Abstract
Heat stress resistance of foliar photosynthetic apparatus was investigated in the Mediterranean monoterpene-emitting evergreen sclerophyll species Quercus ilex. Leaf feeding with fosmidomycin, which is a specific inhibitor of the chloroplastic isoprenoid synthesis pathway, essentially stopped monoterpene emission and resulted in the decrease of the optimum temperature of photosynthetic electron transport from approximately 38°C to approximately 30°C. The heat stress resistance was partly restored by fumigation with 4 to 5 nmol mol−1 air concentrations of monoterpene α-pinene but not with fumigations with monoterpene alcohol α-terpineol. Analyses of monoterpene physicochemical characteristics demonstrated that α-pinene was primarily distributed to leaf gas and lipid phases, while α-terpineol was primarily distributed to leaf aqueous phase. Thus, for a common monoterpene uptake rate, α-terpineol is less efficient in stabilizing membrane liquid-crystalline structure and as an antioxidant in plant membranes. Furthermore, α-terpineol uptake rate (U) strongly decreased with increasing temperature, while the uptake rates of α-pinene increased with increasing temperature, providing a further explanation of the lower efficiency of thermal protection by α-terpineol. The temperature-dependent decrease of α-terpineol uptake was both due to decreases in stomatal conductance, gw, and increased volatility of α-terpineol at higher temperature that decreased the monoterpene diffusion gradient between the ambient air (FA) and leaf (FI; U = gw[FA − FI]). Model analyses suggested that α-pinene reacted within the leaf at higher temperatures, possibly within the lipid phase, thereby avoiding the decrease in diffusion gradient, FA − FI. Thus, these data contribute to the hypothesis of the antioxidative protection of leaf membranes during heat stress by monoterpenes. These data further suggest that fumigation with the relatively low atmospheric concentrations of monoterpenes that are occasionally observed during warm windless days in the Mediterranean canopies may significantly improve the heat tolerance of nonemitting vegetation that grows intermixed with emitting species.
Volatile isoprenoids emitted by emitting vegetation play an important role in atmospheric chemical reactions leading to tropospheric ozone and in photochemical smog formation during summer (Chameides et al., 1988; Fuentes et al., 2000). Apart from importantly affecting regional air quality, volatile isoprenoids alter climate by influencing the residence time of greenhouse gases such as methane and formation of aerosols and cloud condensation nuclei, a major concern of current climate research (Kesselmeier et al., 2002; Peñuelas and Llusià, 2003). At the global scale, biogenic emission of volatile organic compounds is estimated to be severalfold larger than the anthropogenic emission (Guenther et al., 1995; Fuentes et al., 2000).
Although the isoprene and monoterpene emissions are that relevant for air quality and climate, the importance of monoterpenes to the plant remains controversial. After the pioneering work of Sharkey and Singsaas (1995) showing an improvement of leaf thermotolerance by isoprene, several studies have reported a modification of leaf thermotolerance after fumigation with isoprene (Singsaas et al., 1997; Peñuelas and Munné-Bosch, 2005) or with monoterpenes (Loreto et al., 1998; Delfine et al., 2000; Peñuelas and Llusià, 2002). Because heat stress damage to photosynthetic apparatus is primarily associated with enhanced membrane fluidity that results in reduced lipid-lipid and protein-lipid interactions and enhanced membrane leakiness, the improvement of heat stress resistance by volatile isoprenoids has been explained by increases in the stability of membrane liquid-crystalline phase after isoprenoid solubilization in membranes (Sharkey and Singsaas, 1995; Sharkey, 1996).
Isoprene (Loreto and Velikova, 2001; Affek and Yakir, 2002; Velikova et al., 2004; Velikova and Loreto, 2005) and monoterpenes (Perry et al., 2003; Loreto et al., 2004) also appear to act as antioxidants protecting the membranes against peroxidation and reactive oxygen species such as singlet oxygen. Because heat stress is also accompanied by enhanced oxidative stress (Mahoney et al., 1998; Jiang and Huang, 2001; Munné-Bosch et al., 2004; Llusià et al., 2005; Velikova and Loreto, 2005; Velikova et al., 2005), maintenance of membrane integrity and avoidance of antioxidative damage are not necessarily mutually exclusive. Furthermore, membrane disorderness and fluidity increase with the degree of membrane peroxidation (Katynski et al., 2004), and, thus, the possible role of isoprenoids as antioxidants can also contribute to the maintenance of membrane integrity.
Monoterpene-producing species emit a complex mixture of monoterpenes that have widely differing physicochemical characteristics (Niinemets et al., 2004). In particular, the air-liquid-phase (Henry's law constant, H) and lipid-liquid-phase (octanol/water partition coefficient, Kow) partition coefficients vary by more than three orders of magnitude for isoprenoids typically released by emitting vegetation (Niinemets et al., 2004), but little is known of the differences in membrane stabilizing/antioxidative characteristics of these compounds. Given the large variation in compound partitioning among lipid/liquid and liquid/air interfaces, various monoterpenes may differently protect the foliage during thermal stress. We hypothesize that the protective effect is greater for hydrophobic nonoxygenated monoterpenes that can accumulate in plant membranes in larger concentrations than for oxygenated monoterpenes that primarily partition in aqueous phase.
We studied the influences of in vivo monoterpene emissions and fumigations with nonoxygenated monoterpene α-pinene and monoterpene alcohol α-terpineol on the heat stress sensitivity of foliar photosynthesis in Mediterranean evergreen sclerophyll oak Quercus ilex. This species is a dominant Mediterranean forest component that emits approximately 20 different monoterpene species (Kesselmeier et al., 1997) and, contrary to most other oaks, does not emit significant amounts of isoprene. Most of the Q. ilex chemotypes primarily emit α-pinene (Staudt et al., 2001) that is characterized by low water solubility and high partition to lipid phase typical of nonoxygenated monoterpenes (Niinemets and Reichstein, 2002). α-Terpineol is one of the plant monoterpenoids with largest aqueous-phase affinity relative to air (five orders of magnitude larger than that of α-pinene; Niinemets and Reichstein, 2002) and is a minor part of the emissions (1%–2%) from Q. ilex (Niinemets et al., 2002b).
Previous fumigation experiments have been conducted at high air isoprenoid mole fractions of approximately 10 to 10,000 nmol mol−1 that do not generally occur in the ambient air within plant canopies but that under specific experimental conditions resulted in internal leaf isoprenoid concentrations close to those in actively emitting leaves (Singsaas et al., 1997; Delfine et al., 2000; Peñuelas and Llusià, 2002). However, the uptake rates could not be directly measured in these studies due to high isoprenoid background concentrations. We used a relatively low target fumigation concentration of 4.5 nmol mol−1, which may occasionally be reached within the canopies of Mediterranean vegetation on warm calm days and that allowed us to directly measure the uptake. Temperature dependencies of monoterpenoid uptake were combined with the measurements of temperature effects on monoterpene physicochemical characteristics to determine the physicochemical limits of isoprenoid uptake. We hypothesized that, during long-term fumigation, lipid-soluble monoterpenes are taken up from the ambient air to result in physiologically significant internal concentrations and that hydrophobic monoterpene α-pinene is a more potent protector of photosynthetic apparatus during heat stress events than the hydrophilic compound α-terpineol.
RESULTS
Temperature Dependence of Monoterpene Emission
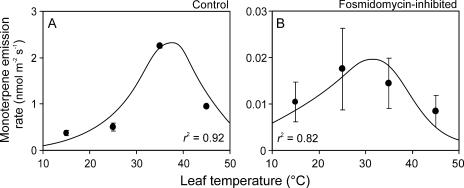
Monoterpene emission rate of control leaves was characterized with an optimum temperature of 37°C to 40°C (Fig. 1A). Fosmidomycin feeding for 7 h suppressed the rate of monoterpene emission by two orders of magnitude (Fig. 1B) to a level that could not be detected by an available gas-chromatographic system equipped with a mass detector (GC-MS) using standard procedures (10–30 min trapping on terpene traps filled with Carbotrap and Carbosieve; for details, see Peñuelas and Llusià, 2002). However, these low emission rates were still measurable with a proton-transfer-reaction mass spectrometer (PTR-MS). The emission rate of fosmidomycin-fed leaves versus temperature response curve was also characterized by a curve with a maximum, suggesting de novo monoterpene production rather than monoterpene emission from a nonspecific storage.
