathway at 30: Much learned, much more to do. Cell185(21), 3857–3876 (2022). [DOI] [PMC free article] [PubMed]
- 44.Choi, N. H., Kim, J. G., Yang, D. J., Kim, Y. S. & Yoo, M. A. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell7(3), 318–334 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hung, R. J. et al. A cell atlas of the adult Drosophila midgut. Proc. Natl. Acad. Sci. U. S. A.117(3), 1514–1523 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo, X. et al. The cellular diversity and transcription factor code of Drosophila enteroendocrine cells. Cell Rep.29(12), 4172–4185 e5 (2019). [DOI] [PubMed]
- 47.Zeng, X. et al. Genome-wide RNAi screen identifies networks involved in intestinal stem cell regulation in Drosophila. Cell Rep.10(7), 1226–1238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Michael, D. R. et al. A Cyclin A-Myb-MuvB-Aurora B network regulates the choice between mitotic cycles and polyploid endoreplication cycles. PLoS Genet. 15(7), e1008253 (2019). [DOI] [PMC free article] [PubMed]
- 49.Green, C. et al. Drosophila necrotic mutations mirror disease-associated variants of human serpins. Development (Cambridge, England)130(7), 1473–1478 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Mazur, M., Zielinska, A., Grzybowski, M. M., Olczak, J. & Fichna, J. Chitinases and Chitinase-like proteins as therapeutic targets in inflammatory diseases, with a special focus on inflammatory bowel diseases. Int. J. Mol. Sci.22(13), 6966 (2021). [DOI] [PMC free article] [PubMed]
- 51.Kim, B. et al. Response of the microbiome-gut-brain axis in Drosophila to amino acid deficit. Nature593(7860), 570–574 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Jin X, et al. A subset of DN1p neurons integrates thermosensory inputs to promote wakefulness via CNMa signaling. Curr. Biol.31(10), 2075–2087 e6 (2021). [DOI] [PubMed]
- 53.Sun, J. et al. Drosophila FIT is a protein-specific satiety hormone essential for feeding control. Nat. Commun.8, 14161 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gotoh, S. et al. eIF5 stimulates the CUG initiation of RAN translation of poly-GA dipeptide repeat protein (DPR) in C9orf72 FTLD/ALS. J. Biol. Chem.300(3), 105703 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao, M. Me31B: A key repressor in germline regulation and beyond. Biosci. Rep.44(5), BSR20231769 (2024). [DOI] [PMC free article] [PubMed]
- 56.Dai, Z. et al. Drosophila Caliban preserves intestinal homeostasis and lifespan through regulating mitochondrial dynamics and redox state in enterocytes. PLoS Genet.16(10), e1009140 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sherazi, S. A. M. et al. Molecular hallmarks of long non-coding RNAs in aging and its significant effect on aging-associated diseases. Neural Regen. Res.18(5), 959–968 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin, L., Song, Q., Zhang, W., Geng, B. & Cai, J. Roles of long noncoding RNAs in aging and aging complications. Biochim. Biophys. Acta Mol. Basis Dis.1865(7), 1763–1771 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Fitzgerald, K. A. & Shmuel-Galia, L. Lnc-ing RNA to intestinal homeostasis and inflammation. Trends Immunol.45(2), 127–137 (2024). [DOI] [PubMed] [Google Scholar]
- 60.Shevelyov, Y. Y., Ulianov, S. V., Gelfand, M. S., Belyakin, S. N. & Razin, S. V. Dosage compensation in Drosophila: Its canonical and non-canonical mechanisms. Int. J. Mol. Sci.23(18), 10976 (2022). [DOI] [PMC free article] [PubMed]
- 61.Keqin, L. et al. Insights into the functions of LncRNAs in Drosophila. Int. J. Mol. Sci.20(18), 4646 (2019). [DOI] [PMC free article] [PubMed]
- 62.Chen, Y., Dai, H., Chen, S., Zhang, L. & Long, M. Highly tissue specific expression of Sphinx supports its male courtship related role in Drosophila melanogaster. PLoS ONE6(4), e18853 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lo Piccolo, L. & Yamaguchi, M. RNAi of arcRNA hsromega affects sub-cellular localization of Drosophila FUS to drive neurodiseases. Exp. Neurol.292, 125–134 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Wang, X. et al. Age-, sex- and proximal-distal-resolved multi-omics identifies regulators of intestinal aging in non-human primates. Nat. Aging4(3), 414–433 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, X. et al. Ageing induces tissue-specific transcriptomic changes in Caenorhabditis elegans. EMBO J41(8), e109633 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wodrich, A. P. K., Scott, A. W. & Giniger, E. What do we mean by “aging”? Questions and perspectives revealed by studies in Drosophila. Mech. Ageing Dev.213, 111839 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tauc, H. M. et al. Age-related changes in polycomb gene regulation disrupt lineage fidelity in intestinal stem cells. Elife10, e62250 (2021). [DOI] [PMC free article] [PubMed]
- 68.Buchon, N., Silverman, N. & Cherry, S. Immunity in Drosophila melanogaster—from microbial recognition to whole-organism physiology. Nat. Rev. Immunol.14(12), 796–810 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kathrin, G. & Thomas, F. The interplay between immunity and aging in Drosophila. F1000Res7(0), 160 (2018). [DOI] [PMC free article] [PubMed]
- 70.Landis, G., Shen, J. & Tower, J. Gene expression changes in response to aging compared to heat stress, oxidative stress and ionizing radiation in Drosophila melanogaster. Aging (Albany N. Y.)4(11), 768–789 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anne, B., Margaret, A. G. & Thomas, A. R. Ageing and rejuvenation of tissue stem cells and their niches. Nat. Rev. Mol. Cell. Biol.24(1), 45–62 (2022). [DOI] [PMC free article] [PubMed]
- 72.Rodriguez-Fernandez, I. A., Tauc, H. M. & Jasper, H. Hallmarks of aging Drosophila intestinal stem cells. Mech. Ageing Dev.190, 111285 (2020). [DOI] [PubMed] [Google Scholar]
- 73.Neely, G. G. et al. A global in vivo Drosophila RNAi screen identifies NOT3 as a conserved regulator of heart function. Cell141(1), 142–153 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq data supporting the findings of this study are available in the NCBI BioProject database under accession number PRJNA1156936 and can be accessed at https://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA1156936.