Abstract
Plants function as an integrated system of interconnected organs, with shoots and roots mutually influencing each other. Brassinosteroid (BR) signaling is essential for whole-plant growth, yet the relative importance of shoot versus root BR function in shaping root system architecture (RSA) remains unclear. Here, we directly tackle this question using micro-grafts between wild-type and BR-null mutants in both Arabidopsis and tomato, assisted by phenotyping, transcriptomics, metabolic profiling, transmission electron microscopy, and modeling approaches. These analyses demonstrate that shoot BR, by determining root carbon availability, allows for a full rescue of mutant root biomass, while loss of shoot BR attenuates root growth. In parallel, root BR dictates the spatial distribution of carbon along the root, through local regulation of growth anisotropy and cell wall thickness, shaping root morphology. A newly developed “grow and branch” simulation model demonstrates that these shoot- and root-derived BR effects are sufficient to explain and predict root growth dynamics and branching phenotype in wild-type, BR-deficient mutants, and micro-graft combinations. Our interdisciplinary approach, applied to two plant species and integrating shoot and root hormonal functions, provides a new understanding of how RSA is modulated at various scales.
Subject terms: Brassinosteroid, Morphogenesis, Plant development
A grafting-based approach combined with modeling uncovers how root system architecture is shaped by the distinct yet essential contributions of the shoot and root through brassinosteroid signaling.
Introduction
Plants act as a coordinated system, with the shoot and the root inherently connected through moving photosynthate, nutrients, hormones and other molecules in the vasculature system. The development of the root system depends on shoot-derived photosynthetic sugars that function as a long-distance energy and signaling source, promoting root elongation, the incipient formation of lateral roots (LRs), and their development within the branching process1–5. Additionally, root morphogenesis is controlled by local hormonal signaling pathways that may integrate with systemic sugar-energy signals6,7.
The plant hormones brassinosteroids (BRs) are essential for growth of above- and below-ground plant organs as evidenced by the typical dwarf phenotype of BR-deficient and insensitive mutants8. BRs synthesis involves multiple enzymatic steps that together form parallel interconnected pathways9,10. BR signaling is initiated upon binding of the hormone to the extracellular domain of the main leucine-rich repeat membrane receptor kinase BRASSINOSTEROID INSENSITIVE (BRI1) and promotes its binding to BRI1 ASSOCIATED KINASE 1/SOMATIC EMBRYOGENESIS RECEPTOR KINASE (BAK1/SERK), leading to BRI1 activation. This triggers a series of regulatory steps that activate the downstream BRI1-EMS-SUPPRESSOR 1 (BES1)/BRASSINAZOLE RESISTANT 1 (BZR1) family of transcription factors, enabling their translocation into the nucleus to induce or repress their target genes11.
The regulation of root development by BR signaling has been studied at various scales12–14. Single-cell-based quantitative morphometric analysis performed in the Arabidopsis primary root meristem, defined the main effect of the BR hormone being on cellular shape, rather than cell size (i.e., volume)15,16. BR signaling achieves this modulation of cell geometry by promoting the anisotropy ratio of axial growth rates, which results in faster growth in the longitudinal axis of the cell (in the direction of root elongation) at the expense of the radial axis, thus restricting root diameter16. In the absence of BR signaling, as in the bri1 mutant, the slow-longitudinally expanding cells have accumulative growth in the radial axis, accompanied by cell divisions in this direction, resulting in a shorter and wider root than wild-type (WT)16. Studies at the root system scale showed that BR signaling promotes LR formation and emergence in young seedlings of Arabidopsis and rice, acting downstream of sugar17 and affecting auxin transport in LR formation, and the transcription of selected LR formation genes18,19. A key open question thus is to what extent, if at all, these multiscale developmental processes are modulated by the growth-promoting activity of shoot BR.
The process of forming LR is an ongoing and iterative phenomenon, often described as a “root clock” in Arabidopsis20. Using multiscale modeling, the frequency of LR formation has been recently proposed to arise from the interplay between root meristem size and growth, and root tip auxin transport21. Given that BR signaling regulates meristem shape and growth function, this strongly suggests a role for BR in the control of root branching that thus far has not been fully explored.
A precise means to separate the effects of shoot and root-borne processes and signals is through grafting experiments, where the apical part of a given plant (scion) is connected to the basal part containing the root (stock) of another plant22,23. In pea, reciprocal grafting between the wild type (WT) and the BR biosynthesis mutant lkb (homolog of the Arabidopsis dwf1) showed no rescue of the dwarf scion phenotype by the WT rootstock24. Consistently, active BR and select BR precursor levels in the mutant scion remained low. However, the phenotype of the grafted roots in this study has not been reported and most BR levels measured in the roots were below detection limits. Thus, the BR hormone does not undergo significant long-distance transport from root to shoot, and hormonal production within the shoot organ is sufficient to drive its own growth24. Whether this is also the case in the roots remains to be addressed.
Here, we approached the aforementioned open questions by dissecting the effects of shoot and root BR signaling on root morphology and branching. Hundreds of micro-grafts between WT and null BR-deficient and insensitive mutants were generated in Arabidopsis and validated in tomato, combining transcriptomics, metabolic profiling, transmission electron microscopy (TEM) and modeling approaches. We found that BR-mediated growth of the shoot fully determines root biomass. In contrast, root BR activity locally regulates anisotropy of growth and cell wall thickness and thus, the spatial distribution of carbon accumulation in the root. Through modeling, we demonstrate that these two BR-mediated effects are sufficient to explain the root branching phenotype of WT and BR-deficient mutants in both Arabidopsis and tomato.
Results
BR activity in the shoot promotes root growth while loss of shoot BR attenuates it
To assess the effect of shoot-derived BR production on root development, we performed reciprocal micrografting in Arabidopsis between WT expressing the plasma membrane marker LTI6B-GFP (hereafter “WT”) and cpd within a workflow that is described in Fig. 1a (Supplementary Fig. 1a). In this workflow, we selected time points that technically allowed for measurements of a developed root system architecture (RSA, in 5-week-old plants), and the measurement of biomass under near-physiological conditions, ensuring that the roots of a single plant could be directly weighed (8-week-old plants, see below). The presence or absence of the LTI6B fluorescence was used to indicate a successful grafting (Supplementary Fig. 1b). We scored success of the procedure at three stages; attachment between the scion and the rootstock 7 days after grafting, elongation of primary root and LR emergence 3 weeks after transferring to agar plates, and survival of the grafts 3 weeks after transferring to pots (see also “Methods”). This revealed the cpd/cpd homograft had a consistently lower success rate from the attachment stage than WT/WT, and the heterograft combinations were more successful in resuming root growth than the mutant homograft alone (Supplementary Fig. 1c, p = 4.4e-05, proportion test, see Supplementary Data 1 for pairwise decrease phase. n = 758 for all graft combinations in this study). These differences may be attributed to a potential necessity for BR signaling in the grafting process. In support, we demonstrate that the BR biosynthesis reporter DWF4pro:N7-NG:DWF4ter25 had an apparent signal 24 h after hypocotyl wounding, while no signal was detected in intact hypocotyls (Supplementary Fig. 1e, f), in accordance with previously reported transcriptome performed on graft-junction26.
Fig. 1. Brassinosteroid (BR) activity in the shoot promotes root branching.
a An illustration depicting the grafted plants and their experimental growth regime. b Scanned images of grafted plants, displaying scion/rootstock of 5-week-old WT/WT, WT/cpd, cpd/cpd, and cpd/WT (1-week recovery after micrografting followed by 3 weeks growth on agar). The representative seedlings were cropped from their corresponding scanned plate images and assembled for a comparison. c–f Root system architecture (RSA) analysis of plants as in (b) are plotted. c Primary root (PR) length, d Number of lateral roots (LRs), e LR length, f Total root length. Note that the cpd shoot restricts the growth of the WT root, while the WT shoot promotes root growth and branching in cpd. In boxplots, the center corresponds to the median, the lower and upper hinges represent the first and third quartiles, and the whiskers extend to the largest and smallest values within 1.5 × the interquartile range (IQR) from the hinges. All data points are plotted individually. Different letters indicate values with statistically significant differences (n: WT/WT = 17, WT/cpd = 21, cpd/cpd = 7, cpd/WT = 19, p ≤ 0.05).
Analysis of the plants 3 weeks after recovery from grafting showed changes in RSA in the heterograft combinations as compared to the homograft whereas the cpd shoot appeared unaffected, maintaining its typical dwarf phenotype (Fig. 1b). The primary root length, number of visible LRs, total LR length, and total root length (total LR length and primary root length) of cpd roots were partially rescued by the WT scion as compared to the homograft cpd/cpd. Analogously, these growth parameters were reduced in WT roots in response to loss of BR in the shoot (cpd/WT) as compared to WT roots in the homograft WT/WT (Fig. 1b–f). Combined this suggests a major role for shoot BR function in root growth.