Figure 1.
Temperature dependencies of the emission rates of total monoterpenes (averages ± se) for control leaves (A) and after feeding with 5 μm fosmidomycin for 7 to 8 h (B). The measurements were conducted under a saturating quantum flux density of 1,000 μmol m−2 s−1. Data were fitted by Equation 5, and the optimum temperature (Topt) calculated by Equation 6 (Topt = 37.5°C for A and Topt = 31.5°C for B).
Fosmidomycin Feeding and Foliar Thermotolerance
Due to relatively large leaf-to-leaf differences in foliage physiological characteristics, we present both actual measurements of stomatal conductance (gw), net assimilation (A), and photosynthetic electron transport rates (Table I) and the characteristics normalized with respect to the maximum value (see “Materials and Methods”).
Table I.
Average (±se) stomatal conductances to water vapor (gw, mmol m−2 s−1), net assimilation rates (A, μmol m−2 s−1), intercellular CO2 concentrations (Ci, μmol mol−1), the capacities for photosynthetic electron transport (Jmax, μmol m−2 s−1), and the optimum temperatures of Jmax (Topt, °C) in nontreated (control) and fumigated and nonfumigated fosmidomycin-fed Q. ilex leaves: maximum values observed during temperature response curves, and the values measured at 25°C and at 45°C
| Characteristic
|
Nontreated Control
|
Fosmidomycin Fed
|
||
|---|---|---|---|---|
| Nonfumigated | α-Pinene Fumigated | α-Terpineol Fumigated | ||
| Max gw | 142 ± 26aa | 175 ± 44a | 182 ± 67a | 107 ± 5b |
| gw at 25°C | 127 ± 32a | 175 ± 44a | 93 ± 24a | 53 ± 4b |
| gw at 45°C | 56 ± 18a | 29 ± 11a | 30 ± 8a | 6.4 ± 0.5bb |
| Max A and A at 25°Cc | 11.3 ± 2.2a | 15.1 ± 1.9b | 8.7 ± 1.2a | 4.5 ± 0.3c |
| A at 45°C | 4.2 ± 1.3a | −0.6 ± 1.1b | 1.8 ± 0.4b | −2.7 ± 0.5cb |
| Ci at 25°C | 261 ± 16a | 250 ± 21a | 263 ± 22a | 271 ± 10a |
| Ci at 45°C | 300 ± 37a | 400 ± 35b | 319 ± 17a | 1,060 ± 30cb |
| Max Jmaxd | 101 ± 11ab | 141 ± 11ab | 72 ± 8a | 44 ± 5c |
| Jmax at 25°C | 76 ± 16ab | 107 ± 10b | 59 ± 10a | 36 ± 5c |
| Jmax at 45°C | 73 ± 15a | 15 ± 8b | 40 ± 8a | 2.2 ± 2bb |
| Topt | 38.8 ± 1.1a | 29.5 ± 0.8b | 37.0 ± 1.1a | 29.6 ± 1.5b |
Means with the same letter are not statistically different (one-way ANOVA followed by Bonferroni test).
Measurements at 40°C.
Maximum A was always observed at 25°C.
Jmax at Topt derived from fitted Jmax versus leaf temperature responses (Eq. 6).
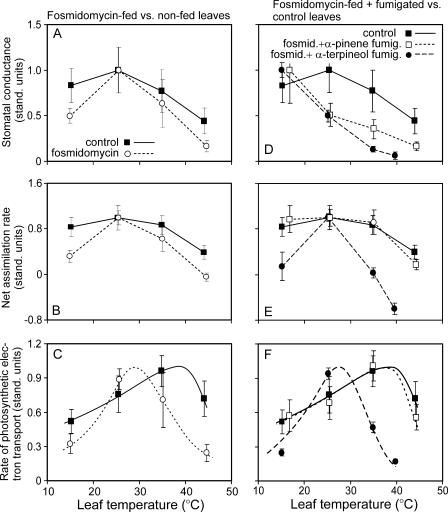
At leaf temperatures of 25°C to 30°C, inhibition of monoterpene emission by fosmidomycin had a minor effect on leaf stomatal conductance (Table I; Fig. 2A) and net assimilation rates (Table I; Fig. 2B). However, at 15°C and 45°C, both the absolute (Table I) and standardized net assimilation rates were larger in control leaves (P < 0.05 for Fig. 2B).
Figure 2.
Average stomatal conductance to water vapor (gw; A and D), net assimilation rate (A; B and E), and the rate of photosynthetic electron transport (Jmax; Equations 4 and 5; C and F) as a function of temperature for nonfumigated non-fosmidomycin-fed (control in all sections) versus fosmidomycin-fed leaves (A–C) and versus fosmidomycin-fed leaves that were fumigated with either α-pinene or α-terpineol (D–F). In fumigation treatments, the leaves were fumigated with specific monoterpenes at concentrations of 3.5 to 5 nmol mol−1 for 2 h at every temperature, and average stomatal conductance and net assimilation rates were calculated. A and gw were measured at saturating quantum flux densities of 900 to 1,000 μmol m−2 s−1 and at ambient CO2 mole fractions of 380 to 420 μmol mol−1. Data in A, B, D, and E were normalized with respect to the average maximum value for a given treatment (Table I) that was observed at 25°C, except for stomatal conductance for fosmidomycin-fed and α-pinene-fumigated (gw = 182 mmol m−2 s−1 at 15°C) or α-terpineol-fumigated (gw = 107 mmol m−2 s−1 at 15°C) leaves in D (corresponding control leaves had a gw of 127 mmol m−2 s−1 at 15°C). Data in C and F were fitted by Equation 5 and standardized with respect to the value at optimum temperature (Eq. 6; Table I). The average optimum temperatures (Topt) are reported in Table I. Error bars provide ± se of four replicate experiments.
The net assimilation rates at current ambient CO2 concentrations also strongly depend on physical conductance to CO2 from ambient air to the internal leaf airspace, i.e. on gw. To further test for the effect of fosmidomycin feeding on leaf photosynthetic apparatus, we calculated the intercellular CO2 concentration (Ci) and the rate of photosynthetic electron transport (J, Eq. 4), which corrects for leaf-to-leaf variation in A due to differences in gw. For our data, maximum gw and J were not correlated (r2 = 0.13, P = 0.18). Foliage net assimilation rates in fosmidomycin-fed heat-stressed leaves were more strongly limited by photosynthetic capacity than by stomatal conductance (larger Ci at 45°C than at 25°C; Table I), but this pattern was not evident for control leaves (values of Ci similar at 45°C and 25°C; Table I). Both the absolute (Table I) and standardized values of J (Fig. 2C) were larger at 45°C in control leaves (Table I; P < 0.01 for Fig. 2C). The optimum temperature of J decreased from 35°C to 40°C in control leaves to 28°C to 32°C in fosmidomycin-fed leaves (Table I; Fig. 2C, P < 0.001 for the difference among the mean optimum temperatures), demonstrating a strong reduction of heat resistance of leaf photosynthetic electron transport rate of leaves with blocked monoterpene synthesis.
Examination of Ci (284 ± 40 μmol mol−1 for control and 339 ± 26 μmol mol−1 for fosmidomycin-fed leaves) and J values (Fig. 2C) suggested that larger net assimilation rates at 15°C of control leaves were primarily attributed to a greater leaf photosynthetic potential in control leaves.
Effects of Fumigation with α-Pinene and α-Terpineol on Heat Sensitivity of Photosynthesis
Examination of relative values suggested that fumigation with 4 to 5 nmol mol−1 concentrations of α-pinene restored the heat stress resistance of photosynthetic apparatus of the fosmidomycin-fed leaves (Fig. 2, E and F). The optimum temperatures of photosynthetic electron transport of control leaves (nonfumigated, non-fosmidomycin-fed) and α-pinene-fumigated leaves did not differ (Table I; Fig. 2F, P > 0.5). However, the absolute values of net photosynthesis and photosynthetic electron transport tended to be lower in fosmidomycin-fed leaves (Table I). The intercellular CO2 concentration at 45°C of α-pinene-fumigated leaves was larger than in nonfumigated non-fosmidomycin-fed controls, but lower than that in nonfumigated fosmidomycin-fed leaves (Table I).
In contrast, the leaves fumigated with α-terpineol had lower stomatal conductances (Fig. 2D), net assimilation (Fig. 2E), and photosynthetic electron transport rates (Fig. 2F) at elevated temperatures, and overall lower optimum temperature (Topt = 28°C–32°C) than those in control and α-pinene-fumigated leaves, suggesting that α-terpineol inhibited rather than protected the photosynthetic apparatus.
Temperature Dependencies of the Uptake and Physicochemical Characteristics of α-Pinene and α-Terpineol
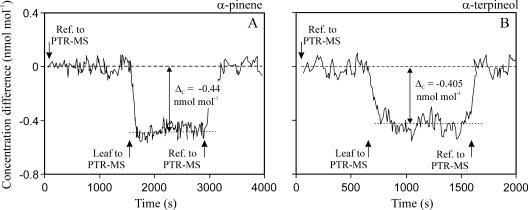
Initial experiments demonstrated that monoterpenes are taken up by the leaves during the fumigation and that the PTR-MS system can be employed for real-time measurements of monoterpene uptake rates (Fig. 3), while the sensitivity and signal-to-noise ratio of available GC-MS systems (Peñuelas and Llusià, 2002) were not enough to detect monoterpene uptake using 10- to 30-min sampling times on terpene traps filled with Carbotrap and Carbosieve (for details, see Peñuelas and Llusià, 2002).
Figure 3.
Sample graphs of monoterpene uptake measurements with PTR-MS. The fosmidomycin-fed leaves were either fumigated with α-pinene (A) or with α-terpineol (B) at 25°C for 2 h. Fumigation concentration (average ± sd) was 4.5 ± 0.5 nmol mol−1 for α-pinene and 3.9 ± 0.5 nmol mol−1 for α-terpineol. Reference air or the air exiting the leaf chamber passed to PTR-MS (Fig. 6), and the rate of monoterpene uptake was calculated from average differences in monoterpene concentrations in the reference and chamber air (ΔC). The leaf was continuously fumigated, while PTR-MS was switched between the reference line and leaf chamber twice or three times, and sample traces are shown for both monoterpenes. A total of 3.64 cm2 leaf area was enclosed in leaf chamber during the α-pinene fumigation and 2.72 cm2 during the α-terpineol fumigation.
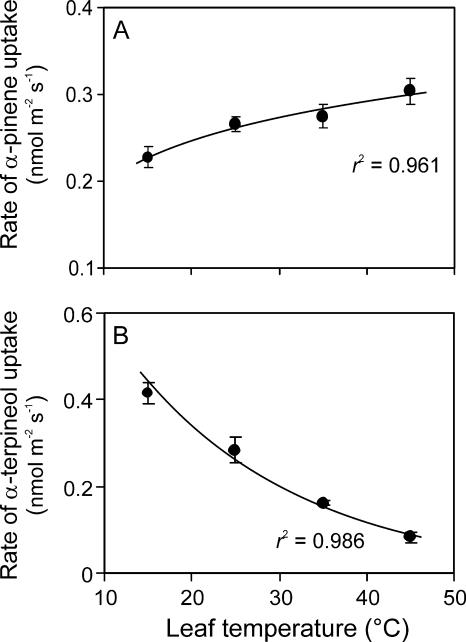
The rate of α-pinene uptake increased with increasing leaf temperature (Fig. 4A), while the rate of α-terpineol uptake decreased with increasing temperature (Fig. 4B). The monoterpene uptake rates at low temperature were approximately one order of magnitude less than the in vivo emissions (compare Figs. 1A and 4). However, the uptake rate of α-pinene at high temperature of 45°C was approximately one-half of the emission rate at that temperature (compare Figs. 1A and 4), demonstrating that even these low ambient atmosphere monoterpene concentrations resulted in physiologically significant uptake rates.
Figure 4.
The rates of uptake of α-pinene (A) and α-terpineol (B) of Q. ilex leaves as a function of temperature (averages ± se). The fosmidomycin-fed leaves were fumigated with either α-pinene (average ± se fumigation concentration of 4.6 ± 0.8 nmol mol−1) or α-terpineol (3.9 ± 0.7 nmol mol−1), and the rate of uptake determined as in Figure 3. Data were fitted by nonlinear regressions in the form of y = aebx. Error bars provide ±se of three experiments.
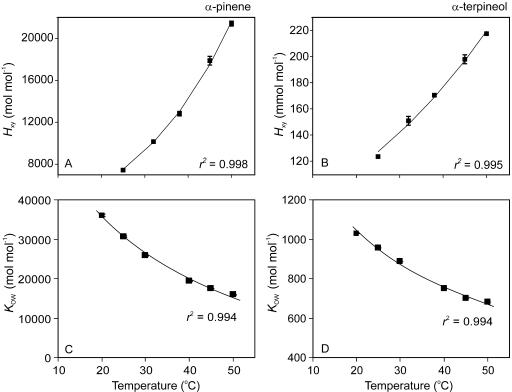
To understand the differences in temperature dependencies of uptake of various monoterpenes and make inferences of the possible protective role of monoterpenes, it is necessary to know the temperature-dependent changes in monoterpene partitioning within the leaf gas, liquid, and lipid volumes. The molar Henry's law constant (Hxy, gas/liquid-phase equilibrium partition coefficient, mol compound/mol air [mol compound/mol water]−1) was five orders of magnitude larger for α-pinene than for α-terpineol (Fig. 5, A and B), demonstrating that α-terpineol is more strongly partitioned in liquid phase. In contrast, the monoterpene octanol/water partition coefficient (Kow, mol compound/mol octanol [mol compound/mol water]−1), which characterizes the monoterpene partitioning between lipid and liquid phases, was 20- to 40-fold larger for α-pinene than for α-terpineol (Fig. 5, C and D). Henry's law constants increased with increasing temperature, while Kow values decreased with increasing temperature, indicating overall greater partitioning of monoterpenes in gas phase (the ratio Hxy/Kow characterizes the monoterpene gas- to lipid-phase partitioning) with increasing temperature (Fig. 5).
Figure 5.
Henry's law constant (Hxy, equilibrium gas/liquid-phase partition coefficient; A and B) and equilibrium octanol/water partition coefficient (Kow; C and D) for α-pinene (A and C) and α-terpineol (B and D) in relation to temperature (averages ± se). Data fitting is as in Figure 4. Hxy (mol monoterpene/mol air [mol monoterpene/mol water]−1) characterizes the monoterpene partitioning between air and water, while Kow (mol monoterpene/m3 octanol [mol monoterpene/m3 water]−1) characterizes the partitioning between lipid and water. Both Hxy and Kow were measured gas chromatographically. Data were fitted by nonlinear regressions in the form of y = aebx in A and B and in the form of y = alog(bx) in C and D. Error bars show ±se of three separate determinations.
DISCUSSION
Protection of the Photosynthetic Apparatus by Monoterpene Emission
High temperatures significantly constrain the growth and productivity and influence the distribution of species in Mediterranean climates (Larcher, 2000). However, plants have a large potential to adjust to a wide range of temperatures (Björkman, 1981). Adaptations to cope with high temperatures involve a wide array of biochemical and physiological modifications, such as higher degree of membrane lipid saturation and xanthophyll and sugar accumulation that collectively decrease membrane fluidity and increase the stability of the liquid-crystalline phase at higher temperatures (e.g. Havaux, 1998; Hüve et al., 2005).
In Mediterranean climates, many species are strong emitters of volatile isoprenoids that may be involved in thermal protection. Previous work of enhanced thermal tolerance of isoprene- and monoterpene-fumigated leaves (Singsaas et al., 1997; Loreto et al., 1998; Delfine et al., 2000; Peñuelas and Llusià, 2002; Peñuelas and Munné-Bosch, 2005) convincingly demonstrates that isoprenoids improve plant heat tolerance.
The importance of isoprenoid emission on heat stress tolerance is further supported by strong reductions in heat resistance of leaves, in which isoprene emission has been inhibited by fosmidomycin (Sharkey et al., 2001; Velikova et al., 2005). Fosmidomycin feeding in our study reduced monoterpene emission rates and internal concentrations by two orders of magnitude (Fig. 1), and this led to reductions in the optimum temperature of photosynthetic electron transport from 36°C to 40°C in control leaves to 28°C to 30°C in fosmidomycin-fed leaves (Fig. 2C). Thus, our data further support the view that isoprenoid emissions significantly alter leaf heat stress resistance.