Shoot BR modulates root gene expression reflecting carbon availability and lateral root regulation
The observed effects of shoot BR and thus shoot size on RSA are likely accompanied by a yet unknown alteration in transcriptional response (Fig. 2a). Hence, we performed RNA-seq on the root system of WT/WT, WT/cpd, cpd/WT, and ungrafted cpd (underwent the steps preceding the hypocotyl cut only, see “methods”), hereafter called cpd/cpdun. The grafting combinations also allowed us to ask if the changes in gene expression in response to shoot BR, depend or not on local root BR. In support of the accuracy of the grafting procedure, the cpd gene had a significantly lower expression level (normalized counts) in WT/cpd and cpd/cpdun roots than in the WT roots (Fig. 2b, Supplementary Data 2). Intriguingly, the clear separation in root gene expression between WT/WT and all other graft combinations along PC1 indicates that the most prominent effect was a shift in gene expression from WT pattern towards cpd pattern. Moreover, all cpd roots (WT/cpd and cpd/cpdun) clustered nearby with respect to PC1 and PC2, suggesting a poor transcriptional rescue by the WT shoot. Next, we verified that known BR target genes (e.g., BR biosynthesis genes) are differentially expressed (DE) between roots of WT/WT and cpd/cpdun (Supplementary Data 2). Based on this control, we considered for further analysis DE genes using a cutoff of log2 fold change of 0.8 and adjusted p value of 0.05 and compared the difference in the transcriptome between cpd/WT and WT/WT (Fig. 2d). This revealed that 684 genes were up-regulated while only 137 were down-regulated. Simply put, hundreds of genes were elevated in WT roots when the shoot had low BR. In accordance with the PCA analysis (Fig. 2c), a high fraction of these genes (322 genes, divided into 168 and 154 genes in the Venn diagram) was also elevated in cpd/cpdun roots as compared to WT/WT (about 63% of the DE genes in cpd/cpdun) (Fig. 2e). GO analysis of these 322 genes revealed enrichment for photosynthesis and carbon fixation genes, categories that have been previously reported to be regulated by sugar starvation (Fig. 2f)27,28. Photosynthesis and carbon fixation genes were the top significant categories also when considering the 168 and 154 genes as separate groups. These results suggest that cpd shoot causes a limited carbon availability which is perceived in the WT root, triggering de-repression of these genes. Interestingly, these genes were constitutively up-regulated in cpd roots, regardless of the shoot genotype (see overlap with WT/cpd, 154 genes, Fig. 2e, Supplementary Data 2). Thus, BR signaling in the root is necessary for regulating carbon starvation responsive genes. Unlike the constitutive de-regulated genes in cpd root, 217 DE genes were rescued by shoot BR (comparing WT/cpd and cpd/cpdun, Supplementary Data 2). These genes included a group related to auxin and LR formation (e.g., YUCCA9, LBD29, PUCHI, Fig. 2g). This agrees with shoot BR dictating sugar availability in the root and suggests that a shoot-derived sugar acting upstream of auxin2 causes the elevated number of LR in WT/cpd as compared to cpd/cpdun (Fig. 1d). Together, shoot BR largely modulates the root transcriptome, depending on active BR signaling in the root (like the response to carbon starvation) and independently of it (as in the control of auxin and LR formation-related genes).
Fig. 2. Shoot BR affects root genes that respond to carbon availability and regulate LR formation.
a An illustration depicting the scientific question. b Normalized counts of the CPD transcripts in the biological replicates corresponding to the four grafting combinations, WT/WT, WT/cpd, cpd/cpdun, and cpd/WT. c PCA analysis displays the biological replicates as above. d A volcano plot displaying –log10 p values vs log2 fold change (FC) of genes in a comparison between cpd/WT and WT/WT. Differentially expressed genes (adjusted p < 0.05, −0.8 > log2FC > 0.8) are colored. e A Venn diagram demonstrating the number of genes with higher expression in roots of WT/cpd, cpd/cpdun, and cpd/WT as compared to WT/WT. Note the high overlap between WT and the cpd (322 genes, divided into 168 and 154 genes in the Venn diagram) upon grafting WT roots to cpd. Common genes across all three pairwise comparisons indicate that this shoot-derived gene regulation depends on root BR. f Functional enrichment of the 322 common genes shown in (e). g LR formation related genes that are rescued in cpd roots by shoot BR were selected from comparing WT/cpd vs cpd/cpd. Adjusted p value is depicted by color and log2FC is noted in parenthesis.
Shoot BR fully rescues the biomass of a BR deficient root
Next, we sought to determine if the RSA in the different graft combinations and the accompanied transcriptomic profiling also reflect the differences in root biomass. To generate sufficient root tissue that can be weighed for comparative analysis, we transferred the plants to pots for an additional 3 weeks (Fig. 1a). Grafts with cpd shoot in both homograft and heterograft had a reduced survival when grown in pots (Supplementary Fig. 1c). Although precise RSA quantification is not feasible for all micrograft combinations in 8-week-old plants, the surviving plants with cpd shoot notably exhibited limited branching, indicating attenuated root growth, and the RSA of cpd roots appeared partially rescued by the WT shoot (Fig. 3a), consistent with the quantified intermediate RSA observed at an earlier age (Fig. 1). Biomass quantification of the roots, both fresh weight (FW) and dry weight (DW), confirmed that the cpd scion arrested the growth of WT roots (cpd/WT), as apparent from the low weight, which was similar to that of cpd/cpd (Fig. 3c, d). Intriguingly, while WT scion only partly rescued cpd root growth parameters (Fig. 1c–f, Fig. 3a), the poor biomass of the cpd root (in the cpd/cpd homograft) was fully rescued by the WT scion (WT/cpd) (Fig. 3c, d). Biomass of the WT shoot appeared largely unaffected by root BR, with slight elevation in the WT/cpd combination (Fig. 3b). To evaluate if the rescue of cpd’s root biomass by shoot BR is also linked to a change in metabolic content, we compared the relative levels of select primary metabolites, using the whole root system of WT/WT, WT/cpd and cpd/cpdun (Fig. 3e). The heterograft cpd/WT could not be included in the analysis due to the low mass of its roots. Among the 31 metabolites analyzed, 16 showed a significant difference in at least one pairwise comparison; among them 13 differed between WT/WT and cpd/cpdun (Fig. 3e, Supplementary Data 1, Supplementary Data 3). This included the elevation of glucose-6-phosphate (G6P) and the reduction of fructose in cpd/cpdun roots vs WT/WT roots, reflecting an overall metabolic imbalance. The levels of most of the tested metabolites were rescued by the WT shoot, in WT/cpd, demonstrating that shoot BR largely controls the root metabolic balance. Finally, to investigate whether the phenotypic effects of shoot BR production (Fig. 1, Fig. 3) could involve a potential hormone transport to the root, in Arabidopsis, or it is the BR signaling and its downstream effects which impact the observed root growth dynamics, we performed a similar analysis in the BR signaling mutant bri1 (Supplementary Fig. 2). The resulting changes in RSA and the root biomass largely recapitulated the cpd experiments, supporting that it is local shoot BR function that is impacting root growth. The lack of rescue in the primary root and the slightly lower biomass rescue of the bri1 root, compared to the cpd root by the WT shoot, may suggest limited hormone transport or regulation due to the accumulation of a specific BR precursor. However, if this is the case, these traits may not be conserved, as an equivalent grafting experiment using the bri1 tomato mutant recapitulated the Arabidopsis cpd results (see Supplementary Fig. 3 below). Together, BR production and signaling in the shoot are critical for the biomass growth of shoot and root and the effect in the latter is likely a consequence of the BR-dependent shoot size and inherent scaled production of photosynthate-derived sugars.
Fig. 3. Shoot BR is a limiting factor for root biomass growth and its metabolic balance.
a Scans of grafted plants showing scion/rootstock of WT/WT, WT/cpd, cpd/cpd, and cpd/WT (8-week-old grown in pots, after washing with water to remove the perlite grains, see scheme in Fig. 1a). b–d Biomass of plants as in (a). b Shoot fresh weight (FW), c root FW and d root dry weight (DW). Note the full rescue of cpd root biomass by the WT shoot and the poor biomass of the WT root when grafted to the cpd scion. In boxplots, the center corresponds to the median, the lower and upper hinges represent the first and third quartiles, and the whiskers extend to the largest and smallest values within 1.5× the IQR from the hinges. All data points are plotted individually. Different letters indicate values with statistically significant differences (n: WT/WT = 19, WT/cpd = 19, cpd/cpd = 2, cpd/WT = 11, p ≤ 0.05). e Metabolite levels in the root depend on BR signaling in the shoot. A heatmap showing metabolites that are significantly different among WT/WT, WT/cpd and cpd/cpdun. (n = 6, p ≤ 0.05). FC fold change. *p ˂ 0.05, **p ˂ 0.01, ***p ˂ 0.001.