As the differences at 15°C demonstrated (Fig. 2, A–C), fosmidomycin feeding also reduced leaf resistance to lower temperatures, suggesting that monoterpene emission confers an advantage over a broad temperature range.
Fumigation with Different Monoterpenes and Heat Stress Sensitivity
In comparison with nonfumigated fosmidomycin-fed control leaves, the fumigation with low ambient air mole fractions of 4 to 5 nmol mol−1 α-pinene increased leaf relative net assimilation (Fig. 2E) and photosynthetic electron transport rates (Fig. 2F) at 45°C. This suggests that α-pinene fumigation improved leaf heat resistance. Most of the previous studies have used higher air concentrations of isoprene (Singsaas et al., 1997; Loreto et al., 1998) and monoterpenes (Delfine et al., 2000; Peñuelas and Llusià, 2002), on the order of 5 to 10,000 nmol mol−1, than the fumigation concentration of 4 to 5 nmol mol−1 used in our study. For isoprene, it was found that the degree of heat resistance conferred by isoprenoid fumigation increased with increasing the air isoprene concentration (Singsaas et al., 1997). Nevertheless, in our study, even at these relatively low air concentrations, the rate of compound uptake at the high temperatures was comparable with the emission rates (compare Figs. 1A and 4A), providing an explanation of the apparent increase of heat stress resistance of α-pinene-fumigated leaves relative to nonfumigated fosmidomycin-inhibited leaves (Fig. 2F).
Furthermore, octanol/water partition coefficient (Kow) of isoprene (263 mol mol−1 at 25°C; Howard and Meylan, 1997) is more than two orders of magnitude less than that of α-pinene (30,670 ± 150 mol mol−1 at 25°C; Fig. 5C), while the Henry's law constant (Hxy) of 4,270 mol mol−1 of isoprene is 1.8-fold lower than that of α-pinene (7,435 ± 11 mol mol−1 at 25°C; Fig. 5A). Thus, the monoterpene lipid-phase-to-gas-phase partition coefficient that is characterized by the quotient Kow/Hxy is approximately 75-fold lower for isoprene. Accordingly, 75-fold larger gas-phase concentrations of isoprene than those of α-pinene are required to achieve the same membrane concentration of these two compounds. Given that the isoprene molecule contains five carbon atoms and α-pinene 10 carbon atoms, this implies approximately 37-fold higher carbon cost of membrane protection by isoprene than by α-pinene. Due to higher membrane solubility, the ambient air fumigation concentrations of 4 to 5 nmol mol−1 of α-pinene correspond to the concentrations of 300 to 400 nmol mol−1 of isoprene that have been found to significantly affect heat resistance of isoprene-fumigated leaves (Singsaas et al., 1997).
From an ecological perspective, the air concentrations used during the fumigation may be occasionally observed in the ambient atmosphere of natural Q. ilex-dominated macchia ecosystems during summer warm windless days (Harrison et al., 2001), when the potential leaf heat stress is the largest. Given that these concentrations appeared to be effective in protecting the leaves from heat stress, our observations suggest that the emission of monoterpenes by emitting species may significantly protect the coexisting nonemitting vegetation during heat events.
Although α-pinene fumigation increased the relative values of net assimilation rates and photosynthetic electron transport at high temperature, the absolute (Table I) versus relative (Fig. 2) data were partly conflicting. There was evidence of overall reduction of stomatal conductance, and net assimilation and photosynthetic electron transport rates at 45°C in fosmidomycin-fed leaves (Table I). Such nonspecific effects of fosmidomycin on foliage photosynthetic apparatus have occasionally been observed, especially if the isoprenoid synthesis pathway is blocked for a long time (Loreto and Velikova, 2001; Velikova and Loreto, 2005), and require further study. Due to the nonspecific effects of fosmidomycin and/or the relatively low α-pinene fumigation concentrations, the fumigation treatment did not fully maintain the photosynthesis values at the control level. Nevertheless, our data collectively suggest that α-pinene fumigation reduced the damage caused by inhibiting isoprenoid synthesis by fosmidomycin (Table I; Fig. 2).
Why Is α-Pinene a More Potent Protector of Photosynthetic Electron Transport than α-Terpineol?
Contrary to α-pinene, fumigation with α-terpineol did not protect the photosynthetic apparatus during heat stress (the same optimum temperature of photosynthetic electron transport as for nonfumigated fosmidomycin-fed leaves) and appeared to be toxic in non-heat-stressed situations. We first suggested that the lower chemical activity of α-terpineol than that of α-pinene explains the difference between these compounds. However, the estimated gas-phase reaction rate constants for reaction with hydroxyl radicals (KOH) for α-terpineol (5.7·1013 cm3 mol−1 s−1) and α-pinene (3.2·1013 cm3 mol−1 s−1) differ by less than the margin of error (Howard and Meylan, 1997). While it can be disputed that the reactivity with hydroxyl radicals is not directly associated with compound antioxidative capacity, KOH is also strongly correlated with the reaction constant for reaction with ozone and active oxygen species (Calogirou et al., 1996; Howard and Meylan, 1997).
Despite similar chemical activity, the temperature dependencies of α-pinene and α-terpineol uptake were strikingly different (Fig. 4), suggesting that lower uptake of α-terpineol at higher temperature (Fig. 4B) explains the lack of protection during heat stress. Furthermore, the physicochemical characteristics of these monoterpenes widely differ. α-Terpineol is mainly partitioned to leaf liquid phase, and α-pinene to gas and lipid phases (Fig. 5). Thus, when the main role of monoterpenes is the stabilization of membrane liquid-crystalline structure or protection from oxidative lesions, lower effective concentrations of α-terpineol in the lipid phase can partly explain the lower heat protection ability conferred.
The apparent inhibitory effect of α-terpineol in non-heat-stressed conditions requires further experimental study, but previous research has demonstrated that several water-soluble monoterpenes may have phytotoxic effects and inhibit plant growth (Müller et al., 1964; Romagni et al., 2000).
Can Monoterpene Physicochemical Characteristics Explain Different Temperature Relations of Monoterpene Uptake?
Provided a major part of monoterpenes is taken up through the stomata, the rate of monoterpene uptake, U, is given by:
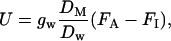 |
(1) |
where gw is the stomatal conductance to water vapor, DM (m2 s−1) is the binary diffusion coefficient of specific monoterpene in the air, Dw (m2 s−1) is the binary diffusion coefficient of water vapor in air, FA is the monoterpene mole fraction in the ambient air, and FI is the monoterpene mole fraction within the leaf air space. The ratio DM/Dw is 0.22 for α-pinene and 0.20 for α-terpineol (Niinemets and Reichstein, 2003a, 2003b), implying that the stomatal conductance is approximately 5-fold less for these monoterpenes than for water vapor.
Equation 1 suggests that monoterpene uptake rate should decrease with increasing temperature because of decreasing stomatal conductance (Fig. 2, A and D), but this does not explain the different temperature responses of uptake of α-pinene and α-terpineol. However, different compound physicochemical characteristics and their temperature responses may alter differently the monoterpene internal concentrations and, thereby, the gradient, FA − FI, from the ambient air to the leaf. Given a major part of the monoterpenoid is stored in leaf liquid volume, the rate of uptake is:
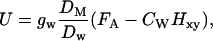 |
(2) |
where CW (mol mol−1) is the monoterpenoid mole fraction in leaf water, and Hxy, the Henry's law constant (gas/liquid-phase equilibrium partition coefficient, mol compound/mol air [mol compound/mol water]−1), converts the liquid-phase mole fraction to a gas-phase equivalent. In turn, when a major part of monoterpene is stored in leaf lipid phase, the rate of monoterpene uptake is expressed as:
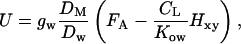 |
(3) |
where CL is the monoterpene liquid-phase concentration in octanol equivalents (mol mol−1), Kow is the monoterpene octanol/water partition coefficient expressed as mol compound/mol octanol [mol compound/mol water]−1, and the ratio Kow/Hxy is the monoterpene octanol to gas partition coefficient.