Root morphology and the biomass distribution along the root are locally regulated by BR signaling
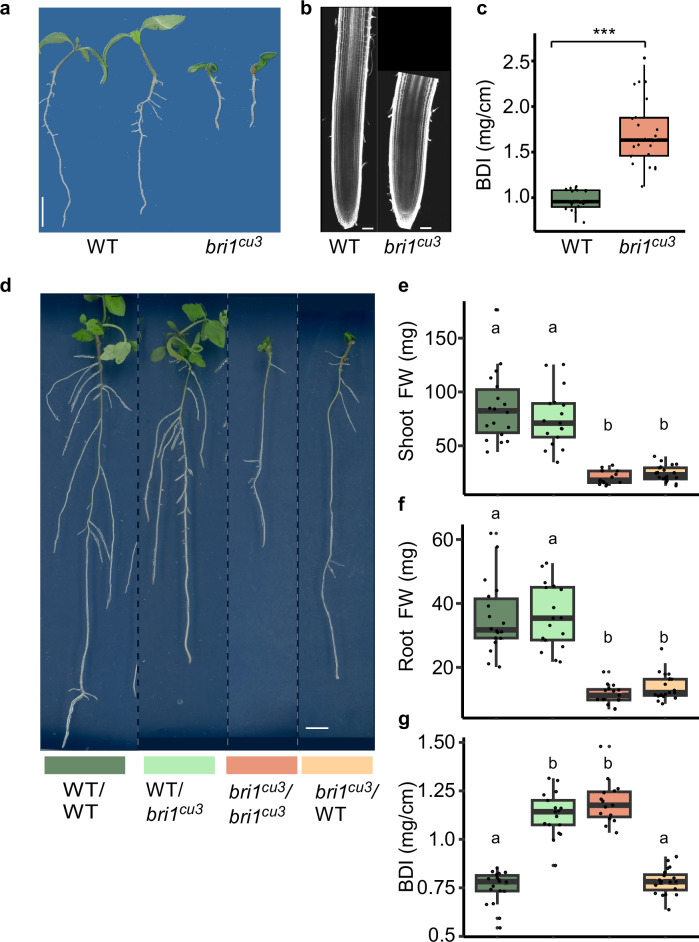
We have demonstrated, assisted by transcriptomics and metabolic profiling, that BR-mediated shoot growth determines root carbon availability. However, while the root biomass of cpd and bri1 mutants was fully rescued by shoot BR (Fig. 3, Supplementary Fig. 2), their RSA parameters had intermediate values (Fig. 1, Supplementary Fig. 2, Fig. 3a), suggesting a distinct carbon distribution along the mutant roots (Fig. 4a). To address this assumption, we imaged the primary root meristems of 5-week-old grafted plants, presuming that biomass rescue was also present at this age (Fig. 4b). We found that the previously reported extended radial growth phenotype of root meristematic cells and the oblique plane of cell division that characterize BR-deficient mutants15,16,29 is similar between cpd/cpd and WT/cpd (Fig. 4b–d). This indicates that the regulation of cellular morphology and anisotropy growth is determined by root-localized BR function, irrespective of shoot-mediated carbon availability. Having more cells per unit root length in the mutant, resulting from reduced cell anisotropic growth rate, which renders shorter and wider cells but with a WT-like volume16, suggests a modified carbon distribution. BR signaling may also affect carbon distribution by controlling the thickness of the primary cell wall. To address this possibility, we performed TEM analysis on longitudinal and cross sections in the root elongation zone of WT and cpd and compared the cell wall thickness in the outer epidermal walls and the inner pericycle walls, to represent outer and inner root cell files respectively (Fig. 4e–j). The analysis revealed that cpd has thicker cell walls compared to the WT across the walls tested. The wider root with thicker cell walls suggests that the mutant must have a higher mass per unit root length (referred to hereafter as biomass density index, BDI). To calculate BDI, we used a segregating population of tomato bri1 (bri1cu3, in the Solanum pimpinellifolium background)30,31, for obtaining a sufficient mass in a single root, allowing us to compare RSA and biomass at the same time after grafting, thus overcoming the limitation of the Arabidopsis root analysis. This demonstrated that bri1cu3 has a vast increase in BDI (~1.6-fold) (Fig. 5a–c) larger than the increases observed for radial and transversal root widths in Arabidopsis (Fig. 4c, d), in agreement with the finding of cpd’s thicker cell walls (Fig. 4e–h). To determine if the elevated BDI is affected by the shoot, we performed micro-grafting between heterozygous BRI1/bri1cu3 or WT and bri1cu3 (Fig. 5d–g, Supplementary Fig. 3, results for 21 days after grafting are shown). These results showed that the shoot biomass remained unaffected by the heterograft combinations (Fig. 5e), suggesting that the slight elevation in shoot biomass observed in WT/cpd (Fig. 3b) may indicate a yet-unknown specific condition in which the mutant rootstock provides an advantage. Unlike the shoot, the mutant root biomass was fully rescued by the WT (i.e., WT or heterozygous BRI1/bri1cu3) shoot (Fig. 5f). Analogously, the accumulation of WT root biomass was hindered by the mutant shoot, recapitulating the Arabidopsis results (Fig. 5f). Importantly, BDI was lower in WT roots than bri1cu3 roots, irrespective of BR signaling in the shoot. Together our data indicates that BR-mediated growth of the shoot determines carbon availability and overall root biomass while root BR controls biomass distribution along the root, in both Arabidopsis and tomato. This distribution, apparent in cell wall thickness and cell shape, provides a new insight into the mode by which BR signaling may promote anisotropic growth rate12.
Fig. 4. Root BR determines the biomass distribution along the root irrespective of shoot BR.
a An illustration depicting equal biomass for a distinct total root length raises the question of how carbon is distributed. b Confocal images showing optical longitudinal and cross-sections taken 50 μm from the quiescent center (QC) of the primary root meristem, in WT/WT, WT/cpd, cpd/cpd and cpd/WT (5-week-old). Scale bar, 50 μm. Note the disorganized cell files in cpd root combinations. c Whole meristem diameter and d stele diameter in roots as in (b) n: WT/WT = 12, WT/cpd = 19, cpd/cpd = 6, cpd/WT = 14. e–i Quantification of cell wall thickness in longitudinal wall of the outer epidermis (e), radial wall of the outer epidermis (f) and wall between neighboring pericycle cells (g), observed in cross-sections in WT (n = 3, green) and cpd (n = 3, magenta) roots. The corresponding cell walls are depicted in pink in the accompanying illustration (h). In outer epidermal radial walls, 15–20 cells were measured, with 20–30 measurements taken along the cell wall of each cell. In outer epidermal longitudinal walls, 2 cells were measured, with 10 measurements taken along the cell wall of each cell. In the pericycle radial walls, 12–15 cells were measured, with 13–18 measurements taken along the cell wall of each cell. Each boxplot represents one plant and each dot represents measurement in a different cell wall. In boxplots, the center corresponds to the median, the lower and upper hinges represent the first and third quartiles, and the whiskers extend to the largest and smallest values within 1.5× the IQR from the hinges. Generalized Linear Mixed Model (glmm analysis, see “methods”) shows that cpd thickness is significantly higher in both epidermis and pericycle. i, j Transmission Electron Microscopy representative images of longitudinal and cross-section of the primary root elongation zone in WT and cpd showing the outer epidermal cell wall (longitudinal section, scale bar, 1 μm; cross section, scale bar, 0.5 μm) and the cell wall between neighboring pericycle cells (scale bar, 1 μm).
Fig. 5. A conserved local BR control of root biomass density.
a Phenotype of 10-day-old tomato WT and bri1cu3 seedlings, grown in pots (background of the image was digitally cleaned to remove the perlite grains for a clear visualization of the roots). b Confocal images showing the root tip of 10-day-old WT and bri1cu3 grown on plates. Scale bar, 100 μm c Quantification of biomass density index (BDI, mass per cm root length) in roots of 10-day-old of WT and bri1cu3, grown in pots (n: WT = 16, bri1cu3 = 22, p ≤ 0.05). d Representative images of grafted tomato seedlings 21 days after grafting (i.e., 31-day-old) of WT/WT, WT/bri1cu3, bri1cu3/bri1cu3, and bri1cu3/WT. Scale bar, 1 cm. e–g Quantification of biomass and BDI in grafts as in (d). e shoot fresh weight, f root fresh weight and g root BDI. In boxplots, the center corresponds to the median, the lower and upper hinges represent the first and third quartiles, and the whiskers extend to the largest and smallest values within 1.5× the IQR from the hinges. All data points are plotted individually. In all boxplots different letters indicate statistically significant differences (n: WT/WT = 18, WT/bri1cu3 = 17, bri1cu3/bri1cu3 = 15, bri1cu3/WT = 20, p ≤ 0.05).
Root branching scales with BR-mediated growth anisotropy and carbon availability
Thus far we have demonstrated that BR signaling promotes two processes, and each is expected to positively drive LR formation: the amount of sugar that reaches the root (evident as biomass, supported by the transcriptional profiling, this study) and anisotropic growth rate and the effect this has on longitudinal meristematic cell numbers and meristematic cell division rates16. We therefore asked if the effect of BR signaling on root branching can be explained by these two physiological and developmental outcomes. To investigate this, we developed a grow-and-branch model (see “methods” for details) to determine if these two combined effects, along with their feedback on one another, are sufficient to explain the observed phenotypes in this study. In this way the model can verify whether all BR effects have been identified, as omitting important effects would result in the model failing to reproduce the observed phenotypes. The model describes root growth rate as a function of root meristem size and meristematic division rate32 and incorporates the reflux-and-growth LR branching mechanism21 (see Supplementary methods and Supplementary Fig. 6 for details on the branching mechanism). Both growth and branching processes depend strongly on meristem parameters. The model can simulate both Arabidopsis and tomato growth over time, outputting important developmental parameters such as LR number, root length, and root FW.
The “grow and branch” model recapitulates a similar branching index
An interesting observation made is that despite a vast difference in the RSA between WT and a range of BR mutants (4-week-old, grown in pots), they all had a similar ratio between LR number and total root length, from hereon called branching index (BI) (Fig. 6a, Supplementary Fig. 4). Indeed, through simply incorporating the smaller meristem size of BR-mutants33–35 and their lower meristematic division rates16, the growth-and-branch model (model 1, Supplementary description of the model) reproduced not only WT and BR-mutant RSAs (Supplementary Fig. 4) but also their highly similar BI (Fig. 6a, Supplementary Figs. 5, 6). This was done with limited model fitting, as eight out of nine parameters were derived from literature and only one was fitted to the experimental data (Supplementary Data 4). This shows that the grow-and-branch model is sufficient to explain the observed constant BI, strongly suggesting a tight coupling between growth and branching through meristem dynamics. Interestingly, we also tested a root-clock model priming implementation, which did not result in a constant BI as was observed in the experiments (Supplementary Fig. 6, Supplementary methods), suggesting that this mechanism cannot fully explain branching in this context. Therefore, we continued to use the reflux-and-growth mechanism for LR priming in this work.
Fig. 6. Root branching scales with BR regulation of growth anisotropy and carbon availability.
a A similar branching index in WT and BR mutants is reproduced by a grow-and-branch root model parametrized to Arabidopsis growth rates and meristem dynamics. b Scatter plot of branching density and total root length in tomato. Orange and blue line plots show model outcomes with the center line representing the mean with a one standard deviation error band (n = 250). The same model was parameterized here to tomato root growth characteristics. The gray line indicates prediction of the branching index according to the WT that is based on regressing lateral root (LR) number as a function of total root length (see “methods”). Inset shows the same data and model outcomes but plotted as a function of plant developmental time. c Network of interactions derived from this study, as implemented in the root growth model to predict graft dynamics. d Heatmaps of predicted and experimental values of all graft combinations, normalized to WT/WT for LR number, total root length over time, and root biomass. Arrows indicate the change in values over time relative to the start. Simulations used 3 model settings (Supplementary description of the model), incorporating root- and shoot-specific BR effects and a transient post-grafting growth halt. e, f Model and experimental graft data at 21 days post grafting for branching index (e) and root FW (f). In boxplots, the center corresponds to the median, the lower and upper hinges represent the first and third quartiles, and the whiskers extend to the largest and smallest values within 1.5× IQR from the hinges. g Scatter plot showing dependency of root biomass (FW), LR number (size of circle/square) and total root length (color intensity) on elevating sucrose concentration. Model outputs are circles; experimental data, squares. Upper-left square/circle correspond to WT. Note that while root FW is almost fully rescued by addition of 2% sucrose (green numbers, % of WT), LR number (blue numbers) and total root length (black numbers) remain lower compared to WT. (n: WT = 7, cpd (0–2%): 13, 12, 11, 10, 10, 12, p ≤ 0.05).
The branching index has temporal dynamics
To generalize the model, we included tomato in our analysis and compared WT and bri1cu3 at three different time points, 10, 14, and 20 days. This revealed changes in BI values and BI variability over time (Fig. 6b, see dots in inset). The model, now parameterized for tomato root growth, but still with minimal fitting (Fig. 6b inset, model 2, Supplementary Fig. 7, Supplementary description of the model), shows that this is caused by a start-up phase in which BI increases once the first LR forms. After the initial BI peak, BI decreases due to the modeled slower growth of higher-order LRs, assumed to be caused by reduced carbon supply. The different temporal BI dynamics of WT and bri1cu3 plants result from their distinct root growth parameters. BIs become similar once the bri1cu3 mutant is past its start-up and the WT has not yet begun its decrease phase (around 14 days, Fig. 6b inset). Note that for the model to reproduce the bri1cu3 mutant data precisely, in addition to lower meristem size and division rate we needed to assume a delay in LR emergence. This is consistent with the limited carbon supply by bri1’s shoot, which is needed to promote auxin-mediated LR regulation2.
The grow-and-branch model predicts temporal dynamics in grafted plants
Next, we refined our model by discerning the effects of shoot-BR-dependent carbon supply, root-BR-dependent growth anisotropy, and BDI (model 3, Fig. 6c, Supplementary description of the model). After incorporating these details (Supplementary Fig. 8), the model predicts that RSA and mass (FW) of WT/bri1cu3 grafts, which start with a small RSA of a full bri1cu3 mutant, is increasingly rescued over time by its new WT shoot due to increased carbon availability. In contrast, RSA and FW of bri1cu3/WT grafts, which start with the large RSA of a full WT, gradually decrease relative to WT/WT due to the decreased carbon supply from the new bri1cu3 shoot. Experiments based on these model predictions showed similar dynamics (Supplementary Fig. 8, Fig. 6d), again showing that the grow-and-branch model incorporating prior knowledge and observations from this study on BR effects (Fig. 6c) is sufficient to explain the tomato phenotypes. The model however did not predict a decline in bri1cu3/bri1cu3 as observed experimentally. This is likely a specific outcome of the grafting procedure, as reflected in the lowest success rates quantified for the mutant homograft in Arabidopsis, which is not included in the model (Supplementary Fig. 1, Supplementary Data 1). Nonetheless, the predicted and experimental RSA development exhibited very similar trends, as evident after normalizing both results for WT/WT (Supplementary Fig. 9), strongly indicating that the proposed model (Fig. 6c) is sufficient to explain the observed phenotypes. Interestingly, the model recapitulated the slightly higher BI value, compared to WT/WT, obtained when WT shoot is grafted over a mutant root (day 21, Fig. 6e), and this outcome was also obtained experimentally in both tomato and Arabidopsis (Supplementary Fig. 10), as well as the root FW differences between the grafts (Fig. 6f).
Addition of sugar rescues cpd’s root biomass but not total root length and LR number
Our data showed biomass rescue of the WT/bri1 but a limited RSA rescue, which suggests that BR-deficient mutants would have a higher carbon demand for root elongation. The model (model 4, Supplementary description of the model) also predicts that for increased carbon, cpd roots are rescued for biomass but not for root length and number, and the experiment confirms these outcomes (Fig. 6g). cpd roots require about 58 mM (2%) sucrose to reach about 90 percent of the WT root mass grown without exogenous sugar (Fig. 6g, Supplementary Fig. 11a, b). The elevation of the WT root biomass peaked at 0.5% sucrose, declining again for higher concentrations (Supplemental Fig. 11a, b). The partial recovery of the cpd root growth parameters combined with its inherently higher BDI allows for a substantial recovery of root biomass, while the smaller meristem size and higher carbon cost per unit length reducing longitudinal division rate, precludes a full recovery of RSA. This recovery of root biomass was dependent on shoot-mediated sugar absorption1, as also supported by our data (Supplementary Fig. 11c–e).
In conclusion, our simulation model demonstrates that shoot BR-dependent carbon availability and root BR-dependent biomass distribution are sufficient to explain RSA phenotype and temporal RSA development in both WT and BR-deficient mutants.