As Hxy increased, and Kow decreased with increasing temperature for both monoterpene species (Fig. 5); these considerations suggest that the gradient, FA − FI, between the ambient atmosphere and the leaf actually decreases rather than increases with increasing temperature. Thus, physicochemical characteristics can explain the stronger temperature-dependent reduction of α-terpineol uptake than can be expected on the basis of reductions in stomatal conductance alone (compare Figs. 2D and 4). However, temperature-dependent modifications in physicochemical characteristics cannot explain the increase of α-pinene uptake rate with temperature.
In fact, as the compounds are taken up from the ambient atmosphere, the internal concentration inevitably increases, resulting in a decreased gradient, FA − FI, and suggesting that a certain steady-state uptake rate, U, cannot be maintained over a long term without biogenic sinks. Pathways of enzymatic consumption have been proposed for acetaldehyde and formaldehyde to explain the foliar uptake rate of these chemicals (Giese et al., 1994; Kondo et al., 1996; Hanson and Roje, 2001; Achkor et al., 2003). Thus, as an alternative explanation of the positive relationship between temperature and α-pinene uptake, we suggest that α-pinene taken up by the leaf reacts with oxidative compounds within the leaf lipid and gas phases, thereby maintaining the gradient FA − FI. This explanation is in line with the evidence suggesting that the improvement of heat resistance by α-pinene emissions and fumigations is due to the antioxidative properties of α-pinene. Protection of foliage photosynthetic apparatus by monoterpene emissions at both high and low temperatures (Fig. 2, A–C) is also in accordance with antioxidative properties of, rather than with increases in, membrane rigidity due to nonoxygenated monoterpenes.
Conclusion
Monoterpene-emitting species all form and emit multiple products. The emission kinetics, storage capacity within the leaf gas, liquid, and lipid phases, as well as the capacity for thermal protection of these different monoterpenes is still poorly known, partly because of the lack of reliable physicochemical characteristics for specific monoterpenes. Our study demonstrates for two widely contrasting monoterpene species that these monoterpenes are differently taken up from ambient air, that they partition differently among leaf air, liquid, and lipid phases, and that their effect on leaf heat stress resistance is different. Several plant species emit oxygenated monoterpenes as major monoterpene species, e.g. broad-leaved tree species Eucalyptus and Melaleuca produce ether 1,8-cineole and monoterpene alcohols α-terpineol and terpinen-4-ol (He et al., 2000; Russell and Southwell, 2003), while the emissions in Mediterranean conifer Pinus pinea are dominated by monoterpene alcohol linalool in certain periods (Staudt et al., 2000; Niinemets et al., 2002a). Our study suggests that weakly membrane-soluble oxygenated monoterpenes should not necessarily affect leaf thermotolerance.
MATERIALS AND METHODS
Plant Material
Seedlings of Quercus ilex were grown in a nursery (Forestal Catalana, S.A., Breda, Catalonia, Spain) in typical Mediterranean conditions (midday photosynthetic quantum flux density of 800 to 1,500 μmol m−2 s−1, air temperature 25°C to 30°C, and relative humidity 40% to 45%) for two years before the experiment. In autumn 2004, 2-year-old plants were transplanted to 2-L pots filled with a mixture of peat and sand (2:1). The plants were well watered and maintained in Mediterranean-like conditions in a greenhouse until the experiments in November 2004 and March 2005. According to gas-chromatographic analyses, the used Q. ilex chemotype primarily emits limonene and α-pinene (>80% of total).
Experimental Treatments
In the evening before the experiments, the shoots with four to five fully expanded leaves were cut under distilled water and put either in 5 μm aqueous solution of fosmidomycin or in distilled water (controls), and maintained overnight in a dark room. We used fosmidomycin as a selective inhibitor of plastidic monoterpenoid synthesis. Fosmidomycin inhibits deoxyxyluose-5-phosphate reductoisomerase, an enzyme in the methylerythritol 4-phosphate pathway of isoprenoid synthesis, thereby blocking the isoprenoid synthesis in plastids (Kuzuyama et al., 1998). If the inhibition of monoterpene emission were specific, fosmidomycin could provide a method of controlling the production of endogenous monoterpene while examining thermotolerance of photosynthesis and measuring the uptake of monoterpenes. Many chemicals can inhibit volatile isoprenoid emission (Loreto and Sharkey, 1990), but fosmidomycin is thought to be unique in inhibiting isoprenoid emission without altering photosynthesis rates (Sharkey et al., 2001; Loreto et al., 2004; Velikova et al., 2005).
Fosmidomycin-fed leaves were either fumigated with α-pinene or α-terpineol or maintained in the ambient atmosphere. Air with different monoterpene concentrations was prepared with a membrane pump that flushed the ambient air through a thermostatted flask containing liquid gas-chromatography grade α-pinene (purity ≥99%; Merk GmbH) or solid α-terpineol (≥97.0%; Merk GmbH). The monoterpene concentration in the evaporation flask outlet was monitored with PTR-MS (Fig. 6), and the evaporating surface area and the thermostat temperature were empirically adjusted to obtain an air monoterpene concentration of 4.5 nmol mol−1. In general, the evaporation flask temperature was 30°C, and the average monoterpene concentration (±se) achieved across the experiments was 4.6 ± 0.8 nmol mol−1 for α-pinene fumigations and 3.9 ± 0.7 nmol mol−1 for α-terpineol fumigations.
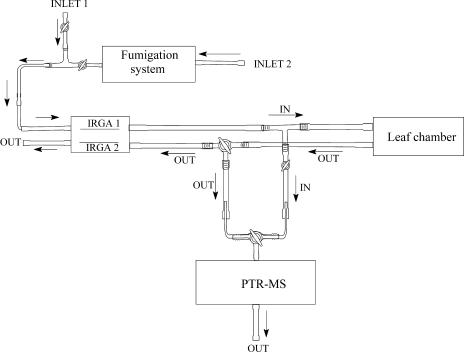
Figure 6.
Schematic overview of the system used for measurements of the rate of monoterpene emission from and uptake by the leaves of Q. ilex. For measurements of the monoterpene emission rates, the air enters into the system from inlet 1, while the second inlet is used for fumigation experiments. The air leaving the chamber is divided between the gas-exchange analyzers of CIRAS-2 to measure CO2 and water vapor exchange and PTR-MS to measure monoterpene concentrations. The fumigation system was calibrated to provide the air with monoterpene concentration of 5 nmol mol−1. Valves before and after leaf chamber were used to monitor the stability of air concentrations of the added monoterpenes.
Photosynthesis and Transpiration Measurements, and Calculation of Photosynthetic Electron Transport Rate
A portable photosynthesis system CIRAS-2 (PP Systems) with an automated gas mixing unit and a Parkinson leaf chamber (Std Broad 2.5) was employed for net assimilation (A) and transpiration rate measurements. The flow rate through the leaf chamber was controlled by the mass flow controller of CIRAS-2 and kept at 0.205 mmol s−1. An intact leaf was clamped in the cuvette, and steady-state gas-exchange rates were estimated at saturating quantum flux densities of 900 to 1,000 μmol m−2 s−1 and at ambient CO2 mole fractions of 380 to 420 μmol mol−1. The dew point of incoming air was 6°C to 8°C during all experiments. Leaf temperature was controlled by CIRAS-2, and leaf gas-exchange rates were measured at 15°C, 25°C, 35°C, and 45°C, resulting in vapor pressure deficits of 0.9 to 15 kPa. The measurements continued for approximately 2 h, and average steady-state gas-exchange rates were calculated. All leaf gas-exchange characteristics were calculated according to von Caemmerer and Farquhar (1981). At every temperature, a different leaf was measured, and altogether 64 leaves were measured for gas exchange (four repetitions at every temperature and four treatments: control, fosmidomycin inhibited without fumigation, and fosmidomycin inhibited with α-pinene or α-terpineol fumigation).