Discussion
Growth is a multiscale process, occurring at the cell and the organ levels, tuned by the environment and by systemic signals across the entire plant body. By dissecting the effects of BR production and signaling in the shoot and root, we uncoupled carbon availability and overall biomass growth from anisotropy growth and revealed that these two processes, governed by BRI1 in the shoot and root respectively, are sufficient to determine the extent of root growth and branching and thus RSA. Our use of two plant species, Arabidopsis and tomato, in experiments and simulation models, support and generalize these findings.
Root biomass growth was found here to be strictly dependent on BR-mediated growth (i.e., irreversible increase in size) of the shoot, which inherently results in an elevated number of photosynthetic cells. The previously reported promoting effect of BR on photosynthesis and sugar production is likely an additional integral component36–40. Our results also imply that the shoot-derived carbon reaches the root, as apparent in the rescue of DW (and FW) in the three independent BR mutants tested, both in agar plates and in pots. This was accompanied by restoring the levels of most of the metabolites measured in cpd roots. In the absence of BR signaling, the dwarf shoot cannot support carbon demand for root growth, as evidenced by the attenuation of the WT root growth and root branching, and by de-repression of genes comprising functional photosynthesis and carbon fixation categories. Interestingly, the elevation of photosynthesis-related genes occurs in non-photosynthetic root cells, as was previously reported for non-photosynthetic Arabidopsis suspension cells27. It remains unclear if this has a physiological relevance beyond mere reporting on starvation. Further analysis of the grafts’ transcriptome revealed that de-repression of these genes in starved WT roots resulted in a transcriptome profile more similar to cpd roots, where these genes are expressed irrespective of carbon availability. This is reminiscent of the constitutive expression of light-responsive genes in BR biosynthesis mutants, even when grown in the dark, which may point to shared regulatory mechanisms with those of sugar starvation41,42. It is conceivable that BR signaling in the root is positively regulated by carbon availability, as in etiolated seedlings, where sugar-TARGET OF RAPAMYCIN (TOR) signaling degrades BIN2 and stabilizes BZR143–45, maintaining the low expression of carbon starvation-response genes.
The developmental program is shown here to determine the carbon distribution within the growing organ. Loss of BR function in the root leads to a higher mass per unit root length, which results from a reduced anisotropic growth rate and its consequential radial extension16. Another outcome of a reduced anisotropy growth rate could be the thicker cell walls observed in cpd root elongating cells, as revealed here. For example, in etiolated hypocotyls, cells initially have thick cell walls, which thin out as they enter the expansion zone and begin to expand46. Growth anisotropy in both shoot and root depends on BR signaling-mediated regulation of the mechanical properties of the epidermis16,47,48. The potential relevance of cell wall thickness for this process may vary between the two organs, as no difference in thickness of the outer epidermal wall was observed in dwf4’s hypocotyl48. Future research is needed to reveal the precise regulation of cell wall modification by the BR signaling and its integration with a biomechanical regulation of growth.
In this study, we developed a grow and branch model simulating RSA development, where we separately specified the effects of shoot and root BR signaling on RSA. This enabled us to demonstrate how shoot-BR-dependent carbon availability affects root cell divisions and how root-BR signaling-dependent cellular anisotropy affects meristem size, cell division rate, and biomass density. These factors largely predict the observed RSA in WT, BR-mutant, and grafted Arabidopsis and tomato plants. Auxin is a key regulator of LR formation49. Given the reported BR-auxin interaction in the root meristem25,50, this hormonal interplay could influence meristem size and cell division rate, thereby affecting branching, according to our model. However, not all auxin effects are included in the model. BR signaling may also directly regulate LR organogenesis17–19, as evidenced when LR is formed in response to local mechanical stress51. In the future, it would be interesting to explore how the integration of effects caused by BR-driven shoot and root system phenotypes, local LR, and carbon availability together shape RSA dynamics, as well as the agricultural potential for obtaining grafting-based short, branched, and radially expanded root system.
Methods
Plant material, growth conditions
All Arabidopsis (Arabidopsis thaliana) lines were in the Columbia (Col-0) background. The following published mutants were used: dwf4 (salk_020761), cpd42, det2-152, br6ox1 (salk_068952); br6ox2 (salk_129352), br6ox1 br6ox2, bri1-116 and bri1cu3 in the Solenum pimpinellifolium background31. For grafting of Arabidopsis, 35S-GFP-LTI6B53 was used as WT rootstock or scion. For Arabidopsis experiments, seeds were sterilized and germinated on one-half-strength Murashige and Skoog (MS) medium supplemented with 0.8% plant agar, 0.46 g l−1 MES (pH 5.8) and 0.2% (w/v) sucrose. Plates with sterilized seeds were stratified in the dark for two days at 4 °C. For long-term growth of un-grafted plants, these plates were transferred to 22 °C and to a 16 h light/8 h dark cycle (70 μmol m−2 s−1) for 7 days, and the seedlings were then transferred to pots containing perlite (Supplementary Fig. 1d) in growth rooms with 16 h light/8 h dark cycle (50 μmol m−2 s−1) for 3 weeks. For sugar experiment, 7 days post germinated seedlings of Col-0 and cpd were transferred to ½ MS media plates containing 0% (0 mM), 0.2% (5.8 mM), 0.5% (14.6 mM), 1% (29 mM), 1.5% (43.82 mM) and 2% (58.42 mM) sucrose (w/v) and grown for 3 weeks on above mentioned conditions. To separate shoots from the media, an independent experiment was conducted in which parafilm strips were sterilized using UV light and 100% ethanol, air-dried, and placed on the media plates. To anchor the shoots on top of the parafilm and prevent them from falling during growth, 50 µl of sterilized 0.8% plant agar dissolved in water was applied to the parafilm, and the shoots were placed on it without embedding. Plates were incubated in growth chambers under the previously described conditions for 2 weeks, after which the seedlings were washed to remove any adhering media, patted dry with Kimwipes, and weighed for FW measurements. For tomato experiments, segregating seeds of bri1cu3 were soaked in water overnight and sterilized using a treatment of 3% sodium hypochlorite and 0.01% Tween-20 for 15 min on shaking and then rinsed five times with sterile water. For root phenotyping, seeds were sown directly on perlite watered with liquid 1/2 MS (pH 5.8) without sugar and grown at a 16 h light/8 h dark cycle (70 μmol m−2 s−1) at 24 °C in growth chambers for required periods.
Micrografting
Arabidopsis micrografting was performed as described in ref. 54 with few modifications. Briefly, sterilized and stratified Col-0, 35S-GFP-LTI6B, cpd and bri1-116 seeds were grown vertically on the agar media for 7 days. Seedlings of similar size were transferred to a 90 mm square petri dish lined with sterile HybondTM-N membrane (Amershem) arranged on two 100 mm discs of Whatman® filter paper grade 3 soaked in sterile water. Grafts were performed in sterile conditions under Nikon SMZ445 zoom stereo microscope using the transverse cut and butt alignment method after removing both cotyledons. Ungrafted controls were also treated with the same procedure except the cut and paste of hypocotyl. Excess water was removed, and grafts were left for healing in the same plates for 7 days. Successfully attached grafts were then transferred to plates with agar media for 3 weeks and were observed for their growth during this time, by scanning weekly. After 3 weeks (i.e., when the plants were 5-week-old), successful grafts were transferred to pots containing perlite soaked in one-half-strength MS for an additional 3 weeks to determine their biomass. For RNA-seq analysis grafting was performed by same method as described above while un-grafted cpd seedlings went through the same procedure except for the hypocotyl cut and joining. Micrografting in tomato bri1cu3 heterozygous and homozygous plants was similarly performed except the grafting was performed on 10-day-old seedlings with wedge and slit method and the growth chamber conditions were kept suitable for tomato.
Root phenotypic analysis
RSA analysis was performed on 5-week-old grafts. More specifically, successful grafted seedlings were removed 3 weeks after transferring to the agar plates and washed in a petri dish filled with water using a soft brush to remove traces of agar sticking to roots and spread on fresh plates with 1% agarose for root visualization. These plates were scanned using Epson perfection V850 pro scanner at 1200 DPI. Root length and LR numbers were measured from these scanned images using ImageJ (www.imagej.net). For biomass measurements in Arabidopsis, grafted and un-grafted seedlings (8-week-old) were taken out from the pots and washed with water to remove the perlite grains. Clean roots and shoots were separated by cutting the root-hypocotyl junction and pat dried with tissue paper to remove excess water and weighed for FW. For DW analysis, roots were freeze-dried for 48 h and weighed again. For the analysis of tomato roots, RSA was analyzed as in Arabidopsis, except that the seedlings were 31-day-old. These seedlings from plates were used for biomass determination after washing and pat drying them, as described for Arabidopsis.