From the gas-exchange measurements, the rate of photosynthetic electron transport, J, was calculated as (Brooks and Farquhar, 1985):
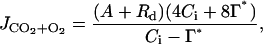 |
(4) |
where Rd (μmol m−2 s−1) is the rate of mitochondrial respiration continuing in the light (μmol m−2 s−1), Γ* (μmol mol−1) is the CO2 compensation point in the absence of Rd, and Ci is the intercellular CO2 concentration (μmol mol−1). Γ* at different temperatures was determined using standard Rubisco kinetic characteristics (Niinemets and Tenhunen, 1997). The temperature dependence of photosynthetic electron transport was fitted as:
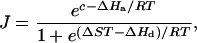 |
(5) |
where c is the scaling constant, ΔHa (J mol−1) is the activation energy, ΔHd (J mol−1) is the deactivation energy, ΔS (J mol−1 K−1) is the entropy term, T (K) is leaf temperature, and R (8.314 J mol−1 K−1) is the gas constant. The optimum temperature for photosynthetic electron transport, Topt, (Niinemets et al., 1999) was further calculated by:
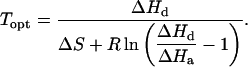 |
(6) |
PTR-MS Measurements
The PTR-MS instrument (IONICON Analytik GmbH) is described in detail in a series of publications (Hansel et al., 1995; Lindinger et al., 1998a, 1998b). The measurement method is based on a proton-transfer reaction from hydronium ions (H3O+) to compounds with a higher proton affinity than water and subsequent detection of the product ions in a quadrupole mass spectrometer. Volatile compounds that have proton affinities larger than the affinity of water (166.5 kcal mol−1) are ionized by proton transfer from H3O+, and the protonated volatiles are mass analyzed. The ion source produces nearly exclusively H3O+ ions (>98%), which are extracted and transferred into the drift tube (Mayr et al., 2003).
The PTR-MS technique has two to three orders of magnitude greater sensitivity than the conventional GC-MS technique, and, thus, the emission rates of organic compounds can be estimated in real time (Cao and Hewitt, 1995; Tani et al., 2003). However, PTR-MS cannot distinguish between the volatile compounds with the same Mr, such as different monoterpenes. We estimated the concentration of monoterpenes from the protonated molecular ions at masses 137 (nonoxygenated monoterpenes) and 155 (terpene alcohols; Tani et al., 2003). In addition, a certain fractionation of nonoxygenated monoterpenes in the drift tube occurs during ionization even under “soft” conditions, resulting in masses 67, 81, and 95 among others (Tani et al., 2003). As a result of fractionation, the total concentration of monoterpenes emitted by Q. ilex is found as the concentration of ions with mass 137 divided by 0.46 (Tani et al., 2003). This correction factor was corroborated by our simultaneous PTR-MS and GC-MS (Carbotrap/Carbosieve trapping) measurements.
The gas-exchange system for combined measurements of net assimilation and transpiration rates as well as monoterpene emissions and for fumigation with specific monoterpenes is outlined in Figure 6. For measuring monoterpene emissions, ambient air entered from inlet 1, and valves before and after the leaf chamber were employed to estimate the monoterpene concentrations in the air entering and leaving the chamber and thus to determine the monoterpene emission rates. As with the photosynthesis measurements, the stable rates were achieved in approximately 20 min after leaf enclosure in the chamber. At every temperature, the steady-state emission rates were monitored for 2 h, and averages were calculated. The temperature dependencies of monoterpene emission rates were fitted with the Arrhenius-type temperature relationship used for the rate of photosynthetic electron transport (Eq. 5), and, further, the optimum temperatures of monoterpene emission were calculated (Eq. 6).
For the fumigation experiments, the system was supplied with the air with the target concentration of specific monoterpene set at 4.5 nmol mol−1 from inlet 2, and the monoterpene concentrations entering and leaving the chamber were monitored by closing or opening the valves before and after leaf chamber (Fig. 6). During the 2-h fumigation, the reference monoterpene concentration entering into the chamber was estimated three to four times and was always found to be constant without any time-dependent drift (Fig. 3). Measurements with empty leaf chamber demonstrated that the chamber itself did not alter the concentration of the entering air (average difference less than 0.03 nmol mol−1). Temperature responses of monoterpene uptake were fitted by nonlinear regressions in the form of y = aebx.
Determination of Temperature Dependencies of Henry's Law Constant for α-Pinene and α-Terpineol
Henry's law constant (Hcc, mol mol−1) is defined as the air-to-water partition coefficient representing the compound's volatility into air from water:
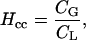 |
(7) |
where CG is the compound concentration in the gas phase and CL the compound concentration in the liquid phase.
The temperature dependencies of the Henry's law constants were estimated by the method of Gossett (1987) as detailed in Copolovici and Niinemets (2005). According to this method, equilibrium monoterpene gas- and liquid-phase concentrations are estimated in two vials with differing gas- and liquid-phase volumes. Four milliliters (VL1) or 50 mL (VL2) deionized water (Millipore water purification system; Millipore) was added to two identical glass vials with a total volume, VT, of 64.5 mL. Thereafter, 0.2 μL of chromatography-grade α-pinene or α-terpineol (Sigma-Aldrich) was injected into each vial with a 1-μL microsyringe (SGE International) under water surface to avoid monoterpene evaporation, and the vial was immediately sealed with a Teflon-coated silicone septum. The vials were further equilibrated in a thermostatic water bath at specific temperature (±0.5°C) for 3 h, and the headspace was sampled for GC analyses with a 0.5-mL sample loop with a syringe (500 μL; Hamilton Bonaduz AG).
The equilibrium for vial 1 is:
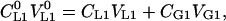 |
(8) |
where CL10 is the initial solute concentration, VL10 is the initial solution volume, CL1 is the solute concentration in the liquid phase, VL1 is the liquid-phase volume after the equilibration, CG1 is the solute concentration in the gas phase (headspace), and VG1 is the equilibrium gas-phase (headspace) volume. The mass balance equation for vial 2 at the equilibrium is:
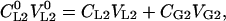 |
(9) |
where the main symbol subscripts are analogous to Equation 8. Changes in liquid-phase volumes are assumed to be negligible before and after reaching the equilibrium in both vials, i.e. 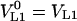 and
and 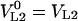 and
and 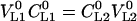 . From Equations 7 to 9, the dimensionless Henry's law constant (Hcc = CG1/CL1 = CG2/CL2) is:
. From Equations 7 to 9, the dimensionless Henry's law constant (Hcc = CG1/CL1 = CG2/CL2) is:
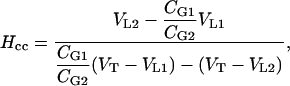 |
(10) |
where VT is the total vial volume (VT = VL1 + VG1 = VL2 + VG2).
The headspace monoterpene concentration, CG, is proportional to the signal peak area (A1 and A2) in the gas chromatogram. Hence,
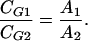 |
(11) |
Combining Equations 10 and 11, we finally obtain:
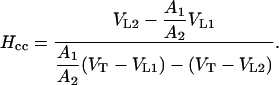 |
(12) |
Henry's law constant expressed as a ratio of mole fractions (Hxy, mol monoterpene/mol air [mol monoterpene/mol water]−1) is given as Hcc (concentration ratio; Eq. 7) times MgρL/(MWρg), where Mg (kg mol−1) is the molecular mass of air (0.029 kg mol−1), ρL is water density (kg m−3), MW is the molecular mass of water (0.018 kg mol−1), and ρg (kg m−3) is air density.
For these measurements, we used a Hewlett-Packard Model 5890 Series II gas chromatograph equipped with a flame ionization detector and with a HP-2 methylsiloxane column (length 20 m, diameter 0.2 mm, film thickness 0.33 μm). The injector temperature was 200°C, and the detector temperature 250°C. The initial temperature of the oven was 70°C, and the final temperature was 120°C with intervals of 10°C/min. The gas flow rates used were 40 mL min−1 for H2, 450 mL min−1 for air, and 45 mL min−1 for N2.
Determination of Octanol/Water Partition Coefficients for α-Pinene and α-Terpineol
Gas chromatography (the same system and setup as for Henry's law constant measurements) was employed for determination of the octanol/water partition coefficient, Kow. A shake-flask method that is based on thorough mixing of the two phases to reach the equilibrium was used (de Bruijn et al., 1989; Dewulf et al., 1999). Stock solutions of monoterpenoids (51.2 mmol/L) were made in high purity analytical grade n-octanol (Fluka Chemie GmbH) presaturated with water. Two milliliters of octanol phase with monoterpenoids was merged with 7 mL of water, and the phases of the solvent system were mutually saturated by shaking for 24 h on a mechanical shaker at the target temperature. To separate the phases, the mixture was centrifuged at specific temperature for 2 h at 9,900g. An aliquot of the octanol phase was used to measure the monoterpene concentration. A mass balance equation was further employed to determine the quantity of substance present at equilibrium in water phase from the amounts of the monoterpene originally present in presaturated monoterpene solution in octanol and the amount of substance remaining in the octanol phase.
Data Analyses
Experimental treatments were compared with one-way ANOVA, and the significant treatment effects were separated by Bonferroni test. All treatments were considered significant at P < 0.05.
In our study, one set of experiments with two repetitions at each treatment were conducted in November 2004, and the entire measurement program was repeated twice in March 2005. The maximum stomatal conductances to water vapor (generally observed at 25°C) were significantly larger (one-way ANOVA) in March (average ± se = 252 ± 23 mmol m−2 s−1) than in November (97 ± 11 mmol m−2 s−1, P < 0.001 for the difference between the averages at different dates), resulting in larger net assimilation rates in March (13.4 ± 1.3 μmol m−2 s−1) than in November (8.8 ± 1.4 μmol m−2 s−1, means are different at P < 0.02). The maximum capacities for photosynthetic electron transport (Jmax = 78 ± 10 μmol m−2 s−1 in November and 102 ± 10 μmol m−2 s−1 in March) were not significantly different between the dates (P = 0.12). This demonstrates that the assimilation rates primarily differed because of larger stomatal conductances in March plants that were fully recovered from previous-year water stress and were also in a different phenological stage.
Due to differences in foliage photosynthetic characteristics between the measurement campaigns, the data were normalized in the figures, but statistical effects of the treatments were tested for both nonnormalized (Table I) and normalized (Fig. 2) data.
This work was supported by the European Commission (contract MC–RTN–CT–2003–504720 “ISONET”), by the Estonian Ministry of Science and Education (grant no. 0182468As03), by the Estonian Science Foundation (grant no. 5702), and by the Spanish Ministry of Education and Science (grant nos. REN2003–04871 and CGL2004–01402/BOS).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.105.065995.
References
- Achkor H, Diaz M, Fernandez MR, Biosca JA, Pares X, Martinez MC (2003) Enhanced formaldehyde detoxification by overexpression of glutathione-dependent formaldehyde dehydrogenase from Arabidopsis. Plant Physiol 132: 2248–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affek HP, Yakir D (2002) Protection by isoprene against singlet oxygen in leaves. Plant Physiol 129: 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman O (1981) The responses of photosynthesis to temperature. In J Grace, ED Ford, PG Jarvis, eds, Plants and Their Atmospheric Environment. The 21st Symposium of the British Ecological Society, Edinburgh 1979. Blackwell Scientific Publications, Oxford, pp 273–301
- Brooks A, Farquhar GD (1985) Effects of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Estimates from gas-exchange measurements on spinach. Planta 165: 397–406 [DOI] [PubMed] [Google Scholar]
- Calogirou A, Larsen BR, Brussol C, Duane M, Kotzias D (1996) Decomposition of terpenes by ozone during sampling on tenax. Anal Chem 68: 1499–1506 [DOI] [PubMed] [Google Scholar]
- Cao XL, Hewitt CN (1995) Detection methods for the analysis of biogenic non-methane hydrocarbons in air. J Chromatogr A 710: 39–50 [Google Scholar]
- Chameides W, Lindsay R, Richardson J, Kiang C (1988) The role of biogenic hydrocarbons in urban photochemical smog: Atlanta as a case study. Science 241: 1473–1475 [DOI] [PubMed] [Google Scholar]
- Copolovici LO, Niinemets Ü (2005) Temperature dependencies of Henry's law constants and octanol/water partition coefficients for key plant volatile monoterpenoids. Chemosphere doi/10.1016/j.chemosphere.2005.05.003 [DOI] [PubMed]
- de Bruijn J, Busser F, Seinen W, Hermens J (1989) Determination of octanol/water partition coefficients for hydrophobic organic chemicals with “slow-stirring” method. Environ Toxicol Chem 8: 499–512 [Google Scholar]
- Delfine S, Csiky O, Seufert G, Loreto F (2000) Fumigation with exogenous monoterpenes of a non-isoprenoid-emitting oak (Quercus suber): monoterpene acquisition, translocation, and effect on the photosynthetic properties at high temperatures. New Phytol 146: 27–36 [Google Scholar]
- Dewulf J, Van Langenhove H, Graré S (1999) Sediment/water and octanol/water equilibrium partitioning of volatile organic compounds: temperature dependence in the 2–25°C range. Water Res 33: 2424–2436 [Google Scholar]
- Fuentes JD, Lerdau M, Atkinson R, Baldocchi D, Bottenheim JW, Ciccioli P, Lamb B, Geron C, Gu L, Guenther A, et al (2000) Biogenic hydrocarbons in the atmospheric boundary layer: a review. Bull Am Meteorol Soc 81: 1537–1575 [Google Scholar]
- Giese M, Baueroranth U, Langebartels C, Sandermann H (1994) Detoxification of formaldehyde by the spider-plant (Chlorophytum comosum L.) and by soybean (Glycine maxima L.) cell suspension cultures. Plant Physiol 104: 1301–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett JM (1987) Measurement of Henry's law constants for C1 and C2 chlorinated hydrocarbons. Environ Sci Technol 21: 202–208 [Google Scholar]
- Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, McKay WA, et al (1995) A global model of natural volatile compound emissions. J Geophys Res 100: 8873–8892 [Google Scholar]
- Hansel A, Jordan A, Holzinger R, Prazeller P, Vogel W, Lindinger W (1995) Proton transfer reaction mass spectrometry: on-line trace gas analysis at the ppb level. Int J Mass Spectrom Ion Process 149/150: 609–619 [Google Scholar]
- Hanson AD, Roje S (2001) One-carbon metabolism in higher plants. Annu Rev Plant Physiol Plant Mol Biol 52: 119–137 [DOI] [PubMed] [Google Scholar]
- Harrison D, Hunter MC, Lewis AC, Seakins PW, Bonsang B, Gros V, Kanakidou M, Touaty M, Kavouras I, Mihalopoulos N, et al (2001) Ambient isoprene and monoterpene concentrations in a Greek fir (Abies borisii Regis) forest. Reconciliation with emissions measurements and effects on measured OH concentrations. Atmos Environ 35: 4699–4711 [Google Scholar]
- Havaux M (1998) Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci 3: 147–151 [Google Scholar]
- He C, Murray F, Lyons T (2000) Monoterpene and isoprene emissions from 15 Eucalyptus species in Australia. Atmos Environ 34: 645–655 [Google Scholar]
- Howard PH, Meylan WM (1997) Handbook of Physical Properties of Organic Chemicals. Lewis Publishers, Boca Raton, FL
- Hüve K, Bichele I, Tobias M, Niinemets Ü (2005) Heat sensitivity of photosynthetic electron transport varies during the day due to changes in sugars and osmotic potential. Plant Cell Environ 28: (in press) [DOI] [PubMed]
- Jiang YW, Huang BR (2001) Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop Sci 41: 436–442 [Google Scholar]
- Katynski AL, Vijayan MM, Kennedy SW, Moon TW (2004) 3,3′,4,4′,5-Pentachlorobiphenyl (PCB 126) impacts hepatic lipid peroxidation, membrane fluidity and beta-adrenoceptor kinetics in chick embryos. Comp Biochem Physiol Part Toxicol Pharmacol 137: 81–93 [DOI] [PubMed] [Google Scholar]
- Kesselmeier J, Bode K, Hofmann U, Müller H, Schäfer L, Wolf A, Ciccioli P, Brancaleoni E, Cecinato A, Frattoni M, et al (1997) Emission of short chained organic acids, aldehydes and monoterpenes from Quercus ilex L. and Pinus pinea L. in relation to physiological activities, carbon budget and emission algorithms. Atmos Environ 31: 119–133 [Google Scholar]
- Kesselmeier J, Ciccioli P, Kuhn U, Stefani P, Biesenthal T, Rottenberger S, Wolf A, Vitullo M, Valentini R, Nobre A, et al (2002) Volatile organic compound emissions in relation to plant carbon fixation and the terrestrial carbon budget. Global Biogeochem Cycles 16: 1126 [Google Scholar]