Confocal microscopy
Root tips (~3–5 mm) of successfully grafted plants 3 weeks after graft recovery were cut, fixed and stained using modified pseudo-schiff propidium iodide (mPS-PI) staining protocol55. Stained roots were placed in a chamber made of 200-μm-thick dual-sided tape (to prevent the sample from changing shape due to coverslip pressure) on a glass slide and a cover slip was glued above. Imaging was conducted using an LSM 510 META confocal laser-scanning microscope with a ×25 oil immersion objective lens (NA 0.8). PI was viewed with an excitation wavelength of 561 nm, and emission was collected with a 575-nm bandpass filter, using a fine pinhole of 1 μm and Z-stacks of 0.8 μM. For tomato meristem, primary roots of 10 days post germinated seedlings were cut ~1 cm from the root tip and were immediately fixed in 4% paraformaldehyde in a 1 × PBS solution with 1 h vacuum on room temperature. After overnight incubation, roots were washed twice in 1 × PBS, the samples were then cleared in ClearSee solution56 for 1 week. To visualize the cell walls, the calcofluor white staining was performed with 0.02% calcofluor-white (sigma) diliuted in the ClearSee solution for 2 days, followed by two washing steps with ClearSee. Samples were incubated in ClearSee solution for a minimum of 2 weeks before imaging. Images were taken with a confocal laser scanning microscope (LSM 710) with 405 nm excitation and detected at 415–500 nm emitted light for visualization of calcofluor-white. Images were later processed in ImageJ (Fiji).
Metabolic profiling
Polar metabolites were extracted following an established gas chromatography-mass spectrometry (GC-MS)-based metabolite profiling protocol57, with slight modifications. Successfully grafted plants grown for 3 weeks in perlite (8 weeks old) were taken out, washed thoroughly with double distilled water and roots were separated from shoots. Roots were pat dried with tissue paper lined with Kim wipes and weighed. Samples were collected into sterile tubes and immediately frozen in liquid nitrogen and lyophilized. About 12 mg DW was used per sample in 6 biological replicates. The samples were ground and incubated in 700 μl methanol containing an internal standard (0.016 mg/ml pentaerythritol), shaken for 20 min at 25 °C, and centrifuged at 20,800 × g for 10 min. 650 μl of the supernatant was transferred to new tubes, and 375 μl chloroform and 750 μl double-distilled water was added. Tubes were then centrifuged at 20,800 × g for 15 min, and aliquots of 150 μl of the upper phase of each sample were transferred to new 1.5 ml tubes and dried by Speedvac (Concentrator Plus, Eppendorf, Hamburg, Germany), overnight. Derivatization was carried out as previously described57.
Polar metabolites (Supplementary data 3) were measured by the Agilent 7200B GC/Q-TOF. The injection and separation procedures were performed according to58 using the DB-35MS column. Metabolite detection and annotation were performed by Quant software (Agilent, Santa Clara, CA, USA), according to an accurate mass library of known markers generated by our group and run in the same system. Following blank subtraction, the peak area of each metabolite was normalized to the internal standard (i.e., ribitol) in each sample, and by the FW of the sample.
RNA extraction
Roots were separated from shoots by dissecting the root-hypocotyl junction of the grafted seedlings 3 weeks after transfer to agar plates. The roots were briefly washed in sterile DEPC-treated DDW and immediately frozen in liquid nitrogen. Three roots were collected to make one biological replicate, a total of 5 replicates were used for each sample type for analysis. RNA was extracted from roots using the RNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s protocol. RNA concentration was determined using a NanoDrop Spectrophotometer (ND-1000). This RNA was further used for RNA -seq.
RNA-seq procedure
The quality of the RNA was evaluated using the TapeStation4200 (Agilent) with the RNA kit (cat no. 5067-5576). The RIN values of all samples were in the range of 8.1–9.8, indicating high-quality RNA. 20 RNA-seq libraries were constructed simultaneously using NEBNext Ultra II Directional RNA Library Prep Kit for Illumina (NEB, cat no. E7760), according to the manufacturer’s protocol. 300 ng total RNA was used as the starting material, with 11 PCR cycles for the amplification step. mRNA pull-down was performed using the NEBNext® Poly(A) mRNA Magnetic Isolation Module (NEB, cat no. E7490). RNA-seq library QC was performed by measuring library concentration using Qubit (Invitrogen) with the Equalbit dsDNA HS Assay Kit (Vazyme, cat no. EQ121) and size determination using the TapeStation 4200 with the High Sensitivity D1000 kit (cat no. 5067-5584). All libraries were then mixed into a single tube with equal molarity. The RNA-seq data was generated on Illumina NextSeq2000, using P2100 cycles (Read1-100; Index1-8; Index2-8;) (Illumina, cat no. 20046811). Reads were aligned to the TAIR10 assembly of the Arabidopsis thaliana genome, and counted using STAR (version 2.7)59. Differential expression of RNAseq was performed using the combination of RUVseq60 and DESeq261.
Statistical analysis
For proportion test (graft success, Supplementary Fig. 1), a Chi-squared test on the equality of proportions was performed followed by pairwise comparisons and holm correction for multiple comparisons. For hypothesis testing for phenotypic variables and metabolites, one-way analysis of variance (ANOVA) was performed using the lm function62. ANOVA assumptions were examined using diagnostic graphs. If ANOVA assumptions were met, pairwise tests were performed between treatments using EMMEANS followed by correction for multiple comparisons using Tukey’s HSD63. If ANOVA assumptions were not met, transformation was performed, followed by assumptions examination and pairwise comparisons. If after transformation ANOVA assumptions were still not met, Kruskal-Wallis test was performed followed by pairwise comparisons and multiple comparisons correction using Dunn test. Treatments significantly different from each other (adjusted p < 0.05) are indicated by different letters, treatments not significantly different are indicated by the same letter. For each test performed, the test, transformation, and adjusted p values are indicated in Supplementary Data 1. In two analyses (Supplementary Fig. 2f, g) the number of observations in bri1/bri1 and bri1/WT was small and as a result the assumption of homoscedasticity could not be achieved. We therefore added simulated data to these groups, from a normal distribution with the group’s mean and the WT/WT standard deviation. This simulation is conservative since the standard deviation of DW and FW increases with total root length, and the length of these groups is significantly smaller than WT/WT. To predict BI (gray line Fig. 6b), we fitted generalized linear model with Poisson distribution and identity link function to WT plants with total root length as independent variable and LR number as the dependent variable. The model was then used to predict LR number for different root lengths and the index was calculated as predicted LR number/total root length. For TEM data that includes repeated measures we used Generalized Linear Mixed Model (glmm), as implemented in lme464, with inverse gaussian family and identity link function. Homoscedasticity of the residuals assumption was checked using F test. For phenotypic data from experiments related to Dynamic tomato model (Supplementary Fig. 7), t test with Welch’s correction for unequal variances was performed, followed by Bonferroni correction for multiple comparisons. All statistical tests are detailed in Supplementary Data 1. All tests performed are two tailed.
Transmission electron microscopy
Roots of Col-0 and cpd (7-day-old) were cut ~2–3 mm from the tip in the puddle of one-half-strength-MS on a glass slide under Nikon SMZ445 zoom stereo microscope and immediately placed under vacuum for 30 min at RT in the fixation solution containing 2% Glutaraldehyde, 2% Paraformaldehyde in 0.1 M Sodium Cacodylate buffer pH 7.4 to allow the root tips sink. Then the fragments were incubated at 4 °C for 16 h, washed with buffer, post-fixed for 1 h on ice with 1% Osmium Tetroxide in Cacodylate buffer, washed again, and immersed in 1% Uranyl Acetate for 60 min. Following dehydration using ethanol, the specimens were infiltrated with epoxy resin for 4 days, oriented in silicon molds and polymerized at 60 °C. Transverse ultra-thin 80 nm sections of the root zone of interest (400 μm and 270 μm for WT and cpd respectively) were cut using ultramicrotome UC7 (Leica), transferred to copper grids and viewed using Talos L120C Transmission Electron Microscope at an accelerating voltage of 120 kV. For longitudinal sections, cuts were made parallel to longitudinal axis of roots and approximately middle section was selected under stereo microscope. Images were acquired at the distances from tip corresponding to cross sections in same zones.
Modeling root growth and branching
A growth-and-branching model simulating the development of RSA was developed and implemented in the Lpy framework for functional-structural plant models65. The baseline model and subsequent extensions are available at https://github.com/BasvdHerik/BR_Branching. Detailed model parameterization and model extensions are elaborated in the Supplementary Methods. The model is used to describe the hierarchically branching tap root architecture of Arabidopsis thaliana and tomato root systems. In the model, root systems consist of three functionally different segment types; root segments, growing root apices and developing early-stage LR primordia, here defined as prebranch sites. Growing root apices represent the part of a root containing the meristem and elongation zone and increase in length (L) every timestep, based on a growth function explained in detail below. When a LR priming event occurs in a growing root apex (also explained in detail below), a new root segment is created, inheriting the state of the apex (length, age, and order) while the apex length is reset and will continue growth of the root. Concurrently, a prebranch site is created forming a side branch between the apex and new root segment that after a time interval needed for LR development and emergence (timeem) will become a growing LR apex. Note that in reality, priming may or may not result in stable prebranch site formation, a stable prebranch site may or may not undergo LR initiation, and a LR primordium formed after LR initiation may or may not result in an emerged LR. To keep our model simple, the timing and chances for each of these transitions are compiled into this single emergence time as well as an emergence parameter translating priming frequency into LR forming frequency.