- Kondo T, Hasegawa K, Uchida R, Onishi M, Mizukami A, Omasa K (1996) Absorption of atmospheric formaldehyde by deciduous broad-leaved, evergreen broad-leaved, and coniferous tree species. Bull Chem Soc Jpn 69: 3673–3679 [Google Scholar]
- Kuzuyama T, Shimizu T, Takashashi S, Seto H (1998) Fosmidomycin, a specific inhibitor of 1-deoxy-D-xylulose-5-phosphate reductoisomerase in the nonmevalonate pathway for terpenoid biosynthesis. Tetrahedron Lett 39: 7913–7916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher W (2000) Temperature stress and survival ability of Mediterranean sclerophyllous plants. Plant Biosyst 134: 279–295 [Google Scholar]
- Lindinger W, Hansel A, Jordan A (1998. a) On-line monitoring of volatile organic compounds at pptv levels by means of proton-transfer-reaction mass spectrometry (PTR-MS): medical applications, food control and environmental research. Int J Mass Spectrom Ion Process 173: 191–241 [Google Scholar]
- Lindinger W, Hansel A, Jordan A (1998. b) Proton-transfer-reaction mass spectrometry (PTR-MS): on-line monitoring of volatile organic compounds at pptv levels. Chem Soc Rev 27: 347–354 [Google Scholar]
- Llusià J, Peñuelas J, Asensio D, Munné-Bosch S (2005) Airborne limonene confers limited thermotolerance to Quercus ilex. Physiol Plant 123: 40–48 [Google Scholar]
- Loreto F, Förster A, Dürr M, Csiky O, Seufert G (1998) On the monoterpene emission under heat stress and on the increased thermotolerance of leaves of Quercus ilex L. fumigated with selected monoterpenes. Plant Cell Environ 21: 101–107 [Google Scholar]
- Loreto F, Pinelli P, Manes F, Kollist H (2004) Impact of ozone on monoterpene emissions and evidence for an isoprene-like antioxidant action of monoterpenes emitted by Quercus ilex leaves. Tree Physiol 24: 361–367 [DOI] [PubMed] [Google Scholar]
- Loreto F, Sharkey TD (1990) A gas-exchange study of photosynthesis and isoprene emission in Quercus rubra L. Planta 182: 523–531 [DOI] [PubMed] [Google Scholar]
- Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127: 1781–1787 [PMC free article] [PubMed] [Google Scholar]
- Mahoney SR, Ghosh S, Peirson D, Dumbroff EB (1998) Paclobutrazol affects the resistance of black spruce to high light and thermal stress. Tree Physiol 18: 121–127 [DOI] [PubMed] [Google Scholar]
- Mayr D, Märk TD, Lindinger W, Brevard H, Yeretzian C (2003) Breath-by-breath analysis of banana aroma by proton transfer reaction mass spectrometry. Int J Mass Spectrom 223/224: 743–756 [Google Scholar]
- Müller WH, Müller CH, Haines BL (1964) Volatile growth inhibitors produced by shrubs. Science 143: 471–473 [DOI] [PubMed] [Google Scholar]
- Munné-Bosch S, Peñuelas J, Asensio D, Llusià J (2004) Airborne ethylene may alter antioxidant protection and reduce tolerance of holm oak to heat and drought stress. Plant Physiol 136: 2937–2947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Loreto F, Reichstein M (2004) Physiological and physico-chemical controls on foliar volatile organic compound emissions. Trends Plant Sci 9: 180–186 [DOI] [PubMed] [Google Scholar]
- Niinemets Ü, Oja V, Kull O (1999) Shape of leaf photosynthetic electron transport versus temperature response curve is not constant along canopy light gradients in temperate deciduous trees. Plant Cell Environ 22: 1497–1514 [Google Scholar]
- Niinemets Ü, Reichstein M (2002) A model analysis of the effects of nonspecific monoterpenoid storage in leaf tissues on emission kinetics and composition in Mediterranean sclerophyllous Quercus species. Global Biogeochem Cycles 16: 1110 [Google Scholar]
- Niinemets Ü, Reichstein M (2003. a) Controls on the emission of plant volatiles through stomata: a sensitivity analysis. J Geophys Res-Atmos 108: 4211 [Google Scholar]
- Niinemets Ü, Reichstein M (2003. b) Controls on the emission of plant volatiles through stomata: sensitivity or insensitivity of the emission rates to stomatal closure explained. J Geophys Res-Atmos 108: 4208 [Google Scholar]
- Niinemets Ü, Reichstein M, Staudt M, Seufert G, Tenhunen JD (2002. a) Stomatal constraints may affect emission of oxygenated monoterpenoids from the foliage of Pinus pinea. Plant Physiol 130: 1371–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets Ü, Seufert G, Steinbrecher R, Tenhunen JD (2002. b) A model coupling foliar monoterpene emissions to leaf photosynthetic characteristics in Mediterranean evergreen Quercus species. New Phytol 153: 257–276 [Google Scholar]
- Niinemets Ü, Tenhunen JD (1997) A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ 20: 845–866 [Google Scholar]
- Peñuelas J, Llusià J (2002) Linking photorespiration, monoterpenes and thermotolerance in Quercus. New Phytol 155: 227–237 [Google Scholar]
- Peñuelas J, Llusià J (2003) BVOCs: plant defense against climatic warming? Trends Plant Sci 8: 105–109 [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Munné-Bosch S (2005) Isoprenoids: an evolutionary pool for photoprotection. Trends Plant Sci 10: 166–169 [DOI] [PubMed] [Google Scholar]
- Perry NSL, Houghton PJ, Sampson J, Theobald AE, Hart S, Lis-Balchin M, Hoult JRS, Evans P, Jenner P, Milligan S, et al (2003) In-vitro activity of S. lavandulaefolia (Spanish sage) relevant to treatment of Alzheimer's disease. J Pharm Pharmacol 53: 1347–1356 [DOI] [PubMed] [Google Scholar]
- Romagni JG, Allen SN, Dayan FE (2000) Allelopathic effects of volatile cineoles on two weedy plant species. J Chem Ecol 26: 303–313 [Google Scholar]
- Russell MF, Southwell IA (2003) Monoterpenoid accumulation in 1,8-cineole, terpinolene and terpinen-4-ol chemotypes of Melaleuca alternifolia seedlings. Phytochemistry 62: 683–689 [DOI] [PubMed] [Google Scholar]
- Sharkey TD (1996) Isoprene synthesis by plants and animals. Endeavour 20: 74–78 [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Chen XY, Yeh S (2001) Isoprene increases thermotolerance of fosmidomycin-fed leaves. Plant Physiol 125: 2001–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Singsaas EL (1995) Why plants emit isoprene. Nature 374: 769 [Google Scholar]
- Singsaas EL, Lerdau M, Winter K, Sharkey TD (1997) Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol 115: 1413–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt M, Bertin N, Frenzel B, Seufert G (2000) Seasonal variation in amount and composition of monoterpenes emitted by young Pinus pinea trees—implications for emission modeling. J Atmos Chem 35: 77–99 [Google Scholar]
- Staudt M, Mandl N, Joffre R, Rambal S (2001) Intraspecific variability of monoterpene composition emitted by Quercus ilex leaves. Can J For Res 31: 174–180 [Google Scholar]
- Tani A, Hayward S, Hewitt CN (2003) Measurement of monoterpenes and related compounds by proton transfer reaction-mass spectrometry (PTR-MS). Int J Mass Spectrom 223/224: 561–578 [Google Scholar]
- Velikova V, Edreva A, Loreto F (2004) Endogenous isoprene protects Phragmites australis leaves against singlet oxygen. Physiol Plant 122: 219–225 [Google Scholar]
- Velikova V, Loreto F (2005) On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ 28: 318–327 [Google Scholar]
- Velikova V, Pinelli P, Loreto F (2005) Consequences of inhibition of isoprene synthesis in Phragmites australis leaves exposed to elevated temperatures. Agric Ecosyst Environ 106: 209–217 [Google Scholar]
- von Caemmerer S, Farquhar GD (1981) Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153: 376–387 [DOI] [PubMed] [Google Scholar]