Initially, primary first order root is growing, with higher order LRs forming over time. The roots are grown on a 2D-plane for graphical output. A newly formed root apex would have an insertion angle between 60 and 75 degrees compared to its parent root and primordia are consecutively formed on both sides of the root. During root growth, roots are growing towards a gravitropic set point angle of 90 degrees.
Growth and branching are both a function of root meristem size and meristematic division rate21,32. Growth of an individual root meristem (groot) is defined as the product of meristematic cell production, the rate at which a meristem produces new cells32, and cell elongation rate, the rate at which cells leaving the meristem increase in size (Eq. 1). Cell production is dependent on meristem size, the number of actively dividing cells in the meristem (Msize) and meristematic division rate (Dfreq) (Eq. 2), whereas cell elongation rate (eloncell) is considered a constant factor. We assume that higher order roots typically have a smaller diameter and correspondingly smaller vascular bundles66, resulting in reduced phloem driven carbon transport and sink strength, and hence a reduced division rate (Eq. 3). The model also incorporates the fact that upon formation or activation of a new root meristem, the number of cells making up the meristem increases over time in a saturating manner towards a maximum meristem size (Msizemax)(Eq. 4)67
| 1 |
| 2 |
| 3 |
| 4 |
where age is the time in hours since the formation of the meristem, and Km is the age at which the meristem size is half maximal, minimum meristem size was set at 1. Upon new apex formation, meristem size is set at this minimum size.
Root system branching, particularly the patterning of prebranch sites competent for LR formation (priming) is modeled based on the reflux-and-growth priming mechanism21. According to this mechanism, priming frequency depends on the meristematic division frequency (Dfreq), meristematic clone number, the number of clones of sibling cells arising from stem cell division in the meristem, (~log2(Msize)), and asynchronicity in division timing between different clones in the meristem (driven by differences between stem cell niche division rate (SCfreq) and the faster (average) division rate in the rest of the meristem Dfreq) (for a detailed derivation see ref. 21). In this work, we estimated SCfreq to be 5-fold lower than Dfreq, based on68. Combined, this leads to the following equation for the frequency of LR formation:
| 5 |
Here, Emratio represents the ratio between emerged LRs and formed priming sites as reported in van den Berg et al.21. This ratio is dependent on the meristem size, where larger meristems have a higher chance for LRs to emerge:
| 6 |
In addition to the reflux-and-growth mechanism for LR priming, our modeling framework also investigated alternative mechanisms, including the clock-and-wavefront and Turing mechanisms. Only the Turing and reflux-and-growth mechanisms produced root branching patterns consistent with experimental data, without requiring additional assumptions. Since the predictions of the reflux-and-growth mechanism were previously validated experimentally21, we proceeded with this mechanism for the rest of our simulations.
To simulate not only RSA but also root FW we modeled the effect of root-BR driven cellular anisotropy and hence root radius as well as root-BR driven cell wall width, which together affect the weight per unit root length (root biomass density). Root lengths and weight per unit length together determine root FW biomass:
| 7 |
See Supplementary information for further details and Supplementary Data 4.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Source data
Acknowledgements
We thank the Biomedical Core Facility (L. Shaulov) at the Technion and the Technion Genomics Center (N. Fourier). We thank Dr. N. Holland for her initial contribution to the project, D. Gasperini for the technical advice on micrografting, I. Efroni for discussions and comments on the manuscript, and S.S-G lab members. This research was supported by the Israel Science Foundation grant (no. 1351/23) to S.S.-G. and by a VICI grant (VI.C.202.011 12268) awarded by the Netherlands Organization for Scientific Research (NWO) to B.v.d.H. and K.t.T.
Author contributions
H.K. and S.S.-G. initiated and designed the experiment. H.K. performed all experiments. G.H. analyzed the data together with H.K. and S.S.-G. and established statistical models. B.v.d.H. and K.t.T. designed, performed, and wrote the grow and branch model. T.L. produced graphs. Y.S. and T.A.-W. performed the metabolic analysis. S.S.-G., H.K., B.v.d.H., G.H., and K.t.T. wrote the paper with input from all the authors.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data needed to evaluate the conclusions in this paper are included in the paper and/or provided in the Supplementary Information/Source Data file. The RNA-seq data used in this study are available in the GEO database under accession code GSE274161. Source data are provided with this paper.
Code availability
Source code for the model is available at: https://github.com/BasvdHerik/BR_Branching/.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hitaishi Khandal, Guy Horev.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-59202-6.
References
- 1.Macgregor, D. R., Deak, K. I., Ingram, P. A. & Malamy, J. E. Root system architecture in Arabidopsis grown in culture is regulated by sucrose uptake in the aerial tissues. Plant Cell20, 2643–2660 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kircher, S. & Schopfer, P. Photosynthetic sucrose drives the lateral root clock in Arabidopsis seedlings. Curr. Biol.33, 2201–2212 e2203 (2023). [DOI] [PubMed] [Google Scholar]
- 3.Kircher, S. & Schopfer, P. Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc. Natl. Acad. Sci. USA109, 11217–11221 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crookshanks, M., Taylor, G. & Broadmeadow, M. Elevated CO(2) and tree root growth: contrasting responses in Fraxinus excelsior, Quercus petraea and Pinus sylvestris. N. Phytol.138, 241–250 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Xiong, Y. et al. Glucose-TOR signalling reprograms the transcriptome and activates meristems. Nature496, 181–186 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reyes-Hernandez, B. J. & Maizel, A. Tunable recurrent priming of lateral roots in Arabidopsis: more than just a clock? Curr. Opin. Plant Biol.76, 102479 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Morales-Herrera, S., Paul, M. J., Van Dijck, P. & Beeckman, T. SnRK1/TOR/T6P: three musketeers guarding energy for root growth. Trends Plant Sci. 29, 1066–1076 (2024). [DOI] [PubMed]
- 8.Clouse, S. D. & Sasse, J. M. Brassinosteroids: essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol.49, 427–451 (1998). [DOI] [PubMed] [Google Scholar]
- 9.Wei, Z. Y. & Li, J. Regulation of brassinosteroid homeostasis in higher plants. Front. Plant Sci.11, 583622 (2020). [DOI] [PMC free article] [PubMed]
- 10.Bajguz, A., Chmur, M. & Gruszka, D. Comprehensive overview of the brassinosteroid biosynthesis pathways: substrates, products, inhibitors, and connections. Front. Plant Sci.11, 1034 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim, E. J. & Russinova, E. Brassinosteroid signalling. Curr. Biol.30, R294–R298 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Ackerman-Lavert, M. & Savaldi-Goldstein, S. Growth models from a brassinosteroid perspective. Curr. Opin. Plant Biol.53, 90–97 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Planas-Riverola, A. et al. Brassinosteroid signaling in plant development and adaptation to stress. Development146, dev151894 (2019). [DOI] [PMC free article] [PubMed]
- 14.Aardening, Z., Khandal, H., Erlichman, O. A. & Savaldi-Goldstein, S. The whole and its parts: cell-specific functions of brassinosteroids. Trends Plant Sci.30, 389–408 (2025). [DOI] [PubMed]
- 15.Graeff, M. et al. A single-cell morpho-transcriptomic map of brassinosteroid action in the Arabidopsis root. Mol. Plant14, 1985–1999 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fridman, Y. et al. The root meristem is shaped by brassinosteroid control of cell geometry. Nat. Plants7, 1475–1484 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta, A., Singh, M. & Laxmi, A. Interaction between glucose and brassinosteroid during the regulation of lateral root development in Arabidopsis. Plant Physiol.168, 307–320 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao, F. et al. Brassinosteroids interact with auxin to promote lateral root development in Arabidopsis. Plant Physiol.134, 1624–1631 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou, J. et al. The histone deacetylase 1/GSK3/SHAGGY-like kinase 2/BRASSINAZOLE-RESISTANT 1 module controls lateral root formation in rice. Plant Physiol.189, 858–873 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno-Risueno, M. A. et al. Oscillating gene expression determines competence for periodic Arabidopsis root branching. Science329, 1306–1311 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Berg, T. et al. A reflux-and-growth mechanism explains oscillatory patterning of lateral root branching sites. Dev. Cell56, 2176–2191 e2110 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Thomas, H. R. & Frank, M. H. Connecting the pieces: uncovering the molecular basis for long-distance communication through plant grafting. New Phytol.223, 582–589 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Feng, M., Augstein, F., Kareem, A. & Melnyk, C. W. Plant grafting: molecular mechanisms and applications. Mol. Plant17, 75–91 (2024). [DOI] [PubMed] [Google Scholar]
- 24.Symons, G. M. & Reid, J. B. Brassinosteroids do not undergo long-distance transport in pea. Implications for the regulation of endogenous brassinosteroid levels. Plant Physiol.135, 2196–2206 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ackerman-Lavert, M. et al. Auxin requirements for a meristematic state in roots depend on a dual brassinosteroid function. Curr. Biol.31, 4462–4472.e4466 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Melnyk, C. W. et al. Transcriptome dynamics at Arabidopsis graft junctions reveal an intertissue recognition mechanism that activates vascular regeneration. Proc. Natl. Acad. Sci. USA115, E2447–E2456 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Contento, A. L., Kim, S. J. & Bassham, D. C. Transcriptome profiling of the response of Arabidopsis suspension culture cells to Suc starvation. Plant Physiol.135, 2330–2347 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheen, J. Metabolic repression of transcription in higher plants. Plant Cell2, 1027–1038 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, Z. et al. Optimal BR signalling is required for adequate cell wall orientation in the Arabidopsis root meristem. Development148, dev199504 (2021). [DOI] [PMC free article] [PubMed]
- 30.Montoya, T. et al. Cloning the tomato curl3 gene highlights the putative dual role of the leucine-rich repeat receptor kinase tBRI1/SR160 in plant steroid hormone and peptide hormone signaling. Plant Cell14, 3163–3176 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koka, C. V. et al. A putative role for the tomato genes DUMPY and CURL-3 in brassinosteroid biosynthesis and response. Plant Physiol.122, 85–98 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beemster, G. T. & Baskin, T. I. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol.116, 1515–1526 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hacham, Y. et al. Brassinosteroid perception in the epidermis controls root meristem size. Development138, 839–848 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Garcia, M. P. et al. Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development138, 849–859 (2011). [DOI] [PubMed] [Google Scholar]
- 35.Vragović, K. et al. Translatome analyses capture of opposing tissue-specific brassinosteroid signals orchestrating root meristem differentiation. Proc. Natl. Acad. Sci. USA112, 923–928 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia, X. J. et al. Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta230, 1185–1196 (2009). [DOI] [PubMed] [Google Scholar]
- 37.Li, X. J. et al. Overexpression of a brassinosteroid biosynthetic gene enhances photosynthetic capacity through activation of Calvin cycle enzymes in tomato. BMC Plant Biol.16, 1–12 (2016). [DOI] [PMC free article] [PubMed]
- 38.Yin, X., Tang, M., Xia, X. & Yu, J. BRASSINAZOLE RESISTANT 1 Mediates Brassinosteroid-Induced Calvin Cycle to Promote Photosynthesis in Tomato. Front. Plant Sci.12, 811948 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlüter, U., Köpke, D., Altmann, T. & Müssig, C. Analysis of carbohydrate metabolism of antisense plants and the brassinosteroid-deficient mutant. Plant Cell Environ.25, 783–791 (2002). [Google Scholar]
- 40.Fabregas, N. et al. Overexpression of the vascular brassinosteroid receptor BRL3 confers drought resistance without penalizing plant growth. Nat. Commun.9, 4680 (2018). [DOI] [PMC free article] [PubMed]
- 41.Chory, J., Nagpal, P. & Peto, C. A. Phenotypic and genetic-analysis of Det2, a new mutant that affects light-regulated seedling development in arabidopsis. Plant Cell3, 445–459 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szekeres, M. et al. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell85, 171–182 (1996). [DOI] [PubMed] [Google Scholar]
- 43.Sheng, H., Zhang, S., Wei, Y. & Chen, S. Exogenous application of low-concentration sugar enhances brassinosteroid signaling for skotomorphogenesis by promoting BIN2 degradation. Int. J. Mol. Sci.22, 13588 (2021). [DOI] [PMC free article] [PubMed]
- 44.Zhang, Z. et al. TOR signaling promotes accumulation of BZR1 to balance growth with carbon availability in Arabidopsis. Curr. Biol.26, 1854–1860 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, Y. Q. et al. Brassinosteroid is required for sugar promotion of hypocotyl elongation in darkness. Planta242, 881–893 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Refregier, G., Pelletier, S., Jaillard, D. & Hofte, H. Interaction between wall deposition and cell elongation in dark-grown hypocotyl cells in Arabidopsis. Plant Physiol.135, 959–968 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savaldi-Goldstein, S., Peto, C. & Chory, J. The epidermis both drives and restricts plant shoot growth. Nature446, 199–202 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Kelly-Bellow, R. et al. Brassinosteroid coordinates cell layer interactions in plants via cell wall and tissue mechanics. Science380, 1275–1281 (2023). [DOI] [PubMed] [Google Scholar]
- 49.Cavallari, N., Artner, C. & Benkova, E. Auxin-Regulated Lateral Root Organogenesis. Cold Spring Harb. Perspect. Biol.13, a039941 (2021). [DOI] [PMC free article] [PubMed]
- 50.Chaiwanon, J. & Wang, Z. Y. Spatiotemporal brassinosteroid signaling and antagonism with auxin pattern stem cell dynamics in Arabidopsis roots. Curr. Biol.25, 1031–1042 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canher, B. et al. The regeneration factors ERF114 and ERF115 regulate auxin-mediated lateral root development in response to mechanical cues. Mol. Plant15, 1543–1557 (2022). [DOI] [PubMed] [Google Scholar]
- 52.Li, J., Nagpal, P., Vitart, V., McMorris, T. C. & Chory, J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science272, 398–401 (1996). [DOI] [PubMed] [Google Scholar]
- 53.Cutler, S. R., Ehrhardt, D. W., Griffitts, J. S. & Somerville, C. R. RandomG. F. P. cDNA fusions enable visualization of subcellular structures in cells of Arabidopsis at a high frequency. Proc. Natl. Acad. Sci. USA97, 3718–3723 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turnbull, C. G., Booker, J. P. & Leyser, H. M. Micrografting techniques for testing long-distance signalling in Arabidopsis. Plant J.32, 255–262 (2002). [DOI] [PubMed] [Google Scholar]
- 55.Truernit, E., Bauby, H., Belcram, K., Barthelemy, J. & Palauqui, J. C. OCTOPUS, a polarly localised membrane-associated protein, regulates phloem differentiation entry in Arabidopsis thaliana. Development139, 1306–1315 (2012). [DOI] [PubMed] [Google Scholar]
- 56.Kurihara, D., Mizuta, Y., Sato, Y. & Higashiyama, T. ClearSee: a rapid optical clearing reagent for whole-plant fluorescence imaging. Development142, 4168–4179 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lisec, J., Schauer, N., Kopka, J., Willmitzer, L. & Fernie, A. R. Gas chromatography mass spectrometry-based metabolite profiling in plants. Nat. Protoc.1, 387–396 (2006). [DOI] [PubMed] [Google Scholar]
- 58.Dhatt, B. K. et al. Metabolic dynamics of developing rice seeds under high night-time temperature stress. Front. Plant Sci.10, 1443 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Risso, D., Ngai, J., Speed, T. P. & Dudoit, S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol.32, 896–902 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol.15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Team RC. R.: A language and environment for statistical computing. R Foundation for Statistical Computing (2018).
- 63.Lenth, R. emmeans: estimated marginal means, aka least-squares means. R. Package Version 1, 2.10 (2024).
- 64.Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw.67, 48 (2015). [Google Scholar]
- 65.Boudon, F., Pradal, C., Cokelaer, T., Prusinkiewicz, P. & Godin, C. L-py: an L-system simulation framework for modeling plant architecture development based on a dynamic language. Front. Plant Sci.3, 76 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu, Q., Pages, L. & Wu, J. Relationships between root diameter, root length and root branching along lateral roots in adult, field-grown maize. Ann. Bot.117, 379–390 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dello Ioio, R. et al. Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr. Biol.17, 678–682 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Rahni, R. & Birnbaum, K. D. Week-long imaging of cell divisions in the Arabidopsis root meristem. Plant Methods15, 30 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data needed to evaluate the conclusions in this paper are included in the paper and/or provided in the Supplementary Information/Source Data file. The RNA-seq data used in this study are available in the GEO database under accession code GSE274161. Source data are provided with this paper.
Source code for the model is available at: https://github.com/BasvdHerik/BR_Branching/.